挺水植物生物炭对硫丹的吸附及催化水解作用
何 琦,曹凤梅,卢少勇,陈方鑫,蔡传伦
挺水植物生物炭对硫丹的吸附及催化水解作用
何 琦,曹凤梅,卢少勇*,陈方鑫,蔡传伦
(中国环境科学研究院,国家环境保护洞庭湖科学观测研究站,湖泊水污染治理与生态修复技术国家工程实验室,北京 100012)
以美人蕉、菖蒲、芦苇、茭白、再力花、芦竹等挺水植物为原料,在限氧升温(550℃)条件下制备6种不同性质的生物炭,研究其组成及结构对硫丹吸附和催化水解作用.结果表明:550℃下热解,湿地植物生物炭所含灰分高于一般农产品废弃物,介于10.88%~31.11%间.生物炭表面芳香类官能团较多,孔隙部分呈碎片化,以介孔为主.6种生物炭对硫丹都具良好吸附性能.吸附行为主要发生在含致密有机质的生物炭表面,包括疏水、扩散及分配作用等.中/碱性条件下,美人蕉生物炭、菖蒲生物炭及再力花生物炭能有效去除水溶液中的硫丹,去除率为96.77%~98.57%.中性条件下,因生物炭对硫丹的催化,对硫丹的去除率提高约16.57%~72.57%.
生物炭;硫丹;催化水解;芳香类官能团
硫丹,属于有机氯农药(OCPs),我国1994~ 2004年施用于棉花,烟草和茶树等农作物的硫丹总量约2.57万t,主要集中在新疆、华东、华中和西南地区[1].目前,我国已公布的“十三五”生态环境保护规划,要求截至2020年基本淘汰硫丹.
硫丹可经地表径流、淋、溶、干/湿沉降等入水,对鱼类的生理生化、内分泌、遗传及代谢机制产生急性或慢性效应[2].江苏省淮河流域的地表水源中α-硫丹达33.3ng/L, β-硫丹达6.7ng/L[3].丹江口水库的间隙水中β-硫丹为22.17ng/L[4].印度恒河流域水体中硫丹总量为166.39ng/L[5].美国南加州地表水、底泥及鱼类也检测出较高的硫丹,且远超美国EPA规定的能引起慢性毒性效应的值(56ng/L)[6].
目前,吸附法较经济高效.去除硫丹的吸附剂有二氧化硅[7]、木炭[7]、粘土[8]、碳纳米管[9]和零价锌[10]等,因其经济费用高或效率低,应用受限.生物炭来源广,价格低,表面吸附位点较多,利于去除有机污染物.目前生物炭去除疏水性有机物(HOMs)的研究多集中在吸附或降解污染物[11-12],利用湿地生物炭去除硫丹的研究鲜有报道.
本文利用傅立叶红外,元素分析及扫描电镜等分析生物炭表面组成、结构及官能团,研究其对硫丹的吸附和催化水解,可为现有水处理设施除硫丹提供高效、低成本且环境友好的吸附剂,为解决人工湿地植物带来的二次污染提供可行方案.
1 材料及方法
1.1 生物炭制备
美人蕉、菖蒲、芦苇、茭白、再力花、芦竹等挺水植物采集于太湖贡湖湾湿地,沥干后,干燥粉碎,过200目筛.氮气氛围中,加热至550℃,热解2.5h,室温下冷却过夜,得到生物炭,分别标记CAI-B(美人蕉)、ACC-B(菖蒲)、PHA-B( 芦苇)、ZIA-B(茭白)、THD-B (再力花)、ARD-B(芦竹).其中管式炉升温程序:3.33℃/min升至400℃,再用40min升至550℃,保持2.5h.氮气速率: 300mL/min.
1.2 硫丹测定
由气相色谱电子捕获检测器(GC-ECD) (5975C, Aglient,美国)测定硫丹,色谱柱为Hp- 5ms毛细管柱(35m´0.32mm´0.25μm, Agilent Inc., USA),升温程序:80℃保持1min,以30℃/min升至180℃,再以3℃/min升至205℃,保持4min,以20℃/min升至290℃,保持2min.检测器温度320℃,氮气尾吹扫60mL/min,进样不分流,进样量1μL.
1.3 吸附实验
1.3.1 吸附等温研究 选系列浓度梯度的硫丹混合液(α-硫丹:β-硫丹=1:1,α-硫丹浓度分别为0.50, 1.00, 2.00, 5.00, 8.00, 10.00mg/L).称15.00mg生物炭于40.00mLEPA小瓶中,加入30.00mL硫丹溶液,置摇床内,室温避光,转速220r/min,平衡7h,取上清液3.00mL,加入等量正己烷,充分摇晃、离心,取上层液体加入过量无水硫酸钠,充分振荡后测GC-ECD,萃取分液两次,硫丹萃取率90.00%以上.并监控溶液pH值变化.
1.3.2 硫丹水解实验 在生物炭-水体系中进行.分别调节1mg/L硫丹溶液(同上),初始pH值为3.20、5.60、7.20、11.00.用磷酸钠、磷酸氢二钠及磷酸配置缓冲液.分别称取15mg生物炭样品于一系列40mL EPA瓶中,加入上述溶液30mL,添加空白组,设置3组平行样.测定平衡浓度及溶液中硫丹二醇浓度,并用水解方程反推发生水解硫丹的量,根据质量守恒定律计算水解、平衡及吸附各部分占比.
1.4 生物炭特征分析方法
测定生物炭的产率[14]、pH值[14-15]、灰分[14,16]、电位[17]等;用元素分析仪(Elementar VarioEL,德国)测定生物炭的元素组成;并借助扫描电镜(SEM)(KYKY-2800B,中国)测定表面结构;傅立叶红外光谱仪(Perkin Elmer 1725X,美国)测定生物炭表面官能团.利用N2吸附等温线Brnauer-Emmett-Teller法,由比表面积仪(Micromeritics, Norcross, USA)测定比表面积.
1.5 数据处理
用Frundlich模型拟合生物炭吸附硫丹的数据,其线性拟合公式[8]是:
loge= logF+×loge(1)
式中:e表示平衡时吸附量mg/g;e表示平衡时溶液的浓度mg/L;F表示Frundlich吸附系数mL/g,表示非线性吸附常数.
吸附分配系数d及有机碳标准化系数oc通过公式(2)和(3)算得

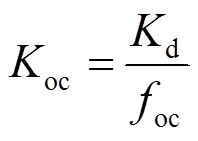
式中:oc为有机碳系数,d和oc(e= 0.001w和0.01w,w为硫丹溶解度)通过以上公式计算.
2 结果与讨论
2.1 不同种类生物炭基本理化特性

表1 生物炭基本特性表
从表1生物炭的理化特性可得:在550 ℃下热解,生物炭产率约32.00%(ARD-B除外),高于500℃下热解的秸秆、玉米芯、棉籽壳等废弃物[18-19],所含灰分10.88%~31.11%,高于玉米芯(4.02%)、柚子皮(9.06%)等[20].呈碱性,为负,与文献[21]相符,可能与生物炭表面所含酸碱官能团及无机碳成分有关.在550℃下热解,生物炭孔径为介孔,比表面积11.02~36.67m2/g,孔径与比表面积的相关性弱(=0.1190),说明炭表面部分孔径可能被灰分覆盖或堵塞[22],与文献[14]一致.
2.2 生物炭结构及表面官能团特性
2.2.1 表面结构与官能团分析 由图1生物炭SEM图谱可知,炭表面具多孔和管状结构,后者可能由植物细胞热解形成[23].总体上,生物炭孔隙发育较成熟,部分呈碎片化,孔壁表面有附着物,可能在烧制中降温过快,部分大孔坍塌,表面粗糙度增加[24],一定程度上减小生物炭比表面积.

图1 生物炭的SEM扫描图像
生物炭的傅立叶红外图谱(图2)表明, THD-B, ACC-B及CAI-B在875cm-1附近具小尖峰,是CaCO3特征峰[24].在1218cm-1附近处谱带为纤维素或半纤维素的官能团,1521cm-1为羧酸官能团的C=O伸缩振动[25].1807cm-1为芳香环的骨架C=C振动[21],碳碳叁键基团位于2179cm-1处,氢氧键大致位于3700cm-1处.在550℃下热解,生物炭表面存在大量芳香结构,仍存在烯烃和炔烃、纤维素和半纤维素结构,部分生物炭表面生成CaCO3沉淀.

图2 生物炭的FTIR图谱
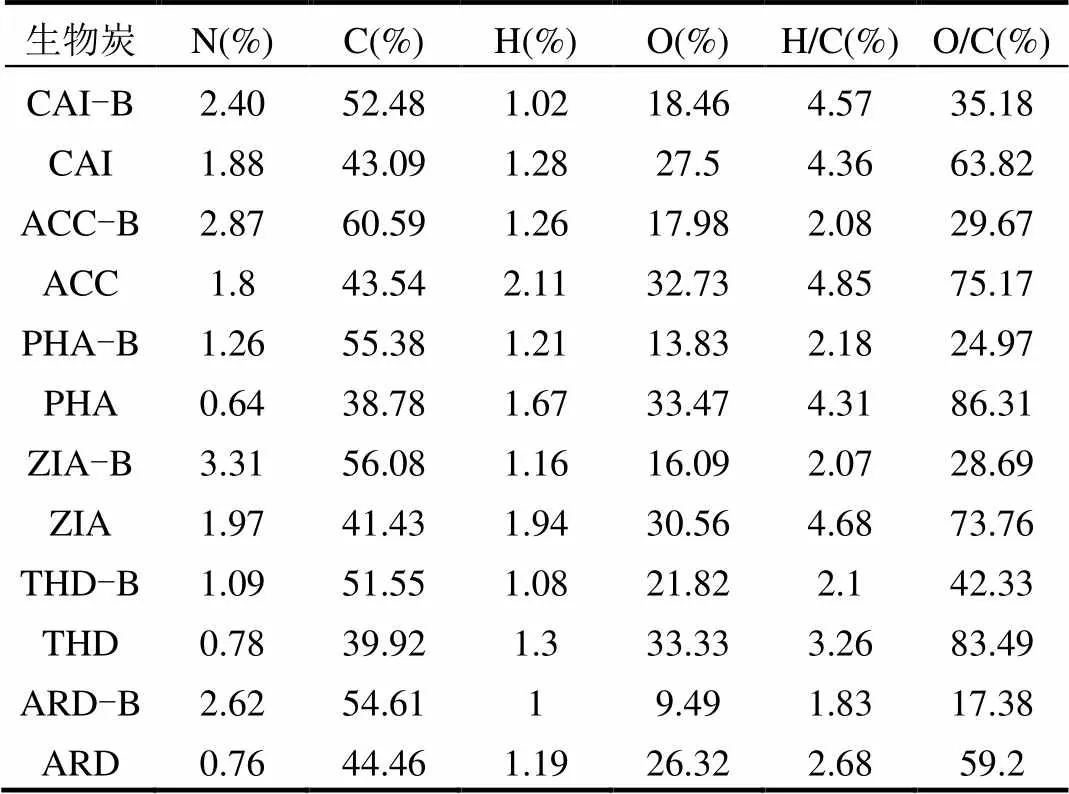
表2 生物炭(质)元素分析
注: CAI、ACC、PHA、ZIA、THD及ARD为相应生物炭原料.
2.2.2 元素组成分析 生物炭(质)的元素组成见表2.可见,各生物炭热解后,N、C等增加,H、O降低.这可能是热解中碳链发生脱羧基、脱氧和脱水等反应,单键变双/叁键[23],与图2红外谱图一致.随热解温度升高O/C原子比降低[22],生物炭表面极性官能团减少,生物炭表面疏水性越强[26-28],越利于吸附硫丹.
2.3 吸附等温实验

图3 生物炭对硫丹的吸附等温线
生物炭对硫丹的吸附等温线拟合的非线性系数为0.53~0.84,非线性化程度高,与沉积物热解后生成生物炭的吸附等温线一致[29].通常,生物炭比表面积越大,其表面微孔越多,提供更多吸附位[24].虽PHA-B、ZIA-B和ARD-B具较大比表面积和较小孔径,但其非线性系数较大,非线性程度低,可能与该类生物炭的弱芳香化有关[30].另外生物炭孔径与α-硫丹和β-硫丹的吸附常数logd较明显正相关(<0.05,=0.7839和<0.01,=0.8003),且比表面积与其相关性较弱(= 0.1644,=0.0368),说明生物炭吸附硫丹,除扩散作用外[31]还有其它作用力.
除PHA-B、ZIAB及ARD-B外,其余生物炭值小于0.60,说明炭表面的芳香性较高,极性也与红外光谱结果一致.硫丹分子与芳香性官能团可通过疏水作用结合[32]而被生物炭吸附.另外,由图2红外谱图可知,生物炭表面也存在类纤维素结构,此类非碳化结构,在吸附过程可能对硫丹具分配作用[31].

表3 生物炭Freundlich等温参数及分配系数表
注:①:生物炭对α-硫丹的等温吸附参数;②:生物炭对β-硫丹的等温吸附参数;表示当e=0.001S时,logoc值(mL/g);表示当e=0.01w时,logoc值(mL/g);*表示F(mL/g);#表示d(mL/g).
CAI-B、ACC-B及THD-B的N值较小,其生物炭表面可提供较多吸附位点,与生物炭具有较小的C/H系数一致,说明硫丹以表面吸附为主.随平衡浓度增加(e=0.001, 0.01w), logoc减小,吸附行为发生在生物炭表面能量较高的致密有机质的吸附位[32].另由表3中logd可知,生物炭对硫丹的亲和力较-硫丹大,可能与-硫丹易在沉积物或植物体富集有关[33].另外,生物炭表面带负电,硫丹为极性非离子型化合物,静电作用也影响吸附[34].
炭-水体系的pH值为5.67~10.07.炭表面的阳离子在溶液中与H+发生离子交换,促进pH值增大[34].在中/碱性时,硫丹会水解产生硫丹二醇和SO32-[35],增加溶液pH值并减轻硫丹毒性.
2.4 生物炭对硫丹催化水解
由图4各pH下生物炭的硫丹去除模式可得:随pH升高,硫丹自身水解程度增加.且pH为3.20、5.60和7.20时,生物炭对硫丹的催化水解较明显,可能因酸性(3.20和5.60)时,硫丹自身水解程度弱[36](小于3.00%),忽略生物炭对水解产物硫丹二醇的吸附,生物炭的硫丹水解效率提高7.15~36.05%,-硫丹和-硫丹仍占优势.中性(pH7.20)时,硫丹水解程度约28.05%,仍高于空白,水解率约提高16.57~72.57%,生物炭对硫丹催化水解较明显.而pH为11.00时, 硫丹自身水解程度大[36](85.65%),硫丹二醇占优势,可与生物炭吸附,因此,发生水解的硫丹浓度明显比空白少.

A:发生吸附硫丹的量(mg/L); B平衡浓度(α-硫丹和β硫丹的总和)(mg/L); C:发生水解硫丹的量(mg/L)
整体上,中/酸性时CAI-B和PHA-B的硫丹吸附效率较好,分别为52.65%~60.51%, 46.57%~ 47.28%.ARD-B及ZIA-B吸附效果较差(10.86%~ 21.22%).而对ACC-B和THD-B,在酸性时,吸附率约21.22%~44.01%,在中/碱性时,吸附效果较好(33.68%~57.95%).偏碱性时,商务炭的吸附效果差别不大(43.12%~50.95%).总之,中/碱性时,生物炭的硫丹去除率较高(78.84%~98.57%). CAI- B、ACC-B和THD-B的硫丹去除率(96.77%~ 98.57%)最高.
3 结论
3.1 6种生物炭对α-硫丹和β-硫丹的去除效果良好, CAI-B(美人蕉), ACC-B(菖蒲)和THD-B(再力花)的硫丹吸附效果(40.17~60.51%)与去除能力(96.77~98.57%)最优.
3.2 生物炭对硫丹的N为0.53~ 0.84,非线性化程度高.且对β-硫丹的亲和力(log)比对-硫丹大.吸附行为以有机质表面吸附为主,除介孔扩散作用外,还有静电、分配及水解催化.
3.3 炭-水体系的pH为5.67~10.07,在中/碱性时,硫丹水解产生硫丹二醇,增加溶液pH并减轻硫丹毒性.当体系pH为3.20、5.60和7.20时,生物炭对硫丹的催化水解较明显.且pH7.20时,对-硫丹和β-硫丹水解效率提高16.57~72.57%.
[1] Jia H, Li Y, Wang D, et al. Endosulfan in China 1-gridded usage inventories [J]. Environmental Science and Pollution Research, 2009,16(3):295-301.
[2] 武焕阳,丁诗华.硫丹的环境行为及水生态毒理效应研究进展[J]. 生态毒理学报, 2015,10(2):113-122.
[3] 王 丽.典型乡镇饮用水源有毒污染物分布特征与健康风险评估[D]. 广州:南方医科大学, 2012.
[4] 吴 敏.南水北调中线丹江口水库调水前有机氯农药的分布研究[D]. 武汉:华中师范大学, 2014.
[5] Leena S, Choudhary S K, Singh P K. Pesticide concentration in water and sediment of River Ganga at selected sites in middle Ganga plain [J]. International Journal of Environmental Sciences, 2012,1(3):260-273.
[6] Quinete N, Castro J, Fernandez A, et al. Occurrence and distribution of endosulfan in water, sediment, and fish tissue: An ecological assessment of protected lands in South Florida [J]. Journal of Agricultural & Food Chemistry, 2013,61(49):11881- 11892.
[7] Sudhakar Y, Dikshit A K. Adsorbent selection for endosulfan removal from water environment [J]. Journal of Environmental Science and Health Part B, Pesticides Food Contaminants and Agricultural Wastes, 1999,34(1):97-118.
[8] Rauf N, Tahir S S, Kang J H, et al. Equilibrium, thermodynamics and kinetics studies for the removal of alpha and beta endosulfan by adsorption onto bentonite clay [J]. Chemical Engineering Journal, 2012,192(2):369-376.
[9] Thomas J, Chitra K R. Nanogold doped TiO2nanotubes: efficient solar photocatalyst for the degradation of endosulfan [J]. Materials Focus, 2014,3(3):233-238(6).
[10] Cong L, Guo J, Liu J, et al. Rapid degradation of endosulfan by zero-valent zinc in water and soil [J]. Journal of Environmental Management, 2015,150:451-455.
[11] Ahmad M, Rajapaksha A U, Lim J E, et al.Biochar as a sorbent for contaminant management in soil and water: a review [J]. Chemosphere, 2014,99(3):19-33.
[12] Chen B, Chen Z, Lv S. A novel magnetic biochar efficiently sorbs organic pollutants and phosphate [J]. Bioresource Technology, 2011,102(2):716-723.
[13] Trigo C, Cox L, Spokas K. Influence of pyrolysis temperature and hardwood species on resulting biochar properties and their effect on azimsulfuron sorption as compared to other sorbents [J]. Science of the Total Environment, 2016,566-567:1454.
[14] Cui X, Hao H, Zhang C, et al. Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars [J]. Science of the Total Environment, 2016,539: 566-575.
[15] Inyang M, Gao B, Yao Y, et al. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass [J]. Bioresource Technology, 2012,110(2):50-56.
[16] Wang F, Sun H, Ren X, et al. Effects of humic acid and heavy metals on the sorption of polar and apolar organic pollutants onto biochars [J]. Environmental Pollution, 2017,231:229-236.
[17] Sun K, Kang M, Ro K S, et al. Variation in sorption of propiconazole with biochars: The effect of temperature, mineral, molecular structure, and nano-porosity [J]. Chemosphere, 2016, 142:56-63.
[18] Abdul G, Zhu X, Chen B. Structural characteristics of biochar- graphenenanosheet composites and their adsorption performance for phthalic acid esters [J]. Chemical Engineering Journal, 2017, 319:9-20.
[19] Lu K, Yang X, Gielen G, et al. Effect of bamboo and rice straw biochars on the mobility and redistribution of heavy metals (Cd, Cu, Pb and Zn) in contaminated soil [J]. Journal of Environmental Management, 2017,186:285-292.
[20] Li J, Liang N, Jin X, et al. The role of ash content on bisphenol A sorption to biochars derived from different agricultural wastes [J]. Chemosphere, 2017,171:66-73.
[21] 王 博,叶 春,李法云,等.水生植物制生物炭对硝态氮的吸附规律研究[J]. 中国环境科学, 2017,37(1):116-122.
[22] 郎印海,刘 伟,王 慧.生物炭对水中五氯酚的吸附性能研究[J]. 中国环境科学, 2014,34(8):2017-2023.
[23] Liu S H, Zeng G M, Niu Q Y, et al. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review [J]. Bioresource Technology, 2017,224: 25-33.
[24] 蒋煜峰,孙 航,胡雪菲,等.添加小麦秸秆生物炭对黄土吸附苯甲腈的影响[J]. 中国环境科学, 2016,36(5):1506-1513.
[25] Miao Q, Bi E, Li B. Roles of polar groups and aromatic structures of biochar in 1-methyl-3-octylimidazolium chloride ionic liquid adsorption: pH effect and thermodynamics study [J]. Environmental Science and Pollution Research, 2017,24(28): 22265-22274.
[26] Hilioti Z, Michailof C M, Valasiadis D, et al. Characterization of castor plant-derived biochars and their effects as soil amendments on seedlings [J]. Biomass and Bioenergy, 2017,105:96-106.
[27] Chen, B. and Z. Chen. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures [J]. Chemosphere, 2009,76(1):127-133.
[28] 赵 炎,郑国灿,朱 恒,等.紫色土对硫丹的吸附与解吸特征[J]. 环境科学, 2015,36(9):3464-3470.
[29] Wang B, Zhang W, Li H, et al. Micropore clogging by leachable pyrogenic organic carbon: A new perspective on sorption irreversibility and kinetics of hydrophobic organic contaminants to black carbon [J]. Environmental Pollution, 2017,220:1349-1358.
[30] Jin J, Wang M, Cao Y, et al. Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: biochar properties and environmental risk from metals [J]. Bioresource Technology, 2017,228:218-226.
[31] Yang K, Jiang Y, Yang J, et al. Correlations and adsorption mechanisms of aromatic compounds on biochars produced from various biomass at 700°C [J]. Environmental Pollution, 2018, 233:64.
[32] Sun K, Kang M, Zhang Z, et al. Impact of deashing treatment on biochar structural properties and potential sorption mechanisms of phenanthrene [J]. Environmental Science & Technology, 2013, 47(20):11473-11481.
[33] Luek J L, Dickhut R M, Cochran M A, et al. Persistent organic pollutants in the Atlantic and southern oceans and oceanic atmosphere [J]. Science of the Total Environment, 2017,583:64-71.
[34] 李 靖.不同源生物炭的理化性质及其对双酚A和磺胺甲噁唑的吸附[D]. 昆明:昆明理工大学, 2013.
[35] Singh S P, Guha S, Bose P, et al. Mechanism of the Hydrolysis of Endosulfan Isomers [J]. The Journal of Physical Chemistry A, 2017,121(27):5156-5163.
[36] Wang J, Li L, Liu J, et al. Distribution mode and environmental risk of POP pesticides such as endosulfan under the agricultural practice of straw incorporating [J]. Environmental Pollution, 2017,220:1394-1399.
Adsorption and catalytic hydrolysis of endosulfan on biochars derived from emergent plants.
HE Qi, CAO Feng-mei, LU Shao-yong*, CHEN Fang-xin, CAI Chun-lun
(State Environmental Protection Scientific Observation and Research Station for Lake Dongtinghu , National Engineering Laboratory for Lake Pollution Control and Ecological Restoration, Chinese Research Academy of Environmental Sciences, Beijing 100012, China). China Environmental Sciences, 2018,38(3):1126~1132
Six kinds of biochars derived from typically wetland plants were pyrolyzed under 550℃ without oxygen, the adsorption and catalytic hydrolysis behavior of these biochars towards endosulfan were investigated .The results showed that the ash contents were higher than other agricultural wastes, rangeing from 10.88% to 31.11%. The results of characterization suggested that the biochars had a mass of aromatic domains and pores on the surface of biochars. The pores were mainly mesopores and partly fragmented. All the biochars have high adsorption capacity toward endosulfan. The adsorption was mainly occurred on the surface of biochars which contained dense organic matter. Hydrophobic, diffusion and distribution effects played significant role in the process of adsorption. Under the conditions of neutral or alkali solutions, CAI-B, ACC-B and THD-B could efficiently remove endosulfan with uptake rates of 96.77% to 98.57%. Moreover, biochar could catalyze the hydrolysis of endosulfan with an increase of hydrolysis efficiency about 16.57%~72.57% under the condition of neutral solution.
biochar;endosulfan;catalytic hydrolysis;aromatic functional group
X5
A
1000-6923(2018)03-1126-07
何 琦(1993-),女,河南南阳人,硕士,主要从事湖泊有机污染调查及控制研究.发表论文7篇.
2017-08-23
典型湖泊有毒有害化学品和水环境调查(2015FY110900)
* 责任作者, 研究员, lushy2000@163.com

