Effect of Inoculation Rhizobium and Response of Soybean-Rhizobium System to Insoluble Phosphate
Li Xiu-ping, Chen Shu-zhen, Zhang Yan-lai Cheng Yan-bo, and Nian Hai*
1 Guangdong AIB Polytechnic College, Guangzhou 510507, China
2 National Center for Soybean Improvement, College of Agriculture, South China Agriculture University, Guangzhou 510642, China
Introduction
Soybean-Rhizobiumsymbiotic system possesses the ability to fix N2and make it available to plants.Some studies suggest that the regulation of N2-fixation has a direct effect on nitrogenase activity in the nodules(Magadlelaet al., 2012; Quernéaet al., 2017; Rejiliet al., 2012).The inoculation ofRhizobiumis known to increase nodulation, growth, nitrogen uptake and yield of crop plants (Elkocaet al., 2008; Zhanget al.,2010).
Rhizobiacan also solubilize organic and inorganic phosphate (Panditet al., 2014; Bargazet al., 2013).Availability of this phosphorus depends largely on microbial activity.A low and limiting P supply eventually reduces plant growth and thus reduces N demand and N2-fixation (Obersonet al., 2013).Soil in South China belongs to acidic and insoluble phosphorus soils, which phosphorus is fixed in soils that are hard to absorb by plants, including Al-P and Fe-P soil, which restrict plant growth by scarcity of P.The phenomena of fixation and precipitation of P in soil, which is highly dependant on pH, cause a low efficiency of soluble P fertilizers such as superphosphate (Abdelnabyet al., 2015; Miaoet al., 2006).P predominantly presents in the forms of inorganic compounds, which belong to those of aluminium (Al-P)and iron (Fe-P) under acidic conditions.Insoluble P may affect the ability of soybean to obtain N that is sufficient to ensure a productive system.There are several ways to promote plant growth and nitrogen fixation under insoluble P.These include not only selection of soybean cultivars that are P efficient,but also inoculation with suitable bacteria, such as,Rhizobiaisolated from wild legumes under stress conditions (Abdelnabyet al., 2015; Togayet al., 2008;Simonet al., 2014) orRhizobiathat are P efficient and phosphate-solubilizing microorganisms.The efficientRhizobiumstrains under insoluble P condition have ability to store large quantities of P, utilization efficiency of internal P, and uptake efficiency at low external concentrations (Singh and Singh, 2014;Fankemet al., 2014; Bekereet al., 2013).
Several studies (Frankow-Lindberg and Dahlin,2013; Zhanget al., 2010; Rejiliet al., 2012; Attaret al., 2012; Vesterageret al., 2007) have indicated thatRhizobiumimprove the growth of plants by nitrogen fixation or solubilizing insoluble P.However,little work had been done on the effect of interactions among insoluble P,Rhizobiumand nitrogen fixation system in the insoluble P soil of South China.The aims of this study were to study the effect of interactions between insoluble P,Rhizobiumand nitrogen fixation system, and to determine the effect of nitrogen fixation system on P uptake.
Materials and Methods
Inoculant
EffectiveRhizobiumstrains were isolated from the nodules of wild or cultivated soybeans, wild strains were designated as W (W5), cultivated strains were designated as C (C46), as well as combined strains of cultivated and wild were designated as CW (CW53,CW54).USDA110 (U110) was used as a control strain(Tayloret al., 1991).The inocula for strains were prepared by resuspending the cells from 2-3 days old yeast extract mannitol agar cultures, in sterile saline(0.85% NaCl) solution containing 1% methyl cellulose(Vanet al., 2014) at 28℃ for 1 week and collected by centrifugation at 3 000 r · min-1for 10 min.Rhizobiumpellets were suspended in N-free Arnon-Hoagland solution containing (mmol · mL-3): 400 CaCl2; 200 MgSO4; 400 K2SO4; 100 NaH2PO4; 50 H3BO4; 50 FeC6H5O7; 20 MnSO4; 2 ZnSO4; 1 Na2MoO4; 0.5 CuSO4; 0.5 NiSO4; and 0.5 CoCl3(Adrianet al., 2012)diluted to 500-fold of packed cell volume.The diluted suspension was used inoculant.
Cultivation of plants
Low-P soil was used with pH 5.5, 65.60 mg · kg-1N, 10.06 mg · kg-1P and 75.20 mg · kg-1K.Plastic pots were filled with 2.5 kg of the soil.Insoluble phosphorus, Al-P (AlPO4) and Fe-P (FePO4· 4H2O),was mixed into pots at 196.7 mg Al-P per pot and 359.5 mg Fe-P per pot (equivalent to 20 mg P per pot),respectively.
The soybean seeds of 'HUAXIA 3' (Glycine maxL.)were sown directly in pots at a depth of 2 cm.Pots were watered to field capacity every other day and maintained free of weeds throughout the experiment.One milliliters of the dilutedRhizobiuminoculant was supplied for each pot at the time of seedlings developed cotyledon, after a week, three milliliters of the dilutedRhizobiumwas inoculated again.Plants were harvested on the 30th day after sowing and separated into root and aerial portions at the soil level.Then the growth parameters of dry weight, P and N contents of plant were determined.Dry weights of plant samples of shoot and root were recorded after drying in an oven at 75℃.P was determined after digestion of 0.1 g dry weight in pure sulfuric acid,following the molybdenum-blue method (Murphy and Riley, 1962).N content was also determined on 0.1 g dry weight digested in pure sulfuric acid, thereafter distilled and titrated according to Kjeldahl analysis method (Bremner and Mulvaney, 1982).
Measurement of nitrogen- fixing activity
Nitrogen-fixing activity of nodulated roots was measured by the acetylene reduction method of Sasakawaet al(1986).
Field evaluation
The filed trial was conducted in Zhanjiang City of Guangdong Province, where the soil belongs to red soil type with pH 5.0, 65.60 mg · kg-1N and 10.06 mg · kg-1P.Inoculants C46 and CW54 were used to inoculate soybean cultivar "Huaxia 3".The inoculants were prepared as mentioned above.
The experimental design was a completely randomized blocks design with three replications.The treatments included CK (without inoculation) and inoculation (106strains ofRhizobiumon the surface of each seed during inoculation).Each plot was 15 m2with row width of 50 cm and plant space of 15 cm.
Five plants were randomly sampled at pod filling stage and maturity, respectively.The biomass of shoot,plant height, the number of nodule, weight of nodule,N and P contents were determined at the first harvest.The yield was measured after harvesting mature plants.The data obtained were statistically analyzed by students'F-test (Stat View 5.0, SAS Institute Inc.).
Results
Pot experiment
Effects of inoculationRhizobiumon soybean root nodule growth
Fifteen strains were researched by nodulation checking, and it was found that all the strains were allRhizobia(data not shown).The results indicated thatRhizobiaof W5 and C46 were more effective in their symbiosis with soybean than others.Plant nodule number, nodule weight, and nodule nitrogenase activity per plant were found to be enhanced by the combined inoculation compared to individual inoculation.The combined strains of inoculation with CW54 had the highest effects, nodule number,nodule weight and nodule nitrogenase activity were markedly stimulated, increasing 89.2%, 57.1% and 81.1%, when compared with CK U110, followed with CW53 in Figs.1, 2 and 3.

Fig.1 Effect of inoculation Rhizobium on root nodule number of soybean
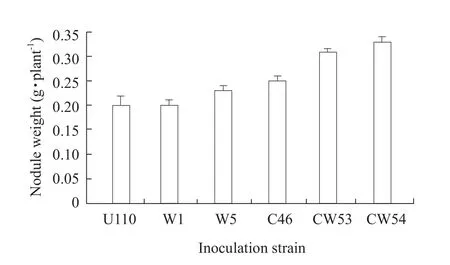
Fig.2 Effect of inoculation Rhizobium on root nodule weight of soybean
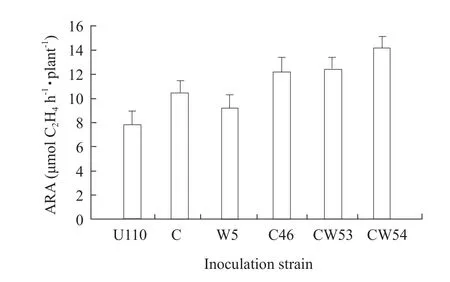
Fig.3 Effect of inoculation Rhizobium on acetylene reducing activity of soybean root nodule
Effects of inoculationRhizobiumon soybean growth
In insoluble P soil, inoculation with CW54, CW53 and C46 strains significantly increased plant dry weight as compared to CK U110.Inoculation in Al-P soil had higher dry weight of shoot or the whole plant than Fe-P.The strains CW54 and CW53 showed the same response to both Al-P and Fe-P.CW54 strain was the most effective on shoot dry weight with an increase of 33.9% in Al-P and 16.2% in Fe-P as compared with CK U110 shown in Figs.4 and 5, respectively.In addition, CW 53 and C46 also significantly enhanced shoot dry weight in Al-P soil, 23.2% and 17.9% as compared with CK U110, but no difference in root dry weight was observed, while in Fe-P conditions, it was significantly promoted only by inoculation W5 strains, increasing approximately 21% as compared with CK U110 shown in Figs.4 and 5.U110 performed well in Fe-P than in Al-P.The results indicated that effects of inoculation were obvious in terms of plant growth.
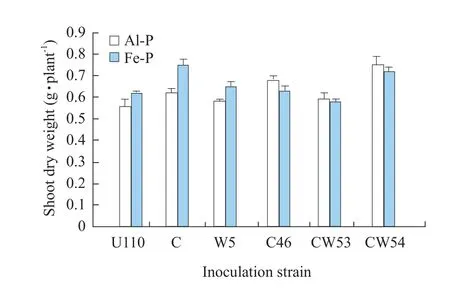
Fig.4 Effect of inoculation Rhizobium on shoot dry weight of soybean
Effects of inoculationRhizobiumon nitrogen content
There was a significant interaction between insoluble P andRhizobiumstrains for N content of plant.Besides,inoculation withRhizobiumstrains and application of insoluble P soil, respectively, had significant difference.However, N content of root was not significant different.Only inoculation with CW54 increased N content of plant in Al-P and Fe-P soil as compared with other inoculants.In Al-P soil, inoculation with CW54 resulted in the highest N content of shoot with 13.325 mg · plant-1or plant with 15.666 mg · plant-1in Table 1.On the average, inoculation withRhizobiumstrains increased higher N content in Al-P soil than Fe-P soil.In Al-P conditions, inoculation with CW54 and C46 strains significantly stimulated N uptake of plant as compared with other strains, increasing 43.2%and 26.9% as compared with CK U110, respectively,while in Fe-P conditions, it was slightly promoted only by inoculation CW54 strains in Table 1.These results suggested that symbiotic nitrogen fixation utilized insoluble P, especially Al-P, in acidic soil.
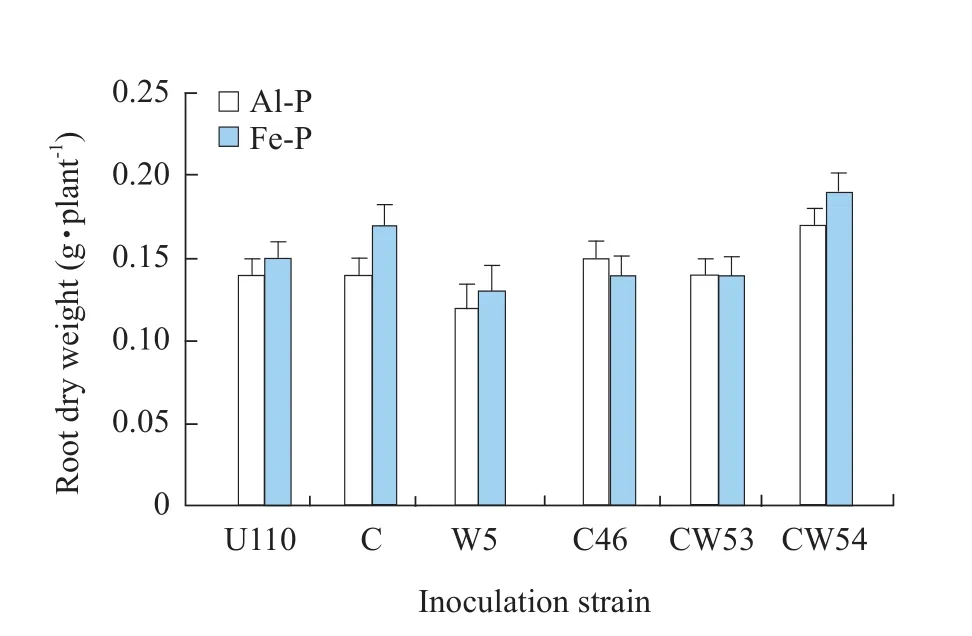
Fig.5 Effect of inoculation Rhizobium on root dry weight of soybean
Effects of inoculationRhizobiumon phosphorus content
Phosphorus appeared essential for both nodulation and N2fixation.Phosphorus was required by soybean for growth and promoted N uptake.Inoculation withRhizobiumstrains affected higher root phosphorus content in Fe-P soil than Al-P soil, but some strains did not increase phosphorus content of root compared to other inoculants.Inoculation with C46 had the highest P content of plant in Al-P soil, increasing 31.6% when compared with CK U110 in Table 2.On the average,inoculation withRhizobiumstrains increased higher phosphorus content of plant in Al-P soil than Fe-P soil in Tables 3-4.However, only inoculation with CW54 significantly enhanced phosphorus uptake of plant in Al-P and Fe-P soil as compared with other inoculants,whereas in Fe-P soil more higher than in Al-P soil,increasing 96.2% (Fe-P) and 8.2% (Al-P), when compared with CK U110 in Table 2, respectively.
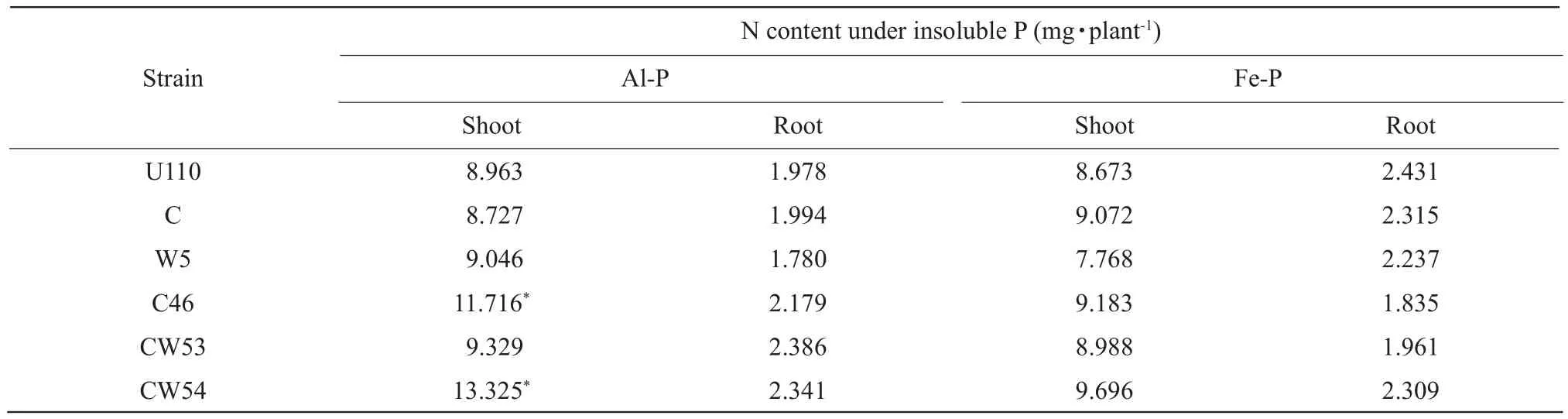
Table 1 Effect of inoculation on N content of plant in insoluble P soil
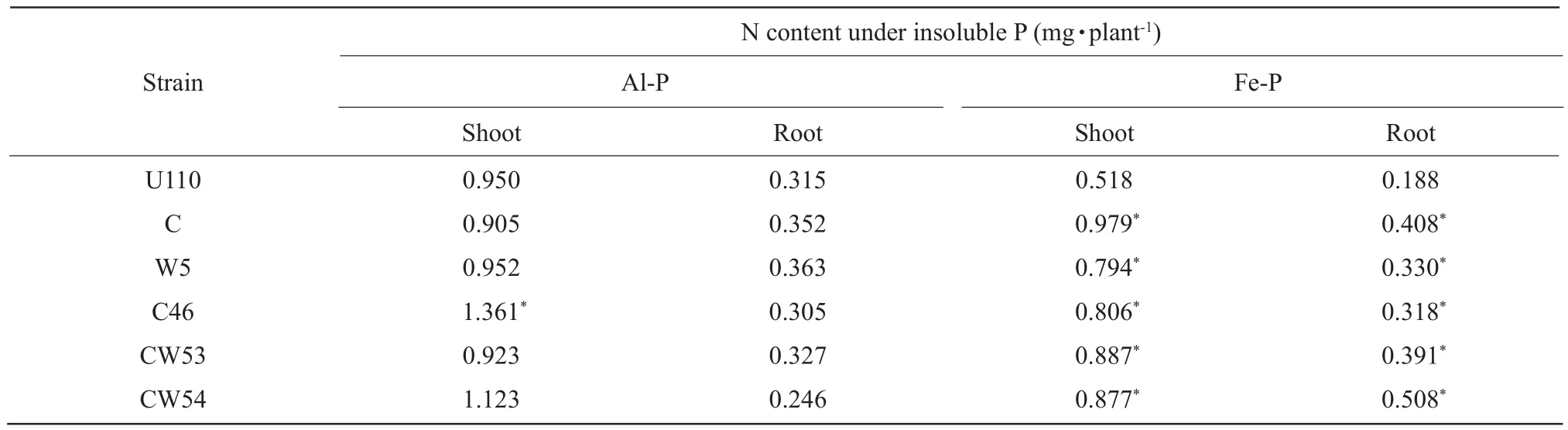
Table 2 Effect of inoculation Rhizobium on P content of plant in insoluble P soil
Field experiment
Evaluation of inoculationRhizobiumunder field conditions
Cultivation experiments of soybean treated withRhizobiumwere achieved several times in a field from November, 2012 to April, 2016.In this paper, the most recent results were shown.Two well-inoculated strains of C46 and CW54 were selected from seven strains.The nodule numbers in the plants treated with C46 and CW54 strains were significantly increased by 150.3%and 157.9% as compared to those of the control at 21-day after inoculating, respectively.Such trend was also clearly observed at the time of soybean podding.Although statistical significance was not observed on the nodule weight, plant dry weight and phosphorus content treated with C46 and CW54 strains at 21-day after inoculating, it was significantly observed in the plants treated with C46 and CW54 strains at the period of soybean podding.The nodule weight, plant dry weight, phosphorus content of pod and the whole plants in the plants treated with CW54 strains inceased 221.1%, 86.4%, 100.3% and 92.4% significantly compared to those of the control, such trend was also clearly observed in the plants treated with C46 strains in Table 3.

Table 3 Effect of inoculation Rhizobium at 21-day after inoculating
The nitrogen contents of the whole plant in the plants treated with C46 and CW54 strains increased 46.6%and 19.4% at 21-day after inoculating, and 105.4%and 125.1% for the time of soybean podding compared to that of the control, respectively.The nitrogen contents of pod in the plants treated with C46 and CW54 strains were also significantly increased in Table 4.The total biomass in the plants treated with C46 and CW54 strains were significantly increased by 81.6%and 86.4%, as compared to those of the control at the time of soybean podding, and the yield were increased by 48.5% and 51.4% in Table 5, respectively.
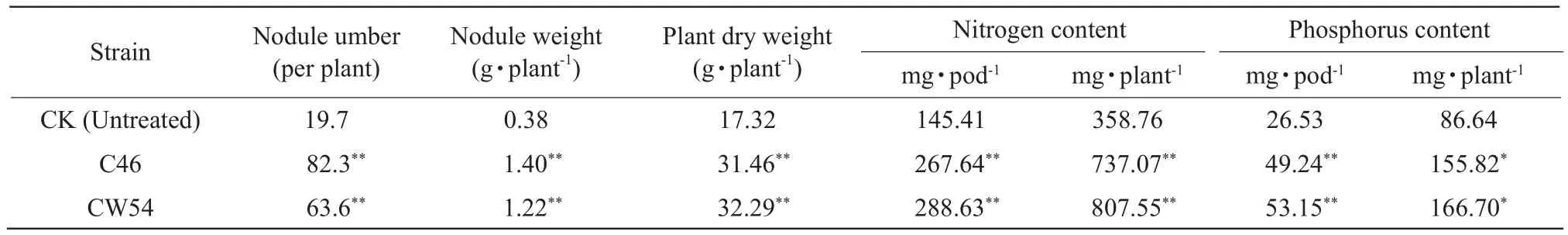
Table 4 Effect of inoculation Rhizobium at period of soybean podding
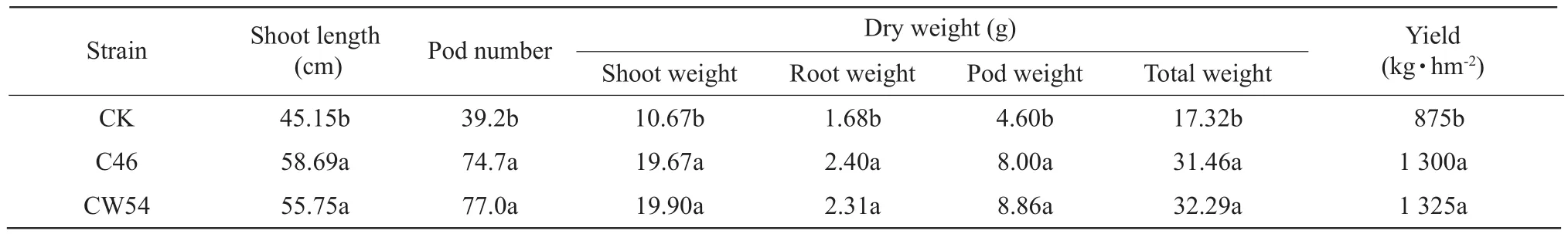
Table 5 Effect of inoculation Rhizobium on yield at period of soybean podding
Discussion
Inoculation withRhizobiumnot only enhanced nitrogen fixation activity, plant biomass, N content in soybean and other legumes (Obersonet al., 2013; Liet al., 2011), but also improved soil fertility and quality.Accordingly, we attempted to analyze the symbiotic nitrogen fixation effectiveness of both W-strains and C-strains.This result showed that most of W-strains were more efficient in terms of nitrogen fixation compared to C-strains.A significant increase of nodule formation and ARA inRhizobiuminoculation was shown in Figs.1, 2 and 3.Among theseRhizobium,CW54 strain gave the highest ARA of 14.21 μmol C2H4h · plant-1in the seedlings shown in Fig.3.A low and limiting P supply eventually reduced plant growth,besides reduced P content and N2-fixation (Obersonet al., 2013).In this study, inoculation with CW54 and W5 strains significantly increased shoot dry weight as compared with CK U110 in insoluble P soil shown in Figs.4 and 5; CW54 strains had a significant effect in Al-P soil and the shoot dry weight was at a maximum 0.75 g · plant-1, increased by 33.9% as compared to that of CK U110 in Figs.4 and 5.These results implied that inoculationRhizobiumwas beneficial for nitrogen fixation and growth of soybean.Nitrogen content could be added to the cropping system by the use of N2-fixing legumes (James and Baldani, 2012;Frankow-Lindberg and Dahlin, 2013; Vesterageret al., 2007).Miaoet al.(2006) reported that Al-P played an important role for nitrogen fixation system of soybean in acidic soil.It could be verified that inoculation with CW54 resulted in the highest N uptake of plant (3.006 mg · plant-1) in Al-P soil, which suggested that the suitableRhizobiuminoculation increased nitrogen fixation, and then improved nitrogen content in the insoluble P soil shown in Table 1.The phosphorus content of the whole plant was followed a similar trend to N uptake.Interestingly, the phosphorus content of root was higher in Fe-P soil, but the phosphorus content of shoot was not significantly different in Table 2.These results suggested it was not easy to utilize Fe-P for increasing phosphorus content of plant compared with Al-P in acidic soil, and also suggested that root was more sensitive than shoot in the insoluble phosphorus soil.
The ratio symbiotic N2fixation (SNF)/phosphorus content was computed to estimate the efficiency of plant to use P for N2fixation (Attaret al., 2012).The results showed that inoculated with CW54 (2.183 mg SNF/mg phosphorus) resulted in higher mean value of SNF/P content compared to CK U110 (0.196 mg SNF/mg P) in Fig.3 and Table 2.This was in agreement with a previous report that the phosphorus content might be involved in regulation of nodule initiation (Magadlelaet al., 2012) and affected N content, SNF of plant (Obersonet al., 2013).The effects of inoculatedRhizobiumin a field were similar to that in a pot.Therefore, inoculationRhizobiumwas beneficial for soybean nutrient uptake, growth and yield in Tables 3, 4 and 5.These results suggested that screeningRhizobiumstrains, especially strains for tolerance to the insoluble P, and later testing soybean cultivars inoculated with these strains might be very helpful in identifying superior symbiotic relationships under insoluble P conditions in acid soil.However,these results needed to be further tested and verified in the field.
Conclusions
The combinedRhizobiuminoculation was more effective than individualRhizobiuminoculation; the biomass in the soybean treated with CW54 strains were significantly increased as compared to those of the control in a field condition; the inodule nitrogenase activity treated with CW54 was markedly stimulated as compared to that of the control U110; the inoculation with CW54 significantly enhanced phosphorus uptake of plant in Fe-P soil higher than that in Al-P soil.Therefore, inoculationRhizobiumwas beneficial for soybean nutrient uptake, growth and yield under insoluble phosphate soil conditions.
The first and second authors contributed equally to this work.
Abdelnaby M, Nasreldeen N, Elnesairy B,et al.2015.Symbiotic and phenotypic characteristics ofRhizobianodulaing cowpea (Vigna unguiculataL.Walp) grown in arid region of libya (Fezzan).Journal of Environmental Science and Engineering B, 4: 227-239.
Adrian U, Goss M J, Simon C,et al.2012.Analysis of matrix effects critical to microbial transport in organic waste-affected soils across laboratory and field scales.Water Resources Research, 48: 154-167.
Attar H A, Blavet D, Selim E M,et al.2012.Relationship between phosphorus status and nitrogen fixation by common beans (Phaseolus vulgarisL.) under drip irrigation.International Journal of Environ-mental Science and Technology, 9(1): 1-13.
Bargaz A, Lazali M, Amenc L,et al.2013.Differential expression of trehalose 6-P phosphatase and ascorbate peroxidase transcripts in nodule cortex ofPhaseolus vulgarisand regulation of nodule O2permeability.Planta, 238(1): 107-119.
Bekere W, Kebede T, Dawud J.2013.Growth and nodulation response of soybean (Glycine maxL.) to lime,Bradyrhizobium japonicumand nitrogen fertilizer in acid soil at melko, South Western Ethiopia.International Journal of Soil Science, 8(1): 25-31.
Bremner J M, Mulvaney C S.1982.Nitrogen-total.In: Page A L,Miller R H, Keeney D R.Methods of soil analysis.II.Chemical and microbiological properties.2nd ed.Am Soc Agron, Madison, Wis.pp.595-624.
Elkoca E, Kantar F, Sahin F.2008.Influence of nitrogen fixing and phosphorus solubilizing bacteria on the nodulation, plant growth, and yield of chickpea.Journal of Plant Nutrition, 31(1): 157-171.
Fankem H, Ngo N L, Nguesseu N G,et al.2014.Maize (Zea mays)growth promotion by rock-phosphate solubilising bacteria isolated from nutrient deficient soils of Cameroon.African Journal of Microbiology Research, 8(40): 3570-3579.
Frankow-Lindberg B E, Dahlin A S.2013.N2fixation, N transfer, and yield in grassland communities including a deep-rooted legume or non-legume species.Plant and Soil, 370(1/2): 567-581.
James E K, Baldani J I.2012.The role of biological nitrogen fixation by non-legumes in the sustainable production of food and biofuels.Plant & Soil, 356: 1-3.
Li Q Q, Wang E T, Zhang Y Z,et al.2011.Diversity and biogeography of rhizobia isolated from root nodules of glycine max grown in Hebei Province China.Microbial Ecology, 61(4): 917-31.
Magadlela A, Kleinert A, Dreyer L L,et al.2012.Low-phosphorus conditions affect the nitrogen nutrition and associated carbon costs of two legume tree species from a mediterranean-type ecosystem.Australian Journal of Botany, 62(1): 1-9.
Miao S J, Qiao Y F, Han X Z.2006.Requirement of phosphorous for soybean cultivars nodulation and nitrogen fixation.System Sciences and Comprehensive Studies in Agriculture, 22(4): 276-278.
Murphy J, Riley J P.1962.A modified single solution method for the determination of phosphate in natural waters.Anal Chem Acta, 27:31-36.
Oberson A, Frossard E, Bühlmann C,et al.2013.Nitrogen fixation and transfer in grass-clover leys under organic and conventional cropping systems.Plant and Soil, 371(1-2): 237-255.
Pandit R, Kunjadia P, Mukhopadhyaya P,et al.2014.Inorganic phosphate solubilizing potential of arthrobotrys conoides and duddingtonia flagrans, a nematode trapping fungi a potential biocontrol agent.International Journal of Agricultural Technology, 10(3): 559-570.
Quernéa A, Battie-laclaua P, Dufoura L,et al.2017.Effects of walnut trees on biological nitrogen fixation and yield of intercropped alfalfa in a mediterranean agroforestry system.European Journal of Agronomy, 84: 35-46.
Rejili M, Mahdhi M, Fterich A,et al.2012.Symbiotic nitrogen fixation of wild legumes in Tunisia: soil fertility dynamics, field nodulation and nodules effectiveness.Agriculture Ecosystems & Environment,157(157): 60-69.
Sasakawa H, Trung B C, Yoshida S.1986.Stem nodulation onAeschynomene indicaplants by isolatedRhizobia.Soil Sci Plant Nutr, 32: 145-149.
Simon Z, Mtei K, Gessesse A,et al.2014.Isolation and characterization of nitrogen fixing rhizobia from cultivated and uncultivated soils of Northern Tanzania.American Journal of Plant Sciences,5(26): 4050-4067.
Singh A P, Singh J B.2014.Differential synthesis of alkaline phosphatase inRhizobiumspecies isolated from the tropics.Zeitschrift Für NaturforschungC, 39(11/12): 1038-1041.
Taylor R W, Williams M L, Sistani K R.1991.Nitrogen fixation by soybean-bradyrhizobium combinations under acidity, low P and high Al stresses.Plant and Soil, 131(2): 293-300.
Togay N, Togay Y, Cimrin K M,et al.2008.Effects of rhizobium inoculation, sulfur and phosphorus applications on yield, yield components and nutrient uptakes in chickpea (Cicer arietinumL.).African Journal of Biotechnology, 7: 776-782.
Van G J, Weissing F J, Kuipers O P,et al.2014.Density of founder cells affects spatial pattern formation and cooperation inBacillus subtilisbio films.The ISME Journal, 8(10): 2069-2079.
Vesterager M, Nielsen N E, Høgh-Jensen H.2007.Nitrogen budgets in crop sequences with or without phosphorus fertilised cowpea in the maize-based cropping systems of semi-arid Eastern Africa.Afr J Agri Res, 2(6): 261-268.
Zhang H T, Guo L Z, Chai Q,et al.2010.Effects of nodule bacteria inoculation on growth of pea/maize system.Agricultural Research in the Arid Areas, 28(5): 92-93.
 Journal of Northeast Agricultural University(English Edition)2018年1期
Journal of Northeast Agricultural University(English Edition)2018年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Characteristics and Degradation Mechanism of Fomesafen
- Comparative Study of Proximate, Chemical and Physicochemical Properties of Less Explored Tropical Leafy Vegetables
- A Comparative Study on Photosynthetic Characteristics of Dryopteris fragrans and Associated Plants in Wudalianchi City, Heilongjiang Province, China
- Effect of Mineral and Vitamin Supplementation on Performance and Haemotological Values in Broilers
- Comparative Research on Facultative Anaerobic Cellulose Decomposing Bacteria Screened from Soil and Rumen Content and Diet of Dairy Cow
- Pharmacokinetics of Milbemycin Oxime in Dogs Following Its Intravenous and Oral Administration
