Prognostic value of cortisol and thyroid function tests in poisoned patients admitted to toxicology ICU
Shahin Shadnia, Nasim Zamani, Hossein Hassanian-Moghaddam, Hamed Shafaroodi, Mina Padandar,Mohammad Hasan Rezaeizadeh
1 Toxicological Research Center, Department of Clinical Toxicology, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
2 Excellence Center of Clinical Toxicology, Iranian Ministry of Health, Tehran, Iran
3 Pharmaceutical Sciences Branch, Islamic Azad University (IAUPS), Tehran, Iran
INTRODUCTION
Determination of prognosis is a major concern for clinical toxicologists managing acutely poisoned patients.Routine prognostic scores in the general intensive care units (ICUs) are often inaccurate for prognostication in this population. Prognostic factors facilitate appropriate disposition to limited ICU beds.[1]
Prognostic value of cortisol and thyroid function tests(TFTs) have previously been evaluated in critically ill internal medicine, pediatric, and traumatic patients.[2-9]In clinical toxicology, previous studies on single poisonings suggest that cortisol and TFTs may have prognostic utility in patients poisoned with aluminum phosphide (ALP),paraquat, and organophosphate.[10-12]These studies suggest the mechanism is related to euthyroid sick syndrome,as well as lipid depletion, hemorrhage, and necrosis in the adrenal cortex in critically ill patients. Other potential mechanisms affecting cortisol include stress,hypotension, and shock in critically ill patients. This may be based on changes in secretion, responsiveness, protein binding, and activity of glucocorticoids.[12]However,no study has evaluated poisoned patients in general to see whether cortisol and TFTs can serve as prognostic factors.
Our center is a large urban hospital with an ICU admission rate of almost 400 per year.[13]Our center routinely sees patients with poisonings from highly lethal pesticides including paraquat, organophosphates and ALP which require intensive support and resources.[1]Adequate prognostic factors are lacking. The aim of the current study was to evaluate the prognostic efficacy of cortisol and TFTs in critically ill poisoned patients admitted to our toxicology ICU.
METHODS
In a prospective observational study, all patients referred to the emergency department (ED) of Loghman Hakim Poison Hospital in Tehran, Iran, with a history of toxin/medication ingestion in need for ICU admission between March 2013 and August 2014, were considered for inclusion in the study.[14]
Those who were admitted to ICU directly from the ED within the first 24 hours post ingestion/exposure were included. All patients with ingestions/exposures or previous history of ingestion of the medications that could affect the cortisol level and TFTs including propylthiouracil, methimazole, lithium, iodide,amiodarone, aminoglutethimide, interferon 2, sunitinib,glucocorticoids, interleukin analogues, dopamine antagonists, somatostatin, rexinoids, carbamazepine,metformin, levothyroxine, oxcarbazepine, metyrapone,furosemide, phenytoin, probenecid, heparin, and nonsteroidal anti-inflammatory drugs were excluded. After ICU admission, the cortisol and TFT tests [total T3, total T4,and thyroid-stimulating hormone (TSH)] were sent between 8 am and 10 am of the first day of ICU stay (in the first 24 hours after ingestion/exposure). Evaluation of both TFTs and cortisol was made by radio-immuno assay techniques.Normal ranges for T3, T4, and TSH were 0.75–2 ng/mL,4.5–11.5 μg/dL, and 5–25 mIu/mL, respectively. Cortisol levels were considered normal if they were about 10 to 20 micrograms per deciliter (μg/dL) in the early morning(within one hour of the usual time of awakening),from 3 to 10 μg/dL at 4 pm, and less than 5 μg/dL after the usual bedtime. Meanwhile, a checklist containing demographic characteristics of the patients, type of the ingested medication/toxin and its dose, time elapsed between its ingestion and hospital presentation, clinical examination and vital signs on arrival, Simplified Acute Physiology Score (SAPS II) and Acute Physiology and Chronic Health Evaluation (APACHE II) scores on ICU admission, days of hospital stay, andfinal outcome of the patients (full recovery, recovery with complications, and death) wasfilled for every single patient. By calculating the SAPS and APACHE scores, we aimed to both have an estimation of the severity of poisoning in our patients and compare these two prognostic factors with cortisol and TFTs. The patients were then followed and their hospital stay and final outcome were also recorded. Data was then inserted into SPSS software (statistical package for social sciences version 17, Chicago, Ill, USA) and analyzed using Kolmogorov Smirnov, studentttest,Mann-WhitneyUtest, logistic regression, and ROC curves. Kolmogorov-Smirnov test was used to determine normality of the variables, for whose description, mean(±SD) and median [interquartile (IQ) range] were given.For qualitative variables, percent of frequency was provided. To compare normally distributed continuous variables,ttest was used. Mann-WhitneyUtest was performed to compare differences between two independent groups when the dependent variable was continuous, but not normally distributed. Logistic model was used to determine independent predictors of death in the patients. APvalue of less than 0.05 was considered to be statistically significant. The study was approved by our local ethics committee and written consent was taken from the patients' next of kin. The comparison wasfirst performed in all patients followed by a comparison between ALP- and non-ALP-poisoned patients. All prognostic factors were then compared to determine the most efficient one in our setting.
RESULTS
Among the 376 patients who were admitted to our ICU during the study period, 152 were excluded because they were sent to ICU from the wards and not directly from the ED. Seventeen were excluded because they had a suspicious history of toxicity but diagnosis of toxicity was never confirmed due to lab test limitations. Another seven patients were excluded due to carbamazepine toxicity (4 patients), previous use of corticosteroids (1 patient), and ingestion/exposure more than 24 hours before hospital presentation (2 patients). In the remaining 200 cases,median age was 31 years (range, 10 to 87 years) and 129(64.5%) were male. The most common causes of poisoning were opioids (78 patients, 39%), sedative-hypnotics (29 patients, 14.5%), and antipsychotics/antidepressants (28 patients, 14%). Twenty-four (12%) were poisoned by ALP. Median time elapsed between ingestion/exposure and hospital presentation was four hours (range 0.5–24 hours). On-arrival Glasgow coma scale (GCS), vital signs,APACHE II and SAPS scores, and cortisol/TFT results are summarized in Table 1. The most common presenting sign/symptom was loss of consciousness (158 patients, 79%)followed by hypoventilation (bradypnea/apnea, 25 patients,12.5%), and agitation (13 patients, 6.5%). Of the patients,163 (81.5%) recovered completely, 26 (13%) died, and 11 (5.5%) recovered with complications (mainly hypoxicischemic sequelae). Of the dead patients, eight (33%) were ALP-poisoned.
After inclusion of all qualitative and quantitative variables that related to death with aPvalue of 0.2 and less, enterlogistic regression was performed. In the ALP-poiosned patients, cortisol (P<0.001) and loss of consiousness (P<0.001) were significantly different between those who survived and those who died. In all patients (ALP- and non ALP-poisoned),the only lab test that could prognosticate the patients' mortality was cortisol (P<0.001;OR0.949; 95%CI0.922, 0.977; cut-off=42.56 µg/dL; sensitivity=69.21%;specificity=81.2%). Comparison of the cortisol and TFTs between the patients with and without hypotension is shown in Table 2. SAPS II and APACHE II could not prognosticate death in patients in toxicology ICU at all.

Table 1. On-arrival characteristics and lab test of all patients (n=200)
In the comparison between ALP- poisoned patients with the others, GCS and cortisol level had the efficacy to prognosticate death in ALP-poisoned patients (Pvalues<0.001 in both uni- and multivariate analyses). Median(interquartile range, IQR) GCS was 7 (6, 10) and 15 (8,15) in non ALP- and ALP-poisoned patient, respectively(P<0.003). Comparison between the dead, alive, and all ALP- and non ALP-poisoned patients has been given in Table 3. Spearman's rho correlation analysis showed a significant correlation between APACHE and cortisol(r=0.189,P=008), APACHE and T4 (r=–0.164,P=023)and SAPS and Cortisol (r=0.148,P=038).
DISCUSSION
Search for prognostic factors is a major concern in clinical toxicology since ICU routine prognostic scores are often inaccurate in this field. This is mainly due to the fact that poisoned patients present with unstable but generally reversible situations affecting the sum of such scores dramatically.
Changes in the peaks and nadirs of cortisol have previously been mentioned in cancer patients.[15]As in cancer patients, poisoning can disrupt the circadian rhythm of cortisol in the body.[10-12]Previous studies detected changed levels of cortisol in poisoned patients inconsistently. Mehrpour et al[16]postulated that glucose disturbances in ALP-poisoned patients were due to stimulation of cortisol, glucagon, and adrenaline secretion, inhibition of insulin, inhibition of glucogenesis in liver, and failure of hepatic glycogenolysis. Chugh et al[17]reported low levels of cortisol in these patients as a result of damages to adrenal cortex such as fat depletion and hemorrhagic necrosis. However, such changes seem to be a problem not only in ALP poisoning but also other intoxications such as paraquat and organophosphate.[10,11]
Although Mehrpour and colleagues had mentioned a high level of cortisol in ALP-poisoned patients, Wahab and associates as well as Farnaghi and coworkers reported different results.[12,18]They found extremely low levels of cortisol in their patients which could be due to either time of sampling or evaluation of the patients at the end stages of the toxicity when probably all adrenal cortex was disrupted.[12,18]Our study specificallyexamined samples drawn between 8–10 am within 24 hours of ingestion/exposure to minimize the effects of such confounding factors on cortisol level. Our data demonstrate that amongst the factors we studied, cortisol is the best prognosticator of outcome for use in our toxicology ICU. Even after performance of regression analysis, it was the only factor that could efficiently prognosticate the patients’ outcome in general and a good tool to prognosticate ALP-poisoned patients. This can facilitate disposition to ICU versus ward in these patients.
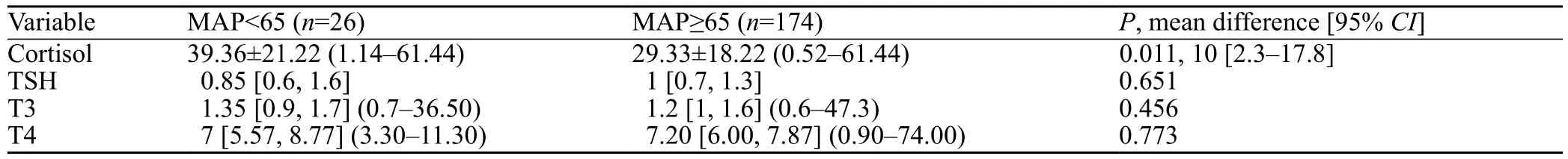
Table 2. Comparison of TFTs and cortisol between those with hyopotention (MAP<6.5) and normal blood pressure (MAP≥6.5)
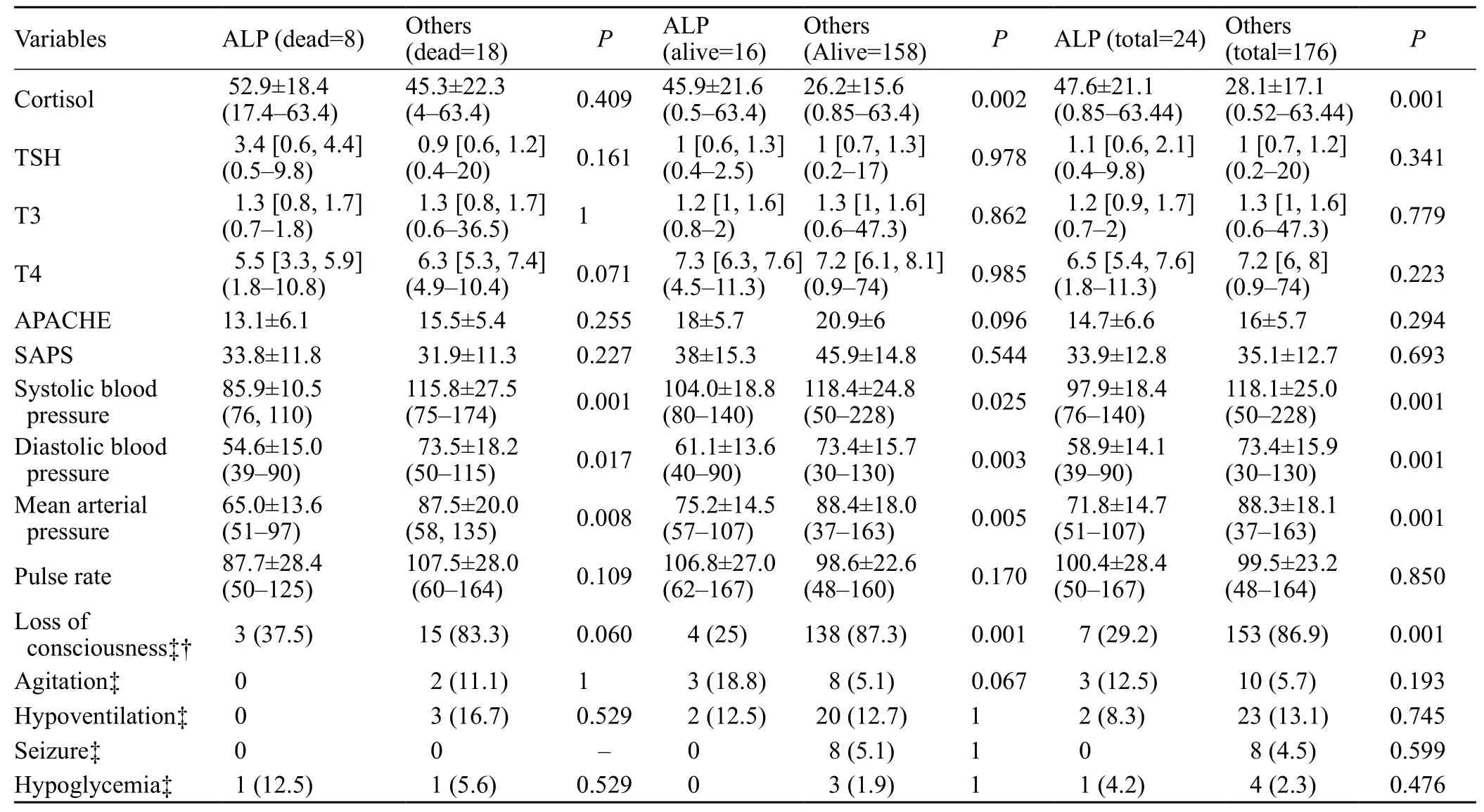
Table 3. Comparison of the ALP- and non ALP-poisoned patients
We could not find any article that has evaluated the prognostic role of TFTs in poisoned patients. Although our results were primarily promising regarding T4, performance of regression analysis rejected the possible value of T4 in prognostication of these patients. This could be due to the fact that we only relied on the patients’ histories for thyroid problems and had no access to the previous profile of their thyroid diseases which may be silent in nature.
The other major finding of the current study was that routine ICU scoring systems that are used in general ICUs are not probably efficient enough to be used in poisoned patients, a result that was somehow concluded in our previous study, as well.[1]This emphasizes the need for determination of better prognostic factors in toxicology ICU.
We also found higher levels of cortisol in ALP-poisoned patients. While previous authors had suggested that cortisol level dropped in these patients, we found completely opposite results. This can be due to the fact that these patients remain conscious until the end stages of toxicity in contrast to other poisonings in which the patients present with early loss of consciousness, ALP-poisoned patients tolerate high levels of physiologic stress, and are aware of the deterioration of their poor condition. It may also be interpreted by their significantly lower MAP compared to the blood pressure of the patients who refer with other types of poisoning. However, lower MAP is per se accompanied by higher cortisol levels and poorer prognosis even in patients intoxicated by drugs or poisons other than ALP.
The major limitation of this study was that the time elapsed between ingestion/exposure and check of cortisol level (8 am to 10 am of the first day of ICU stay) was not the same in all patients although it was always less than 24 hours. This however seemed unavoidable because we meant to measure cortisol level of all patients within the same hours of the day while their time of hospital presentation and ingestion was not similar. On the other hand, we did not follow our patients for more subtle complications and quality of life after discharge (including cognitive decline and depressive symptoms) which is again a limitation of the current study.
CONCLUSION
In conclusion, cortisol was the prognostic factor for all poisoned patients in our toxicology ICU. Cortisol levels are generally higher in ALP-poisoned patients with poorer outcome. Less MAP may accompany a higher cortisol level and is also associated with poorer prognosis in intoxicated patients. More prospective studies on the cortisol circadian cycle and maybe in different time intervals may be beneficial in intoxicated patients.
The authors would like to thank Dr Rama B Rao, MD,FACMT, assistant physician in New York Presbyterian Hopsital and assistant professor of medicine in Weill Cornell Medical College,Cornell University, for her sophosticated editing of this paper.
Funding:This study was not funded.
Ethical approval:The study was approved by our local ethics committee.
Conflicts of interest:The authors state they have no competing interests.
Contributors:SS proposed the study and wrote the first draft.this work. All authors read and approved the final version of the manuscript.
1 Alizadeh AM, Hassanian-Moghaddam H, Shadnia S, Zamani N, Mehrpour O. Simplified acute physiology score II/acute physiology and chronic health evaluation II and prediction of the mortality and later development of complications in poisoned patients admitted to intensive care unit. Basic Clin Pharmacol Toxicol. 2014;115(3):297-300.
2 Van Beek JG, Mushkudiani NA, Steyerberg EW, Butcher I, McHugh GS, Lu J, et al. Prognostic value of admission laboratory parameters in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):315-28.
3 Annane D, Sébille V, Troché G, Raphaël JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA.2000;283(8):1038-45..
4 Lewis LS. Prognostic factors in acute meningococcaemia. Arch Dis Child. 1979;54(1):44-8.
5 O'Neill PA, Davies I, Fullerton KJ, Bennett D. Stress hormone and blood glucose response following acute stroke in the elderly.Stroke. 1991;22(7):842-7.
6 Christ-Crain M, Stolz D, Jutla S, Couppis O, Müller C, Bingisser R, et al. Free and total cortisol levels as predictors of severity and outcome in community-acquired pneumonia. Am J Respir Crit Care Med. 2007;176(9):913-20.
7 Iervasi G, Pingitore A, Landi P, Raciti M, Ripoli A, Scarlattini M,et al. Low-T3 syndrome: a strong prognostic predictor of death in patients with heart disease. Circulation. 2003;107(5):708-13.
8 Vexiau P, Perez-Castiglioni P, Socié G, Raciti M, Ripoli A,Scarlattini M, et al. The 'euthyroid sick syndrome': incidence,risk factors and prognostic value soon after allogeneic bone marrow transplantation. Br J Haematol. 1993; 85:778-82.
9 Riesenbeck LM, Bierer S, Hoffmeister I, Köpke T, Papavassilis P, Hertle L, et al. Hypothyroidism correlates with a better prognosis in metastatic renal cancer patients treated with sorafenib or sunitinib. World J Urol. 2011;29(6):807-13.
10 Giri SN, Curry DL, Stabenfeldt G, Spangler WL, Chandler DB, Schiedt MJ. Effects of paraquat on plasma glucose,cortisol, catecholamines, and insulin in the beagle. Environ Res.1983;30(1):80-8.
11 Kaya E, Yilmaz A, Saritas A, Colakoglu S, Baltaci D, Kandis H, et al. Acute intoxication cases admitted to the emergency department of a university hospital. World J Emerg Med.2015;6(1):54-9.
12 Abder-Rahman H. Effect of aluminum phosphide on blood glucose level. Vet Hum Toxicol. 1999;41(1):31-2.
13 Hassanian-Moghaddam H, Zamani N, Rahimi M, Shadnia S,Pajoumand A, Sarjami S. Acute adult and adolescent poisoning in Tehran, Iran; the epidemiologic trend between 2006 and 2011.Arch Iran Med. 2014;17(8):534-8.
14 Enfield KB, Kirk MA. Use of intensive care unit. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR,editors. Goldfrank’s Toxicologic Emergencies, New York:McGraw Hill Publications Inc; 2015;135-43.
15 Sephton SE, Lush E, Dedert EA, Floyd AR, Rebholz WN,Dhabhar FS, et al. Diurnalcortisolrhythmas a predictor of lungcancersurvival. Brain Behav Immun. 2013;30 Suppl:S163-70.
16 Mehrpour O, Aghabiklooei A, Abdollahi M, Singh S. Severe hypoglycemia following acute aluminum phosphide (rice tablet)poisoning; a case report and review of the literature. Acta Med Iran. 2012;50(8):568-71.
17 Chugh SN, Ram S, Sharma A, Arora BB, Saini AS, Malhotra KC. Adrenocortical involvement in aluminium phosphide poisoning. Indian J Med Res. 1989;90:289-94.
18 Wahab A, Zaheer MS, Wahab S, Khan RA. Acute aluminium phosphide poisoning: an update. Hong Kong J Emerg Med.2008;15(3):152-5.
 World journal of emergency medicine2018年1期
World journal of emergency medicine2018年1期
- World journal of emergency medicine的其它文章
- Perceptions of emergency medicine residents on the quality of residency training in the United States and Saudi Arabia
- Intravenous fluid selection rationales in acute clinical management
- Association between the elderly frequent attender to the emergency department and 30-day mortality: A retrospective study over 10 years
- Differential diagnoses of magnetic resonance imaging for suspected acute appendicitis in pregnant patients
- Ultrasound curriculum taught byfirst-year medical students: A four-year experience in Tanzania
- Falls from height: A retrospective analysis
