Effects of leptin on femoral fracture in rats
Lei Wang,Sixin Sun,Lei Yang,Chun Lu,Xiaojian Cao,✉
1Department of Orthopedics,The First Affiliated Hospital of Nanjing Medical University,Nanjing,Jiangsu 210029,China;
2Department of Immunology,Nanjing Medical University,Nanjing,Jiangsu 211166,China.
Introduction
Leptin is a peptide hormone which is secreted especially by white adipose tissue and encoded by the obese gene.It is produced primarily by adipocytes and acts on the hypothalamus.Moreover,salient nonadipocytic,extra-hypothalamic pathways also exist[1].Recently,the role of leptin in bone metabolism has been identified[2-3].Therole of leptin on the skeletal system is complex.Present information on leptin indicates that it is involved in at least 2 different bone-controlling mechanisms,a direct stimulatory effect on bone growth,and/or an indirect suppressive effect on bone formation through the hypothalamus via the sympathetic nervous system[4].By acting on the hypothalamus and increasing both sympathetic output,and β2-adrenergic receptors on the surface of osteoblasts,leptin exerts an antiosteogenic effect on the central nervous system[5].Recent findings suggested that blocking the central effects of leptin would increase bone mass by stimulating bone formation[6,7]and hypothalamic leptin gene therapy actually increased bone length and total bone mass in growing obese(ob/ob)mice.Overall,the aforementioned findings are congruous with the hypothesis that the central nervous system-mediated actions of leptin are anti-osteogenic.
However,peripherally speaking,some hypothesis demonstrated that leptin has an opposite effect by promoting bone mineralization[8]and osteoblast to osteocyte differentiation in vitro[9-10].Despite this,questions still remain about the concept that peripheral leptin is responsible for the putative bone anabolic effects of the hormone in vivo.Consequently,in this study,we aimed to explore possible effects of leptin on bone growth and fracture healing.
Materials and methods
Adult male SD rats(n=72,250-290 g,purchased from Shanghai Slaccas Laboratory Animal Co,Shanghai,China)were maintained at 23±1°C on a 12-h light/12-h dark cycle.They were all housed individually in specific pathogen-free conditions with unlimited access to water and laboratory food and were treated strictly in accordance with institutional ethical guidelines.These rats were randomly and equally divided into three groups.Group A was treated with 1 mL normal saline(NS)intraperitoneally and used as the operative control group.Group B was treated with 0.3 μg/kg leptin(Leptin Rat,Recombinant,Linco Corp.,USA)in 1 mL NS intraperitoneally.Group C was treated with 0.5 μg/kg leptin in 1 mL NS intraperitoneally.Treatments were started on the day of surgery and lasted for 2 weeks.The average body weights of the rats at the 2,4 and 8 weeks were 262±12.2 g,255±16.5 g,and 265±13.7 g,respectively,and analysis of variance(ANOVA)did not detect a significant difference in average weights.All three groups were divided into three subgroups(n=8 rats)for evaluation at 2,4 and 8 weeks after the operation.These rats were sacrificed by sodium pentobarbital(100 mg/kg,i.p.)overdose,perfused transcardically with NS and 10%neutral formalin.Approval for this study was granted by the Ethics Board of the First Affiliated Hospital with Nanjing Medical University,China.
Femoral fracture model
Femoral osteotomy and fixation were performed in the same manner as previously reported[11].Brie fly,a transverse osteotomy was made at the midshaft of the femur and intramedullary fixation was performed using a stainless steel wire(diameter,1.5 mm).The fracture fragments were reduced and stabilized.Wires were cut on the surface of the intercondylar groove to avoid restriction of knee joint motion.Unrestricted activity was allowed after recovery from anesthesia.
Tissue preparation and histologic analysis
After the rats were sacrificed,the fractured femurs were dissected and carefully cleaned of muscle around the fracture callus to preserve callus integrity.Calluses from weeks 2,4 and 8 after fracture were then fixed in 4% paraformaldehyde,the intramedullary wire was removed,and the specimen was decalcified before paraffin embedding.Sagittal sections,which were chosen blindly and randomly from the middle of each sampled callus and the distance between each section,were 5µm through the fracture site and mounted on Fisher brand Superfrost Plus Slides(Fisher scientific,Pittsburgh,PA,USA).Sections were stained with hematoxylin-eosin for histological evaluation of healing at 2,4 and 8 weeks after fracture.The criteria described by Huo et al.[12]was used for histological evaluation of the specimens.
Radiological evaluation
The bone formation part of the Lane-Sandhu histopathological scoring system was used for radiological evaluation of fracture healing[13].The standard lateralandAPradiographsthatweretaken after euthanasia were evaluated by a double blinded observer.
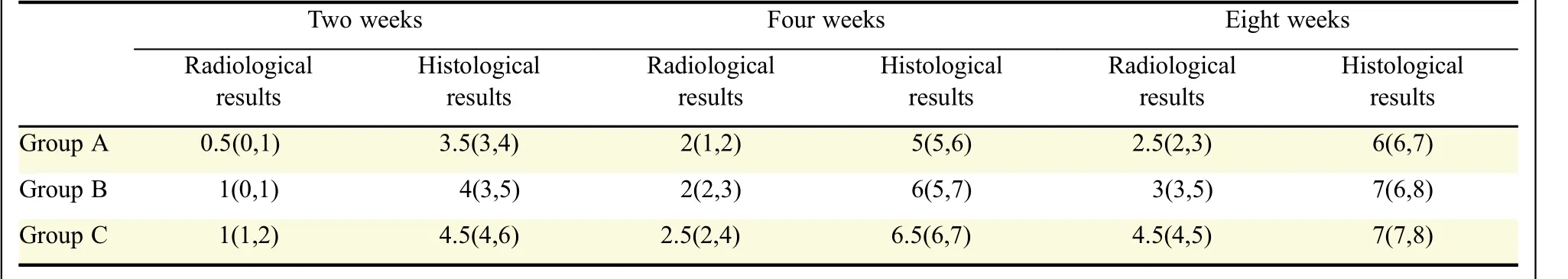
Table 1 Radiological and histological results of the groups*
Microcomputerized tomography(micro-CT)analysis
The explanted femoral bones were scanned by micro-CT using SkyScan 1172 scanner with a voxel size of 20 mm.The data was collected at 100 kVand 100 mA and reconstructed using the cone-beam algorithm.Each femoral bone sample scanning was performed over a 180°rotation with an exposure time of 105 ms.A cylindrical volume of interest with a diameter of 20 mm and a height of 27 mm was selected,which displayed the microstructure of the rat femoral bones(comprising the cortical and cancellous bone).We scanned femoral samples every 1 mm and all the samples were done under the same experimental condition,utilizing the same radiographical machine,the same position and the same people for experimenting and another for measuring.Data analysis was performed using CT Analyzer software(BrukermicroCT,Kontich,Belgium).The region of interest was set at the area of fracture healing,de fined by the fracture area filled with new bone,and the structural indices of the femoral fracture areas were calculated using this software.In three-dimensional(3D)analysis,bone callus volume(BV)and fibrous callus volume were measured.
Statistical analysis
All statistical analyses were performed using SPSS 12.0(SPSS,Inc.,Chicago,IL,USA).The clinical data was expressed as mean±standard deviation.All variables were normally distributed(Shapiro-Wilk test).Differences between body weights of the three groups before operation were detected using one-way analysis of variance(ANOVA),and after the operation we used ANOVA with a post hoc test to analyze data of the three groups.P value less than 0.05 was considered statistically significant.
Results
There were no operation-related complications after anesthesia and intraperitoneal interventions.There were no wound infections and no complications related to surgery.No rats died in the experiment.
Radiological analysis
In all rats different Lane-Sandhu scores of fracture formation were calculated in radiographs(Table1,Fig.1).At 2 weeks after operation,evidence of callus formation was found at the fracture site in these groups,but there was no statistically significant difference.At 4 weeks after operation,callus formation in the three groups was more rapid and the average evaluation scores were higher than 2 weeks;however,we did not detect significant difference in these groups.
At 8 weeks,complete fracture healing was found in both group B and group C.The fracture line became nearly invisible compared to 2 and 4 weeks.The formation of callus in group B and C continued to be significantly better than group A(P=0.04,P=0.013,respectively).There was no significant difference between group B and group C(P=0.197).
Histological results
There was no statistically significant difference between the groups at 2 weeks(P=0.20).
At the 4 week stage,fracture callus from group A showed a well formed callus with areas of newly formed fibrous callus,calcified cartilage,chondrocytes and hypertrophic chondrocytes,group B and C demonstrated more mature trabecular bone formation and larger external callus compared to group A(P=0.01,P=0.002,respectively).
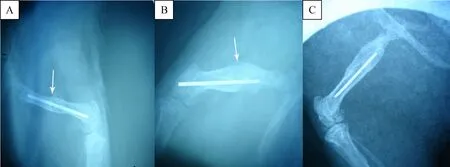
Fig.1 Radiological images at 8 weeks.The formation of callus(white arrow)in group B(B)and group C(C)continued to be significantly better than group A(A).
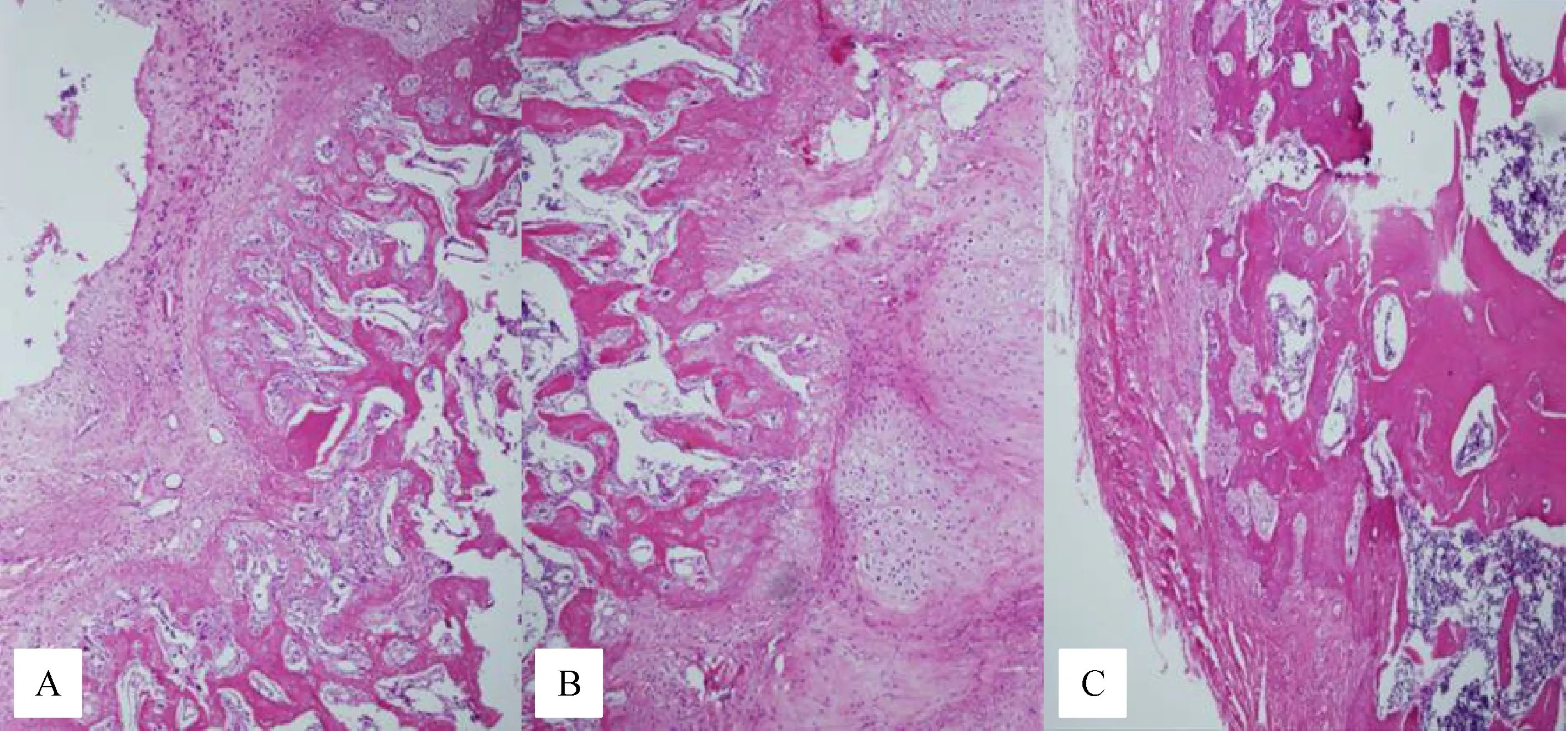
Fig.2 Histological cross-section of the fractured rat femurs obtained 8 weeks after surgery.In group A,predominantly cartilage with some woven bone was observed(A)(black arrows).In group B,predominantly woven bone occurred in the fracture line(B).In group C,predominantly woven bone and lamellar bone were observed(C)(black arrows).Magnification×100.
At the 8 week phase,group A displayed pronounced cartilage with some woven bone.In group B,predominantly woven bone occurred in the fracture line.In group C,predominantly woven and lamellar bone was observed(Fig.2).There was a statistically significant difference between group A and B,and between group A and C(P=0.008,P=0.003,respectively).There was no statistically significant difference between group B and C(P=0.235).
Micro-CT analysis
Computer analysis of the Micro-CT images revealed the volume of new bone and the quality of the femoral fracture area in all rats(Table 2,Fig.3).At 2 weeks,fracture lines were clear and some fibrous calluses were observed in all 3 groups.There was no statistically significantdifferenceamongthethreegroups(P=0.35).At 4 weeks,in group A,the mineralized callus bridging was deemed insufficient.More mature mineralized callus bridging was detected in group B and C compared with group A(P=0.02,P=0.04,respectively).At 8 weeks,some mineralized callus and newly formed bone were seen in group A.A large amount of newly formed bone was seen in group B,greater quantities of bony callus and evidence of bone fusion were observed in group C.Quantitatively,the bone callus volume was higher in group B and C than in group A(P=0.005,P=0.001,respectively).Group C also had better fracture healing than group B at 8 weeks(P=0.01).
Discussion
This study has demonstrated the influence of leptin on femoral fracture healing in rats.The results showed that leptin enhanced fracture healing,as characterized by a significant increase in the parameters regarding callus volume and fracture healing of the fractured femora compared with those in the control group.
Research shows that fracture healing is a varied and complex process[14].It involves an orderly and intricate succession of events,beginning with inflammation,followed by fibrous tissue and cartilage differentiation,and finally,endochondral and intramembranous ossification.Multiple factors are involved in this process,such as hormonal factors(growth hormone and transforming growth factor)and an extracellular matrix comprised of a wound healing process which summarizes skeletal growth and development[15].Therefore,bone union is attributed to two major factors,bone callus formation across the fracture site and mineralization to stabilize the fracture site.
Leptin,the circulating protein product of the obesity(Ob)gene,was initially discovered as a satiety factorregulating food intake and energy expenditure,and it is synthesized and secreted by adipocytes[16-17].As an important hormonal regulatory factor,leptin not only influences lipid metabolism but also has positive effects on peripheral bone metabolism by regulating bone mass and promoting bone mineralization[18-19].
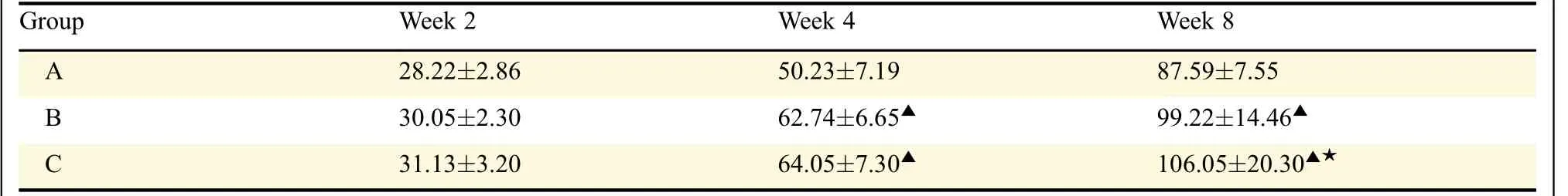
Table 2 Volume of fracture callus in three groups

Fig.3 Micro-CT images of the fractured rat femurs at 8 weeks after surgery.Some mineralized callus and newly formed bone are seen in group A(A).Large volume of newly formed bone is seen in group B(B),greater amounts of bony callus and evidence of bone fusion are observed in group C(C).Quantitatively,the bone callus volume is higher in group B,C than in group A.
Experimental researches showed that the elevation of peripheral serum leptin levels starts immediately after trauma,such as spinal cord injury or traumatic brain injury,by activation of endogenous leptin secretion.These higher serum leptin levels are associated with increased callus formation in the fracture site[20-21].Furthermore,elevated serum leptin may act peripherally to induce myeloid precursor cell differentiation and osteoblast proliferation,and accelerate the mineralization of bone at the fracture site[22-23].Similarly,in our study,because daily application of exogenous leptin might provide consistently higher concentrations of serum leptin levels,better fracture healing was observed in the groups given exogenous leptin(fracture healing of group B,and C were better than group A).We also found that callus volume was higher in the group where more exogenous leptin was administered(group C also had larger bone formation than group B at the 8 week stage)In a study by Wang and colleagues[24],it was reported that elevated leptin expression might also enhance alkaline phosphatase activity,secretion of osteocalcin,and expression of type I collagen mRNA.Turner et al.suggested that leptin acts on growth plate cartilage cells,osteoblasts and osteoclasts to enhance their number and/or activity.Leptin deficiency results in an overall decrease in bone turnover.Therefore,as found with other major regulators of bone metabolism[25],regional changes in bone mass and structure depend upon peripheral serum leptin levels.
Our study,in which leptin was administered intraperitoneally,distinguishes between direct peripheral and indirect central actions of leptin on bone formation because peripheral and central leptin have opposing actions on fracture healing.In all groups,the experimental data obtained continuously without interruption,indicated the suitability of the applied method.The experiments described above suggests that a dose dependent positive effect of leptin on fracture healing when we evaluated radiological,histological and especially micro-CT results.
Several weaknesses exist with respect to paucity in technical explanations of our observations.In addition,a limited number of time points prevented a more detailed view of the stages of healing and the optimal time for administration of leptin.Furthermore,in vivo leptin distribution and local concentration present within the bone were not explored.Future work should investigate the molecular mechanism of action of leptin upon bone healing.In addition,use of impaired fracture healing models,including rats with increased age,diabetes mellitus,or undergoing steroid treatment,could lead to a more complete understanding of the effect of leptin.
[1]Thomas T.The complex effects of leptin on bone metabolism through multiple pathways[J].Curr Opin Pharmacol,2004,4(3):295-300.
[2]Rodrigues L,Mouta R,Costa AR,et al.Effects of high fat diet on salivary a-amylase,serum parameters and food consumption in rats[J].Arch Oral Biol.2015,27;60(6):854-862.
[3]Bertoni L,Ferretti M,Cavani F,et al.Leptin increases growth of primary ossification centers in fetal mice[J].J Anat,2009,215(5):577-583.
[4]Upadhyay J,Farr OM,Mantzoros CS.The role of leptin in regulating bone metabolism[J].Metabolism,2015,64(1):105-113.
[5]Takeda S,Elefteriou F,Levasseur R,et al.Leptin regulates bone formation via the sympathetic nervous system[J].Cell,2002,111(3):305-317.
[6]Bertoni L,Ferretti M,Cavani F,et al.Leptin increases growth of primary ossification centers in fetal mice[J].J Anat,2009,215(5):577-583.
[7]Kume K,Satomura K,Nishisho S,et al.Potential role of leptin in endochondral ossification[J].J Histochem Cytochem,2002,50(2):159-169.
[8]Gaspar AP,Brandão CM,Lazaretti-Castro M.Bone mass and hormone analysis in patients with spinal cord injury:evidence for agonadal axis disruption[J].JClin Endocrinol Metab,2014,99(12):4649-4655.
[9]Bonnet N,Gadois C,McCloskey E,et al.Protective effect of beta blockers in post menopausal women:influence on fractures,bone density,micro and macroarchitecture[J].Bone,2007,40(5):1209-1216.
[10]Li J,Mori S,Kaji Y,et al.Concentration of bisphosphonate(incadronate)in callus area and its effects on fracture healing in rats[J].J Bone Miner Res,2000,15(10):2042-2051.
[11]Li J,Mori S,Kaji Y,et al.Concentration of bisphosphonate(incadronate)in callus area and its effects on fracture healing in rats[J].J Bone Miner Res,2000,15(10):2042-2051.
[12]Huo MH,Troiano NW,Pelker RR,et al.The influence of ibuprofen on fracture repair:biomechanical,biochemical,histologic,and histomorphometric parameters in rats[J].J Orthop Res,1991,9(3):383-390.
[13]Lane JM,Sandhu HS.Current approaches to experimental bone grafting[J].Orthop Clin North Am,1987,18(2):213-225.
[14]Schindeler A,McDonald MM,Bokko P,et al.Bone remodeling during fracture repair:The cellular picture[J].Semin Cell Dev Biol,2008,19(5):459-466.
[15]Park AG,Paglia DN,Al-Zube L,et al.Local insulin therapy affects fracture healing in a rat model[J].J Orthop Res,2013,31(5):776-782.
[16]Mantzoros CS,Moschos S,Avramopoulos I,et al.Leptin concentrations in relation to body mass index and the tumor necrosis factor-alpha system in humans[J].J Clin Endocrinol Metab,1997,82(10):3408-3413.
[17]Mantzoros CS,Qu D,Frederich RC,et al.Activation of beta(3)adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice[J].Diabetes,1996,45(7):909-914.
[18]Cadosch D,Gautschi OP,Thyer M,et al.Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury[J].J Bone Joint Surg Am,2009,91(2):282-288.
[19]Zhang J,Li T,Xu L,et al.Leptin promotes ossification through multiple ways of bone metabolism in osteoblast:a pilot study[J].Gynecol Endocrinol,2013,29(8):758-762.
[20]Hamrick MW,Ferrari SL.Leptin and the sympathetic connection of fat to bone[J].Osteoporos Int,2008,19(7):905-912.
[21]Bigford GE,Bracchi-Ricard VC,Nash MS,et al.Alterations in mouse hypothalamic adipokine gene expression and leptin signaling following chronic spinal cord injury and with advanced age[J].PLoS One,2012,7(7):e41073.
[22]Brock JH,Rosenzweig ES,Blesch A,et al.Local and remote growth factor effects after primate spinal cord injury[J].J Neurosci,2010,30(29):9728-9737.
[23]Aloe L,Bianchi P,De Bellis A,et al.Intranasal nerve growth factor bypasses the blood-brain barrier and affects spinal cord neurons in spinal cord injury[J].Neural RegenRes,2014,9(10):1025-1030.
[24]Wang L,Tang X,Zhang H,et al.Elevated leptin expression in rat model of traumatic spinal cord injury and femoral fracture[J].J Spinal Cord Med,2011,34(5):501-509.
[25]Turner RT,Kalra SP,Wong CP,et al.Peripheral leptin regulates bone formation[J].J Bone Miner Res,2013,28(1):22-34.
 THE JOURNAL OF BIOMEDICAL RESEARCH2018年2期
THE JOURNAL OF BIOMEDICAL RESEARCH2018年2期
- THE JOURNAL OF BIOMEDICAL RESEARCH的其它文章
- Urinary tract infection due to Fusarium oxysporum in an immunocompetent patient with chronic kidney disease
- Distinctive roles of Rac1 and Rab29 in LRRK2 mediated membrane trafficking and neurite outgrowth
- Microglia activation mediated by toll-like receptor-4 impairs brain white matter tracts in rats
- A novel entry point for pedicle screw placement in the thoracic spine
- Pathological significance and regulatory mechanism of lymphotoxin β receptor overexpression in T cells of patients with systemic lupus erythematosus
- Evaluation of cardiolipin nanodisks as lipid replacement therapy for Barth syndrome
