金鱼卵黄原蛋白单抗夹心ELISA的开发及其在环境雌激素检测中的应用❋
马淑伟,王 军,单瑞后,张振忠,汝少国
(中国海洋大学海洋生命学院,山东 青岛 266003)
鱼类卵黄原蛋白(vitellogenin, Vtg)是目前检测环境雌激素活性最常用的生物标志物,其测定通常采用基于Vtg或卵黄脂磷蛋白(lipovitellin, Lv)多克隆抗体建立的酶联免疫吸附方法(ELISA)[1-2]。与多克隆抗体相比,单克隆抗体识别单一抗原表位,具有更高的特异性,利用单克隆抗体建立的ELISA能显著提高检测的精确度,可以更加准确地定量Vtg[3-4]。然而,单克隆抗体的制备成本高,技术难度大,目前只开发了青鳉(Oryziaslatipes)、虹鳟鱼(Oncorhynchusmykiss)等几种鱼类的Vtg或Lv单克隆抗体[3,5-6]。金鱼(Carassiusauratus)是环境雌激素研究常用的受试生物[2,7],研究者已经利用多克隆抗体建立了金鱼Vtg的ELISA[8-9],但是至今未见金鱼Vtg单抗ELISA的报道。同科鱼类的Vtg具有相近的免疫源性,能被同科鱼类Vtg抗体识别,例如鲤鱼(Cyprinuscarpio)Vtg多克隆抗体常被用于检测黑头呆鱼(Pimephalespromelas)和金鱼等鲤科鱼类的Vtg[1,10],推测单克隆抗体也可以检测同科鱼类的Vtg。因此,本研究尝试利用实验室制备的斑马鱼Lv单克隆抗体[11]建立金鱼Vtg的ELISA。
鱼类Vtg ELISA建立后需要开展17β-雌二醇(17β-estradiol, E2)或其它雌激素类物质的暴露实验,以检验方法的可靠性[12]。血浆是检测Vtg常用的样品[2,13],但是血样采集过程会对鱼体造成伤害甚至死亡。随着人们对动物保护与福利的日益关注,开发无伤害的检测方法引起了研究者的关注。Moncaut等[14]提出南美鲷鱼(Cichlasomadimerus)体表粘液中含有Vtg,可以用作检测样品。因此,本研究利用建立的单抗夹心ELISA测定了E2暴露后金鱼血浆和体表粘液中的Vtg含量,评价了以金鱼体表粘液代替血浆,用于Vtg检测的可行性。
1 材料与方法
1.1 实验用鱼
金鱼购自青岛市南山花鸟虫鱼市场,体重(21.6±3.6) g、体长(9.4±0.6) cm,实验室驯养两周后用于实验。实验容器为50 L玻璃水族箱,实验用水为连续曝气24 h的自来水,溶解氧为(7.0±0.1) mg·L-1,光周期(光暗比)为16∶8,每天饲喂适量金鱼颗粒饵料。
1.2 金鱼Vtg与Lv的纯化
金鱼Vtg与Lv的提取和纯化采用此前实验室报道的两步层析法[15]。
1.3 单克隆细胞株的筛选
将对照雄鱼血浆、E2诱导雄鱼血浆、纯化的金鱼Vtg进行SDS-PAGE,将蛋白转印到PVDF膜,封闭后,利用8株斑马鱼Lv单克隆抗体室温孵育4 h;TBST洗3次后,用辣根过氧化物酶标记羊抗兔LgG室温孵育4 h,TBST洗膜3次后,用DAB显色液显色,待条带清晰时,用蒸馏水终止反应。将筛选出的杂交瘤细胞株进行扩大培养。
1.4 抗体的生产与纯化
参照An等[6]的方法制备大量单克隆抗体。向小鼠腹腔注射杂交瘤细胞105个;10 d后取腹水,收集上清,纯化单克隆抗体。利用Bradford法测定蛋白浓度,保存于-80 ℃。
1.5 夹心ELISA的建立与性能评价
参考Mitsui等[16]的方法,将纯化的斑马鱼Lv单克隆抗体用碳酸钠包被缓冲液(0.05 Mol/L, pH 9.6)稀释至5 μg·mL-1后,4℃包被过夜;次日,37 ℃封闭1 h,用PBST清洗3次后,加入100 μL梯度稀释的纯化金鱼Lv,37 ℃孵育1 h,随后加入100 μL不同稀释倍数的HRP-标记金鱼Lv多克隆抗体(1∶5 000、1∶10 000、1∶20 000、1∶40 000),于37°C孵育1 h;最后,加入100 μL TMB单组分显色液(Solarbio, China),37 ℃显色10 min,用2N H2SO4终止反应,测定450 nm下的吸光值。
参照Nilsen等[17]与Maltais等[18]的方法测定ELISA的精确度与检出限。精确度通过组内差异与组间差异评价,检出限定义为12个0标准品孔吸光值的平均值加上两倍标准差所对应的标准品浓度。
1.6 E2对金鱼Vtg的诱导
采用半静态毒性试验方法,E2暴露浓度分别为0、10、100和1 000 ng·L-1,3、21 d后采集血样与体表粘液。体表粘液的采集按照Maltais和Roy[19]的方法略加修改,用刀片轻轻擦拭金鱼尾鳍,将刀片上粘液转移至1.5 mL离心管中,称重,按1∶10(w∶v)加入预冷的含有抑酶肽(0.05 IU·mL-1)的0.01 mol/L PBS (pH 7.4),匀浆后,4℃ 8 000 g离心10 min,取上清。利用Western blot和ELISA检测血浆与体表粘液中的Vtg。
1.7 数据分析
金鱼(n=6)血浆与体表粘液中Vtg含量经Tukey’s检验后进行单因素方差分析(ANOVA)。所有数据以平均值±标准差的形式表示,当P<0.05时,定为差异显著,P<0.01时,定为差异极显著。数据统计检验采用SPSS软件(version 18)进行。
2 结果
2.1 单克隆细胞株的筛选与抗体获取
Western blot结果显示,H2H6、H1C8、H4C11、H3A8检测到纯化Vtg与E2诱导组雄鱼血浆的多条清晰条带,但与对照雄鱼血浆不发生交叉反应(见图1)。将这4株细胞扩大培养后,利用Protein G纯化获得了H2H6、H1C8、H4C11、H3A8单克隆抗体的IgG组分。
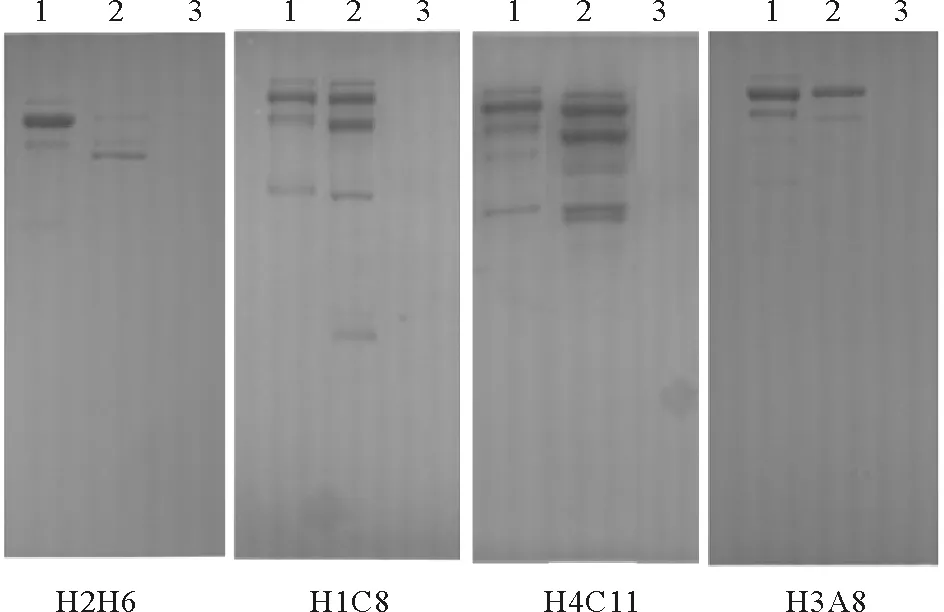
(1:纯化的Vtg;2:E2诱导组雄鱼血浆;3:对照组雄鱼血浆。1: purified Vtg; 2: E2-induced male plasma; 3: control male plasma.)
图1 Western blot测定斑马鱼Lv单克隆抗体对金鱼Vtg的特异性
Fig.1 Specificity ofanti-zebrafish Lvmonoclonal antibodies to goldfish Vtg by Western blot analysis
2.2 夹心ELISA的建立
以纯化的4种单抗分别包被酶标板,以HRP标记的金鱼Lv多抗(稀释倍数为10 000倍)为检测抗体建立了夹心ELISA。以单抗H2H6与H4C11包被时,金鱼Lv标准品的吸光值很低,且不呈线性;在利用单抗H3A8与H1C8建立的ELISA中,金鱼Lv的吸光值较高,具有较好的线性,相比之下,在基于单抗H3A8建立的夹心ELISA中Lv曲线的斜率更大(见图2)。
当单抗H3A8包被浓度为5 μg·mL-1时,不同稀释倍数HRP标记抗体获得的曲线如图3A所示。当HRP标记抗体稀释1∶10 000时,曲线具有很宽的工作范围,呈现较好的线性,并且最大吸光值在3.0左右,在该反应条件下,ELISA的工作范围为15.6~1 000 ng·mL-1(y=1.664 9x-2.106 2,R2=0.990 4),检出限为9.6 ng·mL-1(见图3B)。

图2 利用夹心ELISA筛选单克隆抗体Fig.2 Screening monoclonal antibody by sandwich ELISA

图3 HRP标记抗体稀释倍数的确定(A)与夹心ELISA的标准曲线(B)Fig. 3 Determination of the optimal dilution of HRP-labeled antibody (A) and a standard curve of sandwich ELISA (B)
利用单抗H3A8建立的夹心ELISA同时检测了纯化的金鱼Vtg与Lv,发现它们的标准曲线几乎重合(见图4)。

图4 金鱼Vtg和Lv免疫同源性检测Fig. 4 Test of immunologic similarities between goldfishVtg and Lv
利用Lv标准品测定了ELISA的精确度。其组内差异为1.30%~4.99%,组间差异为2.30%~4.37%(见表1),均低于5%。

表1 ELISA组内差异与组间差异的测定
2.3 E2暴露对金鱼血浆与体表粘液Vtg的诱导
2.3.1 E2暴露3 d Western blot结果显示,单抗H3A8在对照组、10和100 ng·L-1E2暴露3 d后的雄鱼血浆、体表粘液均未检测到Vtg条带;在1 000 ng·L-1暴露组雄鱼血浆、体表粘液均检测到两条与纯化Vtg相同的条带(见图5)。

(1:对照组;2:10 ng·L-1组;3:100 ng·L-1组;4:1000 ng·L-1组;5:纯化的金鱼Vtg。Land 1: control; land 2: 10 ng·L-1; land 3: 100 ng·L-1; land 4: 1 000 ng·L-1; land 5:purified goldfishVtg.)
图5 Western blot检测E2暴露3 d后雄鱼血浆(A)和体表粘液(B)中的Vtg.
Fig.5 Vtg inductionin plasma(A)and surface mucus(B) of male goldfish exposed to E2for 3 days detected by Western blot
对照组、10 ng L-1和100 ng L-1E2暴露3 d后的雄鱼血浆和体表粘液均未检测到Vtg,1 000 ng L-1暴露组雄鱼血浆和体表粘液中Vtg含量分别为(168.5±32.2) μg·mL-1和(9.9±1.7)μg·mL-1,显著高于对照组(P<0.01,见图6)。
2.3.2 E2暴露21 d Western blot结果显示,对照组与10 ng·L-1E2暴露21 d后的雄鱼血浆和体表粘液未检测到Vtg条带,100 ng·L-1E2暴露组雄鱼血浆检测到两条带,1 000 ng·L-1E2暴露组出现了多条清晰条带(见图7A)。100和1 000 ng·L-1E2暴露组雄鱼体表粘液检测到2条清晰条带及1条较弱条带(见图7B)。
ELISA结果显示,对照组与10 ng·L-1E2暴露组雄鱼血浆和体表粘液中未检测到Vtg,100与1 000 ng·L-1E2暴露后雄鱼血浆Vtg含量显著升高至(14.6±4.7)、(1 216.0±525.2)μg·mL-1(P<0.01,见图8A)。100与1 000 ng·L-1暴露组雄鱼体表粘液中的Vtg含量显著升高至(17.2±0.2)、(288.53±125.6)μg·mL-1(P<0.01,见图8B)。

图6 利用ELISA测定不同浓度E2暴露3 d后雄性金鱼血浆(A)和体表粘液(B)中的Vtg浓度Fig. 6 Vtgconcentrations in plasma (A) and surface mucus(B) of male goldfishexposed to different E2for 3 daysdetected by ELISA.
3 讨论
本研究利用斑马鱼Lv单克隆抗体建立了金鱼Vtg的夹心ELISA,为金鱼Vtg的检测提供了新的方法。Western blot结果显示,单抗H2H6、H1C8、H4C11、H3A8能够检测到E2诱导组雄鱼血浆与纯化Vtg的阳性条带,但是与对照组雄鱼血浆无交叉反应,表明它们对金鱼Vtg具有很高的特异性,这与斑马鱼Vtg的研究结果相近[20];并且金鱼Vtg在以上4种单抗的检测下都显示一条清晰的主带与多条弱带,这与金鱼Vtg多克隆抗体的Western blot结果相一致[21],表明斑马鱼Lv单抗能够识别金鱼Vtg的多个亚基,可以用于金鱼Vtg的检测。ELISA的结果显示单抗H3A8对金鱼Lv具有更宽的检测范围,且斜率明显高于其它抗体,表明该抗体对金鱼Lv具有更高的亲和力。以单抗H3A8为包被抗体,以纯化的金鱼Lv为标准品,以HRP标记金鱼Lv多克隆抗体为检测抗体建立了夹心ELISA,发现金鱼Vtg与Lv的标准曲线几乎完全重合,可见单抗H3A8对金鱼Vtg与Lv具有相同的结合能力;ELISA的组内与组间差异均低于5%,低于金鱼Vtg多克隆抗体建立的ELISA方法[21],表现出很高的精确度。以上结果证实基于斑马鱼Lv单克隆抗体建立的夹心ELISA能够准确定量金鱼Vtg。本研究建立的ELISA工作范围为15.6~1 000 ng·mL-1,检出限约为9.6 ng·mL-1,与鲤鱼和纹鳢(Channastriata)等鱼类Vtg ELISA的研究结果相近[22-23], 但是高于金鱼Vtg多克隆抗体建立的ELISA[21]。为进一步提高检测方法的敏感度,今后有必要开发金鱼Vtg的单克隆抗体。

(1:对照组;2:10 ng·L-1组;3:100 ng·L-1组;4:1 000 ng·L-1组;5:金鱼Vtg。Land 1: control; land 2: 10 ng·L-1; land 3: 100 ng·L-1; land 4: 1 000 ng·L-1; land 5: goldfish Vtg.)
图7 Western blot检测E2暴露21 d后雄鱼血浆(A)和体表粘液(B)中的Vtg
Fig.7 Vtg induction in plasma (A)and surface mucus (B) of male goldfish exposed to E2for 21 days detected by Western blot
有研究报道鱼类血浆与体表粘液中的Vtg含量在外源雌激素的诱导下存在正相关性[18,24],并且鱼类体表粘液取样较血浆取样方便,对鱼体没有伤害,更适合用作Vtg的检测样品[24-25]。为检验金鱼体表粘液中的Vtg是否能够有效指示外源化合物的雌激素活性,本研究利用建立的ELISA测定E2暴露后雄性金鱼血浆和体表粘液中的Vtg含量。E2的环境浓度通常在5~199.0 ng/L之间[26-27],本研究据此设计了E2的暴露浓度为10、100与1 000 ng·L-1。检测结果显示,100与1 000 ng·L-1E2暴露21 d能诱导雄性金鱼血浆产生Vtg,这与E2对斑马鱼Vtg的诱导结果相一致[28],证明基于斑马鱼Lv单抗建立的ELISA能够用于检测外源化合物对金鱼的雌激素活性。此前,有研究报道腹腔注射E2能够诱导雄性罗非鱼(Oreochromismossambicus)和金鱼体表粘液产生Vtg[21,29]。本研究在1 000 ng·L-1E2暴露3 d、100和1 000 ng·L-1E2暴露21 d后雄鱼体表粘液检测到了Vtg,表明E2水体暴露同样会诱导金鱼体表粘液生成Vtg。在1 000 ng·L-1E2暴露3 d时,雄鱼血浆中Vtg含量远高于体表粘液,约为体表粘液Vtg含量的17倍,但是100 ng·L-1E2暴露21 d后雄鱼血浆中Vtg的含量是体表粘液的1.2倍,可见在外源化合物质长期暴露下,体表粘液与血浆中的Vtg含量更为接近,可以有效指示外源化合物的雌激素活性。

图8 利用ELISA测定不同浓度E2暴露21 d后雄鱼血浆(A)和体表粘液(B)的Vtg浓度Fig.8 Vtg concentrations in plasma (A) and surface mucus (B) of male goldfish exposed to different E2 for 21 days detected by ELISA
综上,本研究首次建立了金鱼Vtg的单抗夹心ELISA,并且发现金鱼体表粘液Vtg可以用作环境雌激素的无伤害检测指标,为金鱼Vtg指标在环境雌激素研究中应用提供了重要的方法学参考。
[1] Ishibashi H, Tachibana K, Tsuchimoto M, et al. In vivo testing system for determining the estrogenic activity of endocrine-disrupting chemicals (EDCs) in goldfish (Carassiusauratus)[J]. Journal of Health Science, 2001, 47(2): 213-218.
[2] Deguchi Y, Wu N X, Toyoizumi T, et al. Application of a new bioassay technique using goldfish for assessment of water toxicity[J]. Environmental Toxicology, 2008, 23(6): 720-727.
[3] Kordes C, Rieber E P, Gutzeit H O. An in vitro vitellogenin bioassay for oestrogenic substances in the medaka (Oryziaslatipes)[J]. Aquatic Toxicology, 2002, 58(3): 151-164.
[4] Luo W, Zhou Q, Jiang G. Development of enzyme-linked immunosorbent assays for plasma vitellogenin in Chinese rare minnow(Gobiocyprisrarus)[J]. Chemosphere, 2011, 84(5): 681-688.
[5] Marx A, Sherry J, Hansen P D, et al. A new monoclonal antibody against vitellogenin from rainbow trout (Oncorhynchusmykiss)[J]. Chemosphere, 2001, 44(3): 393-399.
[6] An L, Hu J, Zhu X, et al. Crucian carp (Carassiuscarassius) VTG monoclonal antibody development and application[J]. Ecotoxicology and Environmental Safety, 2007, 66(2): 148-153.
[7] Yan Z, Lu G, Liu J, et al. An integrated assessment of estrogenic contamination and feminization risk in fish in Taihu Lake, China[J]. Ecotoxicology and environmental safety, 2012, 84: 334-340.
[8] Wang J, Wang W, Zhang X, et al, Development of a lipovitellin-based goldfish (Carassiusauratus) vitellogenin ELISA for detection of environmental estrogens[J]. Chemosphere, 2015, 132: 166-171.
[9] 邴欣.金鱼卵黄原蛋白多克隆抗血清制备及在内分泌扰乱化学物质检测中的应用[D].青岛: 中国海洋大学, 2006: 31-42.
Bingxin.Preparation of Polyclonal Antiserum Against Goldfish Vitellogenin and Its Application in Studies of Endocrine Disrupting Chemicals. [D]. Qingdao: Ocean University of China, 2006: 31-42.
[10] Mylchreest E, Snajdr S, Korte J J, et al. Comparison of ELISAs for detecting vitellogenin in the fathead minnow (Pimephalespromelas)[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2003, 134(2): 251-257.
[11] Wang J. Wang W, Tian H, et al.A novel enzyme-linked immunosorbent assay based on anti-lipovitellin monoclonal antibodies for quantification of zebrafish (Daniorerio) vitellogenin [J]. Ecotoxicology and Environmental Safety, 2017, 136: 78-83.
[12] Ishibashi H, Tachibana K, Tsuchimoto M, et al. In vivo testing system for determining the estrogenic activity of endocrine-disrupting chemicals (EDCs) in goldfish (Carassiusauratus)[J]. Journal of Health Science, 2001, 47(2): 213-218.
[13] Brodeur J C, Woodburn K B, Zhang F, et al. Plasma sampling and freezing procedures influence vitellogenin measurements by enzyme-linked immunoassay in the fathead minnow (Pimephalespromelas) [J]. Environmental Toxicology and Chemistry, 2006, 25(2): 337-348.
[14] Moncaut N, Nostro F L, Maggese C. Vitellogenin detection in surface mucus of the South American cichlid fishCichlasomadimerus(Heckel, 1840) induced by estradiol-17β. Effects on liver and gonads[J]. Aquatic Toxicology, 2003, 63(2): 127-137.
[15] Wang J, Bing X, Yu K, et al. Preparation of a polyclonalantibody against goldfish (Carassiusauratus) vitellogenin and its application todetect the estrogenic effects of monocrotophos pesticide [J]. Ecotoxicol Environ Safe, 2015, 111: 109-116.
[16] Mitsui N, Tooi O, Kawahara A. Sandwich ELISAs for quantification ofXenopuslaevisvitellogenin and albumin and their application to measurement of estradiol-17βeffects on whole animals and primary-cultured hepatocytes [J]. Comp Biochem Physiol C, 2003, 135(3): 305-313.
[17] Nilsen B M, Karin B, Eidem J K, et al. Development of quantitative vitellogenin-ELISAs for fish test species used in endocrine disruptor screening[J]. Analytical & Bioanalytical Chemistry, 2004, 378(3): 621-633.
[18] Maltais D, Roy R L, Couillard C M. Hybrid ELISAs for vitellogenins of the endangered copper redhorseMoxostomahubbsiand the shorthead redhorseMoxostomamacrolepidotum(Cypriniformes, catostomidae)[J]. Ecotoxicol Environ Safe, 2010, 73(5): 883-892.
[19] Maltais D, Roy R L.A lateral flow immunoassay for rapid evaluation of vitellogenin levels in plasma and surface mucus of the copperred horse(Moxostomahubbsi)[J]. Environ Toxicol Chem, 2007, 26, 1672-1676.
[20] Wang J, Zhang X N, Shan R H, et al. Lipovitellin asan antigen to improve the precision of sandwich ELISA for quantifying zebrafish(Daniorerio) vitellogenin [J]. Comp Biochem Physiol, 2016, 185C: 87-93.
[21] Wang J, Shan R H, Zhang X N, et al.Development of a lipovitellin-based sandwich ELISA for quantificationof vitellogenin in surface mucus and plasma of goldfish (Carassiusauratus)[J]. Ecotoxicology and Environmental Safety, 2015, 120: 80-87.
[22] Hennies M, Wiesmann M, Allner B, et al. Vitellogenin in carp (Cyprinuscarpio) and perch (Percafluviatilis): Purification, characterization and development of an ELISA for the detection of estrogenic effects[J]. Science of the Total Environment, 2003, 309(1): 93-103.
[23] PrakashO, Goswami SV, et al. Establishment of ELISA for murrelvitellogenin and choriogenin, as biomarkers of potential endocrine disruption[J]. Comparative Biochemistry and Physiology, Part C, 2007, 146: 540-551.
[24] Meucci V, Arukwe A. Detection of vitellogenin and zona radiata protein expressions in surface mucus of immature juvenile Atlantic salmon (Salmosalar) exposed to waterborne nonylphenol[J]. Aquatic Toxicology, 2005, 73(1): 1-10.
[25] Maltais D, Roy R L. Purification and partial characterization of vitellogenin from shorthead redhorse (Moxostomamacrolepidotum) and copper redhorse (Moxostomahubbsi) and detection in plasma and mucus with a heterologous antibody[J]. Fish PhysiolBiochem, 2009, 35(2): 241-254.
[26] Manickum T, John W, Terry S. Determination of selected steroid estrogens in treated sewage effluent in the Umsunduzi (Duzi) River water catchment area[J]. Lecture Notes in Statistics, 2011, 2(3): 139-195.
[27] ManickumT, John W. Occurrence, fate and environmental risk assessment of endocrine disrupting compounds at the wastewater treatment works in Pietermaritzburg (South Africa)[J]. Science of the Total Environment, 2014, s 468-469(1): 584-597.
[28] Wang J, Zhao F, Shan R H, et al. Juvenile zebrafish in the vitellogenin blank period as an alternative test organism for evaluation of estrogenic activity of chemicals[J]. Environ Toxicol Chem, 2016, (35): 1783-1787.
[29] Kishida M, Jennifer L.Vitellogenin in the surface mucus of tilapia(Oreochromismossambicus): Possibility for uptakeby the free-swimming embryos[J]. Experimental Zoology, 1994, 268: 259-268.

