植物抗灰霉病菌分子机制的研究进展
张燕 夏更寿 赖志兵
(1. 丽水学院生态学院,丽水 323000;2. 华中农业大学作物遗传改良国家重点实验室,武汉 430070)
灰霉病菌(B. cinerea)是典型的死体营养型真菌,杀死植物细胞后获取养分,引起植物组织腐烂,又因发病植物组织表面后期形成灰色霉层,而被称为灰霉病菌[1]。B. cinerea能在500多种植物中致病,其中作物超过200种,包括番茄、草莓、葡萄等众多重要经济作物[2]。该菌喜温湿,不仅危害植株上的茎、叶和果实,同样危害采后贮藏的果实,严重影响产量和品质,全球每年因灰霉病造成的经济损失达100-1 000亿美元,被列为十大植物真菌病害之一[3]。灰霉病的有效防治主要依赖于化学药剂,但存在农药毒性、残留、环境污染等问题。此外,化学药剂的长期使用,导致田间抗药性灰霉病菌的出现[4],而且,德国田间鉴定到一种新的引起草莓灰霉病的种——B. fragariae,对杀菌剂也表现出高频抗药性[5]。因此,一直以来,关于植物自身对灰霉抗病性的研究相当重视,以期找到替代化学药剂的其它物质,或者利用植物自身基因提高对灰霉病的抗性。随着研究的深入,对灰霉病菌隐秘不易察觉的毒性策略和植物复杂的细胞及分子抗病反应机制都有一定程度的认识。
植物对抗B. cinerea等寄主范围广的死体营养型 真 菌(Broad host-range necrotrophs,BHNs), 通常采用多层面的抗病反应,应对病原菌纷繁复杂的毒性策略,且在遗传学组成上呈现出多基因控制的数量性状的特征。迄今为止的研究表明P/DAMP(Pathogen/damage-associated molecular patterns,P/DAMP)激发的免疫反应PTI(P/DAMP triggered immunity,PTI)是植物抗灰霉病菌机制的主要组成部分[6-7]。近几年,在植物对B. cinerea的抗性反应的研究不仅加深对PTI的认识,还找到一系列可供开发利用的防治灰霉病菌的因素和方法,包括植物生长环境条件、微生物自身或来自微生物的分子、颇有时代烙印的代谢物如青蒿素及白藜芦醇、寄主诱导的基因沉默防治方法等。本文从识别PTI信号、信号传导、转录相关因子和表观遗传事件、抗灰霉细胞学事件及次生代谢产物等方面的研究进展作一综述,特别指出可在防治实践中利用的潜在因素和方法,以期解决单一使用化学农药导致的抗药性、环境污染等问题。
1 PAMP/DAMP信号及识别
1.1 激发PTI的信号分子
PTI是植物抗灰霉的主要机制,在识别信号分子PAMP/DAMP后激发的数量抗性反应,是包括 激 活 MAPKs(Mitogen-activated protein kinases,MAPKs)途径、camalexin的合成及乙烯信号途径等系列反应的叠加。信号分子PAMP指的是来自病原微生物且进化缓慢的特征性模式分子,而DAMP则是指由病原微生物降解寄主细胞成分的产物。真菌和植物细胞壁的降解产物,分别成为能激发PTI的PAMP和DAMP分子。
1.1.1 真菌细胞壁成分几丁质 几丁质是灰霉病菌等真菌细胞壁的成分,由多个N-乙酰-D-葡萄糖胺组成。植物几丁质酶作用于真菌细胞壁,生成不同长度的几丁质分子,6个及以上的N-乙酰-D-葡萄糖胺组成的几丁质分子都能激发有效的PTI[8]。近期的研究发现,过表达水稻几丁质酶基因RCH10能提高百合对灰霉的抗性,而不影响百合植株的开花和发育[9]。芥菜几丁质酶BjCHI1也能提高植物抗灰霉的能力,BjCHI1基因的表达受转录因子BjMYB1的调控,且找到芥菜的类受体激酶的部分编码序列,含LysM基序,意味着芥菜中很可能存在几丁质受体蛋白[10-11]。此外,表达桑葚的几丁质结合蛋白MLX56的拟南芥显示出更高的灰霉抗性[12]。灰霉病菌在入侵植物细胞时,被植物几丁质酶降解自身细胞壁,同时也分泌CWDEs(Cell walldegrading enzymes,CWDEs),降解植物细胞壁,生成DAMPs。
1.1.2 植物细胞壁、表皮组分降解产物 果胶酶(Pectinase)是主要的CWDEs,B. cinerea基因组有一个内源多聚半乳糖醛酸酶(PG)的基因家族,该家族的6个成员基因编码基础的或酸性的PG异构酶[13],作用于植物细胞壁的果胶,生成DAMP分子寡聚半乳糖醛酸(OGs),诱导拟南芥的免疫反应,提高对灰霉病菌的抗性,OGs激发产生瞬时和长期的一氧化氮(NO),NO调节OGs激发的活性氧的爆发和防卫基因的表达,并且参与对灰霉的基本防卫反应[14-15]。细胞壁成分的修饰则降低果胶甲基酯化和阿魏酸化,果胶甲基化通过影响多聚半乳糖醛酸的水解及水解后OGs的长度,提高拟南芥对灰霉的敏感性[16-17]。
除了细胞壁,植物表皮也是DAMP的来源。灰霉病菌泌出角质酶,作用于植物表皮,生成另一种DAMP分子表皮素单体,诱导典型的PTI反应,包括碱性化、合成乙烯、ROS爆发和防卫基因的上调表达[13,18-19]。与灰霉病菌角质酶活性产物相类似,植物表皮发育、脂质的组成及表皮素多聚体等发生异常状况,会被植物自身更快识别,而且防卫信号更易扩散或活性氧更快爆发而提高对灰霉病菌的抗性[20-22]。编码 AP2/ERF 转录因子的 DEWAX(Decrease Wax Biosynthesis)基因,偏好在植物表皮表达且受黑暗诱导,负向调控表皮蜡质的合成;DEWAX基因过表达提高拟南芥表皮透性和ROS积累,且与PDF1.2基因的启动子区直接结合而促进其表达,增强拟南芥和亚麻对灰霉病菌的抗性[23-24]。
1.1.3 植物细胞内蛋白类DAMPs 植物受灰霉病菌侵染后,植物体内一些蛋白前体经过剪切产生能激活抗病反应的小肽,这些小肽被称之为蛋白类DAMPs,如番茄产生的系统素(Systemin)和拟南芥产生的 PEPs(Peptides)。
系统素仅存在于茄科植物中,其前体蛋白prosystemin(PS)含有200个氨基酸,当有病原菌入侵或者外界环境刺激条件下,PS蛋白被蛋白酶剪切形成18个氨基酸的系统素被分泌到细胞外,植物细胞受体蛋白识别PS蛋白并激活茉莉酸(JA)响应基因和其他抗病基因的表达,增强植物对灰霉病菌的抗病性[25]。虽然PS蛋白不能被系统运输,但该蛋白的mRNA是一个可移动的信号分子,能在植物体内长距离运输,因此,远离入侵点的叶片对灰霉病菌的抗病性也增强[25]。至于PS蛋白剪切形成系统素、系统素被分泌到胞外及系统素的受体蛋白均有待于进一步分析鉴定。
PEP1蛋白的前体ProPEP1含有92个氨基酸,受病原菌、MeJA和损伤等的诱导后表达。ProPEP1被剪切而形成23个氨基酸的PEP1蛋白,细胞膜上的受体蛋白激酶PEPR1和PEPR2识别泌出胞外的PEP1并被激活而磷酸化BIK1,从而激活下游乙烯(ET)信号途径,增强植物对灰霉病菌和其他病菌的抗病性[26]。拟南芥中存在5个PEP1的同源蛋白PEP2-PEP6,PEP2被PEPR1和 PEPR2识别,PEP3-6被PEPR1识别,提高植物的灰霉抗性[27-29]。
1.2 PTI信号的识别
不论是PAMP还是DAMP分子,都是特征性的模式分子(Pattern),长期进化过程中,植物拥有了一系列的模式识别受体(Pattern recognition receptors,PRRs),特异性地识别模式分子,拉响危险警报,导致免疫反应的发生。灰霉病菌侵染过程中形成的PAMP/DAMP分子几丁质、OGs、表皮单体及PEP1,分别被CERK1(Chitin elicitor receptor kinase 1,CERK1)、WAK1(Wall- associated kinase 1,WAK1)、EIX1(ET-inducing xylanase 1,EIX1)和 PEPR1/PEPR2(PEP1 receptor 1/2,PEPR1/2)识别[6,30],这些PRRs都是定位在细胞膜的类受体激酶RLKs(Receptor-like kinases)。值得一提的是,BAK1(Brassinosteroid insensitive 1-associated kinase 1,BAK1)也是一种RLK,在不同的PRR特异性识别相应的Pattern过程中,起共同受体的作用,缺失BAK1导致对灰霉病菌的敏感性增强[31-32]。拟南芥PEP1和PEP2被PEPR1识别过程中,PEP1和PEP2诱导BAK1磷酸化,而PEPR1磷酸化激酶BIK1(Botrytis induced kinase 1,BIK1), 由 此 传 导 PTI信号[26,33]。
2 灰霉病菌信号识别后的传导
2.1 BIK1在PTI信号传导中的作用
拟南芥接种灰霉病菌早期BIK1被诱导,编码一个类受体胞内激酶(Receptor-like cytoplasmic kinase,RLCK),在抗灰霉病菌反应中是必需的[34]。BIK1的磷酸化受到来自病原菌的乙烯分子的调控,且乙烯信号途径重要的调节因子EIN3也直接调控BIK1,而BIK1是表达乙烯反应基因所必需的[34-35]。此外,BIK1压制水杨酸(SA)和油菜素内酯(BR)信号途径,但是BIK1如何调控SA信号途径以及如何在ET信号途径中起作用仍不清楚。BIK1和BAK1一样,与多个PRRs互作,调节植物对灰霉病菌的抗性,因此,BIK1独立于MAPKs途径,把多个PRRs和下游的免疫反应联系起来[26,36-37]。
2.2 MAPKs传递PTI及其它灰霉病菌的信号至转录因子
MAPKs途径通过MPKKK-MPKK-MPK链式反应放大植物接收到的PTI及其它灰霉病菌的识别信号,并将信号传递给转录因子,诱导抗病相关基因表达[38]。其中,MPK3和MPK6是MAPKs途径中调控拟南芥对灰霉病菌抗性的主要成分,MPK3或MPK6基因突变显著抑制OGs诱导的抗病性,但突变体mpk3抗灰霉能力减弱,而mpk6接种灰霉后表型与野生型相差不大,故对灰霉菌的基础抗性主要由MPK3介导,而对DAMPs诱导产生的PTI途径则需要MPK3和MPK6共同参与[38-39]。编码核孔复合体的Nup88/MOS7基因突变减少MPK3在核内的积累,显著降低植物对灰霉病菌的抗病性[39]。ERF6和WRKY33是抗灰霉病菌PTI反应的重要转录因子,MPK3/MPK6直接磷酸化ERF6和WRKY33,增强两个转录因子的稳定性,传递PTI信号的同时,使得下游抗病基因表达更持久[40]。
3 抗灰霉病菌转录再编程
不同PAMP/DAMP信号及其它灰霉病菌的识别信号,传递给转录因子后,植物通过转录再编程调控抗病相关基因表达,发生下游的免疫反应。转录再编程主要通过转录因子、转录媒介体和表观修饰3个方面进行调控。
3.1 抗灰霉病菌的转录因子
在植物抗灰霉病菌的过程中WRKY类转录因子发挥重要的调控作用。WRKY57直接靶向JAZ1和JAZ5的启动子促进转录,WRKY70和WRKY54负向调控细胞壁相关的防卫反应,从而抑制JA信号途径,增强拟南芥对灰霉的感病性[41]。与上述3个WRKY因子的作用相反,拟南芥的WRKY3、WRKY4和WRKY33正向调控灰霉抗性[42-43]。
WRKY33在植物抗灰霉病PTI反应中起关键作用,拟南芥wrky33突变体对灰霉病菌表现极感表型,过量表达WRKY33明显提高植物的抗病性;WRKY33的在PTI反应中的作用主要是促进ET合成(靶标基因ACS2和ACS6)和调控ET信号途径(起作用的蛋白GDSL lipase1,GLIP1)、调控JA信号途径(靶标基因ORA59、JAZ1、JAZ5)、以及抑制SA、脱落酸(ABA)的合成和SA信号途径;接种灰霉病菌后,WRKY33结合到NCED3和NCED5基因(ABA合成的关键基因)的启动子区域抑制它们的转录,wrky33突变体中SA合成酶基因ICS1表达量和SA水平显著高于野生型植株,SA信号途径的相关基因NPR1、EDS1、PAD4、PR1和PR2的表达量也明显高于野生型植株[42,44-45]。此外,WRKY33促进植保素camalexin的合成,camalexin合成关键因子PAD3是WRKY33的直接靶基因,受WRKY33蛋白正向调控[42]。拟南芥HOOKLESS(组蛋白乙酰化酶,HLS1)乙酰化WRKY33组蛋白H3,并且招募MED18,加强WRKY33的表达,提高灰霉抗性[46]。不仅仅拟南芥的WRKY33,番茄和烟草中的WRKY33同样在抗灰霉病菌PTI中起关键性的作用。番 茄 中 的 SlDRW1(Solanum lycopersicum defenserelated WRKY1,SlDRW1) 与 拟 南 芥 WRKY33序列同源性达到50.5%,VIGS沉默SlDRW1基因明显减弱植物对灰霉的抗性[47]。此外,烟草的MAPKWRKY途径也增强对灰霉病菌的抗性[48]。植物抗灰霉病菌PTI反应是MAPKs途径激活、camalexin的合成和乙烯的交叠,AP2/ERF类和MYB转录因子也是与乙烯或camalexin合成等密切相关的抗灰霉PTI重要的转录因子。
AP2/ERF类转录因子受乙烯调控,含有57-66个氨基酸组成的DNA结合域,结合启动子的顺式作用元件GCC-box(AGCCGCC),调控基因表达。拟南芥5个ERF基因 ERF1、RAP1.2、ORA59、ERF5和ERF6都受灰霉菌的诱导表达,同时也受ET和JA诱导表达,通过ET/JA信号途径增强植物对灰霉的抗性[40,49-50]。拟南芥 AtERF014负向调节灰霉抗性[51];番茄的 SlERF.A1、SlERF.B4、SlERF.C3和SlERF.A3、青蒿的AaERF1和AaORA均正向调控植物对灰霉病菌的抗性[52-53]。
MYB类转录因子也通过调节DAMP信号或乙烯相关转录因子等途径参与调控植物对灰霉病菌的PTI免疫反应。Atmyb46突变体植株导致植物次生细胞壁缺陷,芥菜转录因子BjMYB1激活几丁质酶BjCHI1的表达,无疑MYB转录因子AtMYB46和BjMYB1都参与调控DAMP-PTI;而MTF1(又名:MYBC)突变,则乙烯调控的转录因子基因ORA59表达量显著高于野生型,且突变植株高抗灰霉病菌[54-55]。MYB51则通过转录激活吲哚葡糖异硫氰酸盐的合成而影响抗灰霉病菌PTI组成camalexin的合成[56]。
除WRKY、ERF和MYB三大类重要的转录因子外,还有其他转录因子在植物与灰霉互作中起作用。如拟南芥的GBF1(G-BOX BINDING FACTOR1)负向调节病原菌诱导的CATALASE 2(CAT2)基因表达,正向调控PHYTOALEXIN DEFICIENT 4(PAD4)的表达,从而增加拟南芥对灰霉的感病性[57]。
3.2 转录媒介体和转录延伸复合物
媒介体是真核生物进化上保守的多个蛋白亚基组成的复合体,也是基因转录调控复合体的重要组成部分[58]。媒介体亚基通过与转录因子互作、改变RNA聚合酶II与DNA的结合能力或调节表观修饰而调控抗灰霉病菌的免疫反应。受MED25调控的转录因子包括ABI5、ERF1、ERF15、RAP2.2、ERF98、ORA59、EIN3/EIL1和 MYC2,因此,MED25作为抗灰霉病菌相关的ET和JA信号平衡点起作用[59-63]。不同于 MED25,MED18除了激活抗病基因PTR3的表达外,还与抗病转录因子YY1互作直接抑制感病基因TRX-h5、GRXS13和GRX480的转录从而增强植物抗病性;另一方面,MED18增强RNA聚合酶II与靶基因的启动子、编码区和终止子区的结合,介导组蛋白H3K36me3修饰水平,达到增强抗病基因表达的目的[64]。CDK8是媒介体磷酸激酶区的蛋白亚基,与MED25互作正向调控依赖于ERF1的ET信号途径或者依赖于ORA59的JA信号途径,CDK8增强AACT(Agmatine coumaroyltransferase,AACT)基因的表达,促进抗性次生代谢产物HCAA(Hydroxycinnamic acid amides,HCAA)合成积累,在拟南芥抗灰霉病菌过程中起作用[65]。此外,MED33也正向调控拟南芥对灰霉病菌的抗性[66]。
转录过程中的延伸复合体(Elongator)是与RNA聚合酶II互作的复合物,也参与拟南芥抗灰霉病菌的过程。延伸复合物亚基 2(Elongator Protein 2,ELP2)是诱导表达WRKY33、ORA59及PDF1.2所必需的,正向调控拟南芥对灰霉的抗性;DRL1(Deformed root and leaves 1,DRL1)与延伸复合物互作,同样是诱导表达ORA59和PDF1.2基因所必需的,增强拟南芥对灰霉病菌的抗性[67-68]。
3.3 表观修饰调控植物灰霉抗性
表观修饰包括DNA甲基化、组蛋白乙酰化、组蛋白赖氨酸甲基化和组蛋白泛素化等。甲基转移酶SDG(SET domain group,SDG)甲基化组蛋白H3K4和H3K36,已经阐明SDG8提高MKK5的组蛋白H3K36me3修饰水平,促进JA信号途径,且SDG8和SDG25共同调控抗病基因CER2(ECERIFERUM 2)和CER3(ECERIFERUM 3)的组蛋白H3K4和H3K36的甲基化水平,从而增强植物对灰霉病菌的抗性[69-70]。甲基化修饰调节植物对灰霉病菌抗性的另一个途径是,RNA介导的DNA甲基化途径(RNA-dependent DNA methylation,RdDM),该途径由小的干扰RNA(small interfering RNAs,siRNAs)驱动,直接介导DNA甲基化修饰,同时影响目标区段的组蛋白修饰水平,正向调控植物对灰霉病菌的抗性。RdDM途径中关键基因的突变体nrpd1、nrpd2、nrpe1、ago4、drd1和rdr2均感灰霉,且nrpd2突变体中受灰霉病菌诱导的PDF1.2基因表达水平受抑制,而PR1基因表达提高,由此推测,RdDM途径可能正向调控JA途径或负向调控SA途径,增强植物对灰霉病菌的抗性[71]。尽管RdDM途径对组蛋白的修饰机制影响植物对灰霉病菌抗性尚不明确,已有研究证实了组蛋白的乙酰化和泛素化对植物灰霉抗病性的影响。
HLS1(Hookless1,HLS1)直接介导拟南芥基因组组蛋白乙酰化水平,HLS1直接靶向抗病基因WRKY33的转录起始和编码区,增强组蛋白H3乙酰化水平;同时HLS1招募MED18亚基,共同促进WRKY33基因转录;hls1突变体受灰霉病菌诱导后,PDF1.2基因转录水平远远高于野生型植株,但hls1突变体高感灰霉病菌[46]。与HLS1类似,转录延伸复合体也能介导抗病基因WRKY33、ORA59和PDF1.2基因的组蛋白H3K9乙酰化水平促进基因表达,提高植物灰霉抗性[67]。组蛋白的泛素化与甲基化和乙酰化效果类似,同样影响植物对灰霉病菌的抗性。介导组蛋白H2B单泛素化酶基因HUB1(Histone monoubiquitination1,HUB1)或HUB2突变,显著降低拟南芥对灰霉病菌的抗性[72]。同样,番茄的组蛋白单泛素化酶SLHUB1和SLHUB2通过调节SA和JA/ET信号通路的平衡增强番茄对灰霉病菌的抗性[73]。
4 植物激素的调控
植物激素是完整的植物免疫不可或缺的成分,灰霉病菌的侵染提高了植物体内SA、ABA、ET、JA的水平,激素的内在平衡和信号途径的反应是正常的植物免疫的关键。SA对植物灰霉抗性的影响和植物品种有关,SA信号途径中的成分PR1在抗灰霉病菌的系统获得抗性(SAR)和诱导系统抗性(ISR)中起重要作用,是SAR的分子标记,SAR对拟南芥抗灰霉没有作用,但能提高番茄和烟草对灰霉病菌的抗性。SA途径被灰霉利用而增强致病性,如灰霉泌出胞外的多糖(EP)激活SA途径,与JA信号途径对抗,增强感病性[74]。ABA对植物抗灰霉病菌的影响和灰霉病菌侵染的阶段、受侵染的组织有关,缺少ABA的sitinen番茄突变体和ABA响应因子ABI5的突变体植株均高抗灰霉病菌[75];而番茄ABA诱导的转录因子AIM1控制对灰霉病菌的基本防卫,正向调节早期对灰霉的防卫反应[76]。由此可见,SA和ABA在植物对灰霉病菌的抗性中作用复杂。
与SA和ABA不一致,JA和ET则能提高植物抗灰霉能力。ET通过调控PAMP受体复合物成分、不同的转录因子、防卫基因的表达、MAPKs和BIK1调节灰霉病生长和病症。最近的研究结果表明,VIGS沉默番茄乙烯响应因子B3亚组成员基因SlERF.A1、SlERF.A3、SlERF.B4和SlERF.C3均显著降低植物对灰霉病菌的抗性[77]。JA调控下游抗病基因表达的分子机理也取得最新的进展,灰霉病菌入侵诱导植物体内JA的大量积累,光敏色素phyB也通过激活JA途径增强对灰霉病菌的抗性。活性态的JA-Ile与受体蛋白COI1结合,促进JA信号抑制子JAZ(Jasmonate ZIM-domain)的降解而激活转录因子基因ORA59、MYC2等的表达[78]。过量表达茉莉酸诱导的氧化酶基因JOXs(Jasmonate-induced oxygenases,JOXs)或者茉莉酸氧化酶基因JAO2(Jasmonic acid oxidase 2,JAO2),导致植物体内活性态的JA-Ile转化为非活性态的12OH-JA,显著抑制植物对灰霉病菌的抗性[79-80]。拟南芥JA信号途径中的转录因子ORA59调控抗病相关基因,而MYC2则调控损伤响应基因,损伤响应基因通常促进植物对灰霉病的感病性,因此,拟南芥中JA与ET信号途径互作,抑制MYC2促进ORA59基因表达,从而增强抗灰霉病基因表达;番茄中MYC2与JA2-like(MYC2-targeted TFs,MTFs)组成转录复合体调控损伤响应基因的表达,与MTF ERF.C3(ethylene response factor.C3,ERF.C3)互作调控抗灰霉病相关基因的表达[81]。
5 抗灰霉相关细胞学事件及次生代谢产物
5.1 细胞死亡、自噬和活性氧爆发
植物抗病过程中最具代表性的细胞死亡是过敏性反应(HR),目的在于限制病原菌的生长,但恰恰有利于死体营养型真菌灰霉的侵染。细胞自噬是降解和再循环胞质内成分的细胞过程,也是植物抗灰霉病菌必不可少的一个环节,自噬途径将失去功能的蛋白消解,维持植物正常的生理生化过程,增强植物对灰霉病菌的抗性,自噬途径的关键基因ATG5、ATG7、ATG18a等突变都导致植物高感灰霉病菌,WRKY33 与 ATG18a互作,增强灰霉抗病性[82]。过量表达BAG6(Bcl-2 associated athanogene6,BAG6)基因激活自噬体的形成,能有效增强植物对灰霉病菌的抗性[83-84]。
ROS在植物免疫中作为信号分子诱导抗性、加速细胞死亡或者直接抗菌。抗或感灰霉植株接种后均大量积累ROS,因此,ROS在抗灰霉病过程中作用复杂。ROS发生的时间不同、积累的水平高低都影响着植物对灰霉的敏感性和灰霉入侵信号的感知等方面[7]。近期的研究发现,不同细胞器产生的ROS对抵抗灰霉病菌的入侵作用差异很大,NADPH氧化酶Rbohs是植物中产生ROS的关键酶,位于番茄细胞膜上的SlRbohB正向调控植物对灰霉病菌的抗性;烟草中过量表达SlRbohB基因显著增强灰霉抗性[85]。拟南芥过氧化物酶产生的非原生质体ROS损害了表皮完整性,导致DAMP激发的防卫反应[86]。而在线粒体和叶绿体产生的ROS能促进灰霉病斑拓展和植物感病性,突变编码神经酰胺激酶的ACD5(ACCELLARATED CELL DEATH 5)基因导致线粒体产生大量的ROS,并增强植物对灰霉的感病性[87];烟草叶绿体中定点表达蓝细菌黄素氧化还原蛋白(cyanobacterial flavodoxin)抑制叶绿体产生的ROS,能显著限制灰霉病病斑的拓展和病菌生物量的累积[88]。
5.2 抗灰霉的次生代谢产物
植物与灰霉病菌互作进化过程中,为对抗灰霉
病菌名目繁多的侵入方式,除了上述多种防卫反应之外,还会泌出毒性次生代谢产物抑制灰霉病菌的生长,另一些细胞内的次生代谢产物则参与不同的抗病途径,增强或减弱植物对灰霉的抗性。
5.2.1 抑制灰霉生长的次生代谢产物 众所周知,camalexin(3-thiazol-2-yl-indole)在植物抗灰霉病菌中发挥非常重要的作用,camalexin合成途径中关键 酶CYP79B2、CYP79B3、CYP71A13、CYP71B15基因突变都显著减弱植物对灰霉病菌的抗性。Camalexin合成受灰霉病菌诱导激活,合成途径中多个关键酶的基因转录水平受WRKY33、MPK3和MPK6的调控[89]。合成产生的camalexin由位于植物表皮细胞膜上的转运蛋白AtABCG34运到胞外抑制灰霉病菌的生长[90]。
葡糖异硫氰酸盐(Glucosinolates,GSs)是植物体内重要的次生代谢产物,包括吲哚葡糖异硫氰酸盐(Indole glucosinolates,IGSs)和脂肪酸葡糖异硫氰 酸 盐(Aliphatic glucosinolates,AGSs)[91]。IGSs合成关键酶包括CYP79B2、CYP79B3、CYP83B1、SUR2、UGT74B1、ST5a、CYP81F2、CYP81F3、IGMT1、TGMT2,芥子苷酶水解GSs生成异硫氰酸盐等生物活性物质,对微生物、线虫和昆虫有毒性[92]。近期的研究发现IGSs类化合物能有效拮抗灰霉病菌,油菜中过量表达IGSs合成途径基因BnUGT74B1,有效提高叶片中IGSs含量,增强对灰霉的抗性[93]。整个IGS生物合成途径都受MPK3、MPK6和ERF6的调控,CYP81F2、IGMT1和IGMT2基因受MPK3/MPK6磷酸化激活的ERF6正向调控;另一方面,ERF6通过其他未知转录因子间接调控MYB51和MYB122基因表达,而转录因子MYB51和MYB122再调节CYP83B1、CYP79B2和CYP79B3基因的表达;产生的IGSs被PEN2(Penetration2)等芥子苷酶(myrosinases)水解释放出活性态的不稳定化合物,由转运蛋白PEN3(Penetration3)分泌到胞外抑制灰霉病菌的生长[94]。
5.2.2 其它参与灰霉抗病反应的次生代谢产物 与上述直接抑菌的次生代谢产物不同,近期的研究揭示一些次生代谢产物通过参与不同的抗病反应途径而影响植物抗灰霉。拟南芥脯氨酸脱氢酶ProDH1和ProDH2受SA或JA的正向调控,提高植物对灰霉的抗性,维生素B6和蔗糖运输蛋白(STP13)有助于对灰霉和其它菌的抗性[95-97];拟南芥的基质金属蛋白酶At2-MMP参与PAMP激发的免疫提高灰霉抗性,相应地,番茄的基质金属蛋白酶Sl3-MMP提高ROS水平和防卫基因的表达,增强对灰霉抗性[98-99];番茄全代谢组分析鉴定到1-甲基色氨酸涉及植物对灰霉抗病性,海藻糖-6-磷酸合成酶在对灰霉抗性中起重要作用[100-101];烟草叶片表达甜菜红碱显著提高对灰霉的抗性[102];葡萄的奇异果甜蛋白TLP29可能涉及SA或JA/ET途径负向调控拟南芥对灰霉抗性[103];此外,拟南芥中表达葡萄白藜芦醇关键合成酶VaSTS19提高对灰霉抗性,但表达VaSTS21提高拟南芥对灰霉感病性[104-105]。由此可见,植物在感受到灰霉病菌侵染时,会通过代谢过程协调生长与抗病免疫之间的关系,并且多种次生代谢产物参与抗灰霉病菌的过程。
6 可供开发利用的抗灰霉病菌信号因子
鉴于目前对灰霉病的防治仍主要依赖于化学药剂的使用,又因环境、抗药性等问题,促使人们寻找更多的提高植物抗灰霉病反应的因素,除上述PAMP/DAMP信号外,外界生长条件、其它微生物及其产物、外界物理刺激、大分子化合物等,诱导系统抗性(ISR)、系统获得抗性(SAR)等不同的抗灰霉病菌的反应,可以为开发生物农药或其它防治方法奠定理论基础。
6.1 激发抗灰霉病反应的生长条件
光是植物免疫重要的调节因子,当细胞色素B(phyB)因红光/远红光(R∶FR)的比值低而失活时,拟南芥对灰霉病菌的抗性下降,这是由于phyB失活,植株对JA敏感性降低,从而抑制了对灰霉病菌的抗性,且该抑制作用是与SA无关,依赖于Coronatine Insensitive1(COI1)和JAZ10(压制JA信号的家族);R∶FR低比值减少了拟南芥吲哚芥子油苷和植保素camalexin的合成[106-107]。而mono-heme细胞色素b,位于拟南芥细胞质膜上,敲除该蛋白的基因AIR12,提高对灰霉的抗性[108]。有趣的是,天竺葵Geranium robertianum比G. pyrenaicum更感灰霉,R∶FR低比值抑制G. pyrenaicum对灰霉的抗性,相反,增强G. robertianum对灰霉抗性[109]。由此可见,光(R∶FR低比值)对植物抗灰霉病菌的影响与植物自身的遗传背景密切相关,很可能在协调植物生长与植物抗病二者之间的关系中起作用。
工业发展造成对流层CO2浓度的提高,生长在高CO2浓度环境中的拟南芥则更抗灰霉,与拟南芥体内抗灰霉相关基因PAD3转录水平的提高有关;JA的合成和JA信号通路中的LOX3、OPR3、JAZ10、PDF1.2转录水平都明显高于非高CO2环境中的拟南芥植株;此外,高CO2激活SA途径,体内SA水平及PR1、PR2、PR5、ICS1转录水平均高于非高二氧化碳环境中的植株,因此,高CO2不仅提高拟南芥抗灰霉,也提高对细菌的抗病性[110]。
除了光和二氧化碳两种因素以外,轻柔的触碰诱导ROS的发生、胼胝质的沉积、触碰诱导基因(TCH)的表达和防卫相关基因FaPR1、FaCHI2-2、FaCAT、FaACS1和FaOGBG-5的表达,提高草莓对灰霉的抗性[111]。辣椒上的伤口诱导局部灰霉抗性,但却诱导系统感病性[112]。伤口激发ATP的释放,胞外ATP施用能诱导JA表达和乙烯的生物合成,并且诱导JA信号通路中防卫基因的表达,提高拟南芥对灰霉的抗性[113]。此外,曾有混合肥料提高拟南芥抗灰霉的研究,结果表明橄榄渣和橄榄树叶的堆肥与珍珠岩不同,能诱导拟南芥对灰霉抗病性。GO分析显示,生长在堆肥中的拟南芥相对于生长在珍珠岩的植株,接种灰霉后,在生物胁迫、SA和ABA刺激、氧化胁迫、细菌、真菌等刺激相关反应基因显著富集;而且,堆肥和接种灰霉病菌均能诱导PR1的转录,生长在堆肥中的拟南芥接种后更促进PR1的表达[114]。可见,堆肥的确能提高拟南芥对灰霉的抗病能力。
6.2 影响灰霉抗性的微生物及其代谢产物
微生物或来自微生物的代谢产物能通过激发诱导系统抗性(ISR)、系统获得抗性(SAR)等抗病反应提高植物对灰霉病菌的抗病性。
6.2.1 激发ISR等抗病反应的微生物 植物诱导系统抗性ISR指的是植物在恰当的刺激下而具备的防卫能力增强的状态,对真菌、细菌、病毒及昆虫的植物抗性都有效。ISR需要JA和乙烯(ET)信号途径并且和编码植物防卫素基因PDF1.2的表达相关,也有研究发现ISR依赖SA、JA/ET途径及NPR1[115]。一些根围微生物能诱导ISR,豆科根瘤中分离的革兰氏阳性细菌Micromonospora菌株能诱导不同番茄栽培品种的持久的灰霉抗性,通过诱导JA调控的防卫作用,包括防卫基因LOXA、PinII的诱导表达而抗灰霉病菌[116]。Micromonospora菌株不仅让番茄具备增强防卫能力的状态,而且具有直接抗真菌能力,因此,是生物防治最值得考虑的微生物材料。另一根围细菌Bacillus cereus AR156通过及时增强PR1蛋白的表达、过氧化氢积累和胼胝质的沉积,快速激活MAPKs信号和FRK1/WRKY53基因表达,达到提高拟南芥抗灰霉的目的;这些反应依赖于JA/ET途径和NPR1,而与SA自身水平高低无关[115]。
灌木根围真菌Rhizophagus irregularis定殖在番茄根部,大量的代谢发生改变,番茄内聚集更高水平的维生素叶酸、核黄素、吲哚衍生物和酚类化合物;当B. cinerea侵染R. irregularis定殖后的番茄时,LOX途径起到关键抗病作用[117]。Burkholderia phytofirmansPsJN是高效诱导抗病性的植物内生细菌,PsJN能移动到葡萄的叶片,在B. cinerea周围形成生物膜,限制灰霉生长。除了直接的抗灰霉菌的作用外,PsJN能诱导胼胝质沉积、过氧化氢的产生,且当灰霉侵染有PsJN存在的葡萄部位时,启动表达 PR1、PR2、PR5和 JAZ蛋白[118]。Bacillus subtilisGB03的挥发性化合物VOCs激发拟南芥的PR1和PDF1.2的表达,提高灰霉抗性[119]。由上可见,有益的根围细菌和真菌或植物内生细菌,诱导ISR、激发植物自身与JA、ET或SA有关的抗病反应,部分有益微生物还具有直接抗灰霉的作用。
6.2.2 激发SAR的灰霉病菌(糖)蛋白等物质 灰霉病菌泌出的一些代谢产物和蛋白能产生过敏性反应症状,如草酸、葡双醛霉素、类坏死和乙烯诱导的肽蛋白(NLPs)和木聚糖酶 Xyn11A[7,120]。拟南芥接种灰霉病菌不能诱导SAR,而近期研究表明灰霉病菌B. cinerea的另一些(糖)蛋白分子能激发植物的SAR或其它抗性反应,提高番茄或烟草对灰霉病菌的抗性。B. cinerea的BcSpl1基因编码的cerato-platanin家族的泌出蛋白,处理烟草后,能激发两个受NPR1(SAR主要的调控子)控制的基因PR1-a和PR-5的表达[121]。B. cinerea的另一个泌出蛋白BclEB1,同样能够激发被处理的烟草中的PR1-a和PR-5的表达[121]。BcGs1,B. cinerea的泌出糖蛋白,诱导众所周知的SAR标记基因PR1-a的表达,也诱导乙烯介导且不依赖于JA的死体营养型真菌抗性信号途径中TPK1b的表达,此外,还诱导JA信号途径的激发子系统素原(系统素的前体)的表达,说明BcGs1同时诱导植物的SAR、ET介导的抗灰霉信号通路成分以及JA信号通路成分的表达[93]。B. cinerea泌出的木糖葡聚酶激发大豆的防卫基因Pvd1、PvPR1和PvPR2的表达,激发PTI和SAR[122]。
除了上述灰霉病菌B. cinerea的泌出蛋白及其它代谢物以外,疫霉菌Pythium oligandrum的类似elicitin的蛋白Oli-D1和Oli-D2激发烟草的HR,诱导番茄JA/ET介导的信号途径中SlLapA1、SlPin2、SlLOX-E和SlERF2基因的表达而抗灰霉[123]。
7 总结与展望
寄主范围极其广泛的死体营养型灰霉病菌,采用多种策略侵染寄主植物,形成对作物营养体和果实破坏性很强的灰霉病。生产实践中对灰霉病的防控仍以使用化学药剂为主要防治方式,一方面造成环境污染和食用隐患;另一方面,田间B. cinerea抗药性菌株不断出现,导致化学防治效果差。因此,全面深入理解植物抗灰霉的分子机制,掌握抗灰霉遗传组分,开发利用植物自身基因进行种质抗病遗传改良是有利于保持绿色生态环境的有效措施,但这个过程需要很长时间。而近几年的研究找到具有实践应用潜力的灰霉抗性因素和成分(图1-A和B),可作为开发替代化学药剂的不错选择。此外,随着分子生物学手段的拓展和提高,也找到可以应用来防治灰霉的新的方法。
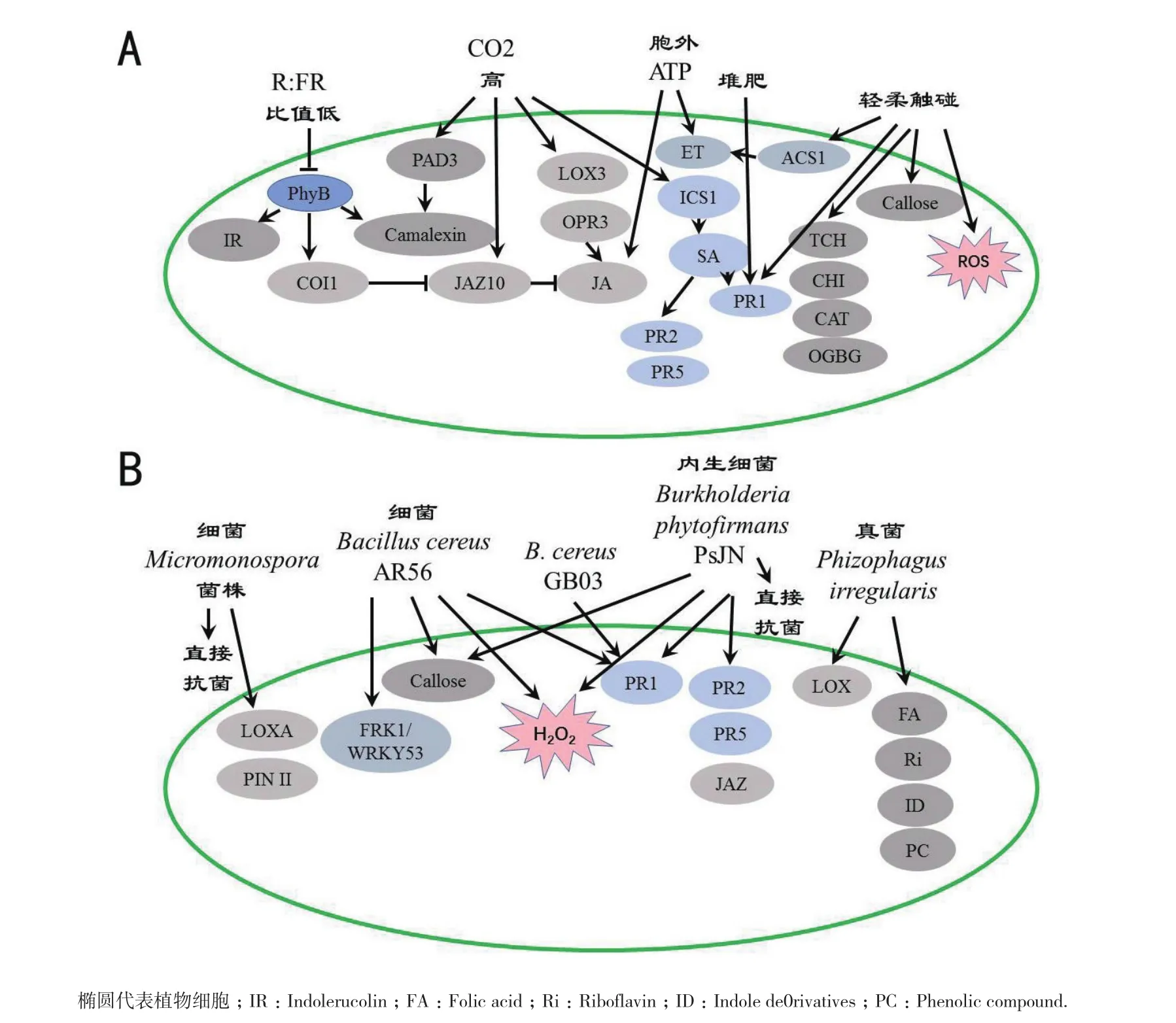
图1 植物细胞外激发抗B. cinerea反应的信号
寄主诱导的基因沉默技术HIGS(Host-induced gene silencing,HIGS)已经被开发和利用来保护作物免受真菌的侵染。灰霉病菌与植物互作过程中,输出sRNA到寄主植物破坏免疫反应[124],而拟南芥表达双链RNA(dsRNA),互补真菌DCL(Dicer-like,DCL)家族基因,显著提高对灰霉病菌的抗性[125]。因此,HIGs可用来培育抗灰霉病菌的作物品种的有效方法之一。可见,随着植物抗灰霉病分子机制研究的不断深入,更多的有效基因能用于抗灰霉病菌种质遗传改良,结合上述提高植物抗灰霉病菌的因素和方法,逐步取代单一的化学防治方法,解决化学农药抗性、环境污染、安全绿色食品等问题。
[1] Williamson B, Tudzynski B, Tudzynski P, et al. Botrytis cinerea:the cause of grey mould disease[J]. Molecular Plant Pathology, 2007,8:561-580.
[2] Elad Y, Pertot I, Cotes Prado AM, Stewart A. Plant hosts of Botrytis spp[M]//Fillinger S, Elad Y, eds. Botrytis-the fungus, the pathogen and its management in agricultural systems. Heidelberg.Germany:Springer, 2016:413-486.
[3] Dean R, Van Kan JA, Pretorius ZA, et al. The top 10 fungal pathogens in molecular plant pathology[J]. Molecular Plant Pathology, 2012, 13:414-430.
[4] Veloukas T, Kalogeropoulou P, Markoglou AN, et al. Fitness and competitive ability of Botrytis cinerea field isolates with dual resistance to SDHI and QoI fungicides, associated with several sdhB and the cytb G143A mutations[J]. Phytopathology, 2014, 104 :347-356.
[5] Rupp S, Plesken C, Rumsey S, et al. Botrytis fragariae, a new species causing gray mold on strawberries, shows high frequencies of specific and efflux-based fungicide resistance[J]. Applied and Environmental Microbiology, 2017, 83(9). pii:e00269-17.
[6] Lai Z, Mengiste T. Genetic and cellular mechanisms regulating plant responses to necrotrophic pathogens[J]. Current Opinion in Plant Biology, 2013, 16:505-512.
[7] Mengiste T. Plant immunity to necrotrophs[J]. Annual Review of Phytopathology, 2012, 50:267-294.
[8] Liu T, Liu Z, Song C, et al. Chitin-induced dimerization activates a plant immune receptor[J]. Science, 2012, 336 :1160-1164.
[9] Núñez de Cáceres González FF, Davey MR, Cancho Sanchez E, et al.Conferred resistance to Botrytis cinerea in Lilium by overexpression of the RCH10 chitinase gene[J]. Plant Cell Reports, 2015, 34 :1201-1209.
[10] Gao Y, Jia S, Wang C, et al. BjMYB1, a transcription factor implicated in plant defence through activating BjCHI1 chitinase expression by binding to a W-box-like element[J]. Journal of Experimental Botany, 2016, 67:4647-4658.
[11] Gao Y, Zhao K. Molecular mechanism of BjCHI1-mediated plant defense against Botrytis cinerea infection[J]. Plant Signaling &Behavior, 2017, 12:e1271859.
[12] Gai YP, Zhao YN, Zhao HN, et al. The latex protein MLX56 from mulberry(Morus multicaulis)protects plants against insect pests and pathogens[J]. Frontiers in Plant Science, 2017, 8 :1475.
[13] Prins T, Tudzynski P, Tiedemann A, et al. Infection strategies of Botrytis cinerea and related necrotrophic pathogens. In Fungal Pathology[M]// JW Kronstad Dordrecht. The Netherlands:Kluwer, 2000:33-64.
[14] Rasul S, Dubreuil-Maurizi C, Lamotte O, et al. Nitric oxide production mediates oligogalacturonide-triggered immunity and resistance to Botrytis cinerea in Arabidopsis thaliana[J]. Plant,Cell & Environment, 2012, 35:1483-1499.
[15] Davidsson P, Broberg M, Kariola T, et al. Short oligogalacturonides induce pathogen resistance-associated gene expression in Arabidopsis thaliana[J]. BMC Plant Biology, 2017, 17:19.
[16] Lionetti V, Raiola A, Camardella L, et al. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea[J]. Plant Physiology, 2007, 143:1871-1880.
[17] Reem NT, Pogorelko G, Lionetti V, et al. Decreased polysaccharide feruloylation compromises plant cell wall integrity and increases susceptibility to necrotrophic fungal pathogens[J]. Frontiers in Plant Science, 2016, 7:630.
[18] Fauth M, Schweizer P, Buchala A, et al. Cutin monomers and surface wax constituents elicit H2O2in conditioned cucumber hypocotyl segments and enhance the activity of other H2O2elicitors[J]. Plant Physiology, 1998, 117:1373-1380.
[19] Schweizer P, Felix G, Buchala A, et al. Perception of free cutin monomers by plant cells[J]. The Plant Journal, 1996, 10 :331-341.
[20] Voisin D, Nawrath C, Kurdyukov S, et al. Dissection of the complex phenotype in cuticular mutants of Arabidopsis reveals a role of SERRATE as a mediator[J]. PLoS Genetics, 2015, 5(10):e1000703.
[21] L’Haridon F, Besson-Bard A, Binda M, et al. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity[J]. PLoS Pathogens, 2011, 7(7):e1002148.
[22] Bessire M, Chassot C, Jacquat AC, et al. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea[J].The EMBO Journal, 2007, 26:2158-2168.
[23] Ju S, Go YS, Choi HJ, et al. DEWAX transcription factor is involved in resistance to Botrytis cinerea in Arabidopsis thaliana and Camelina sativa[J]. Frontiers in Plant Science, 2017, 8 :1210.
[24] Go YS, Kim H, Kim HJ, et al. Arabidopsis cuticular wax biosynthesis is negatively regulated by the DEWAX gene encoding an AP2/ERF-Type transcription factor[J]. The Plant Cell, 2014,26:1666-1680.
[25] Zhang H, Yu P, Zhao J, et al. Expression of tomato prosystemin gene in Arabidopsis reveals systemic translocation of its mRNA and confers necrotrophic fungal resistance[J]. The New Phytologist,2017, 217:799-812.
[26] Liu Z, Wu Y, Yang F, et al. BIK1 interacts with PEPRs to mediate ethylene-induced immunity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110:6205-6210.
[27] Ross A, Yamada K, Hiruma K, et al. The Arabidopsis PEPR pathway couples local and systemic plant immunity[J]. The EMBO Journal, 2014, 33:62-75.
[28] Tang D, Zhou JM. PEPRs spice up plant immunity[J]. The EMBO Journal, 2016, 35:4-5.
[29] Yamada K, Yamashita-Yamada M, Hirase T, et al. Danger peptide receptor signaling in plants ensures basal immunity upon pathogeninduced depletion of BAK1[J]. The EMBO Journal, 2016, 35:46-61.
[30] Miya A, Albert P, Shinya T, et al. CERK1, a LysM receptor kinase,is essential for chitin elicitor signaling in Arabidopsis[J].Proceedings of the National Academy of Sciences of the United States of America, 2007, 104:19613-19618.
[31] Chinchilla D, Zipfel C, Robatzek S, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence[J]. Nature, 2007, 448:497-500.
[32] Kemmerling B, Schwedt A, Rodriguez P, et al. The BRI1-associated kinase 1, BAK1, has a brassinolide-Independent role in plant celldeath control[J]. Current Biology, 2007, 17 :1116-1122.
[33] Schulze B, Mentzel T, Jehle AK, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1[J]. The Journal of Biological Chemistry, 2010, 285:9444-9451.
[34] Veronese P, Nakagami H, Bluhm B, et al. The membrane-anchored BOTRYTIS-INDUCED KINASE1 plays distinct roles in Arabidopsis resistance to necrotrophic and biotrophic pathogens[J]. The Plant Cell, 2006, 18:257-273.
[35] Laluk K, Luo H, Chai M, et al. Biochemical and genetic requirements for function of the immune response regulator BOTRYTIS-INDUCED KINASE1 in plant growth, ethylene signaling, and PAMP-triggered immunity in Arabidopsis[J]. The Plant Cell, 2011, 23:2831-2849.
[36] Zhang J, Li W, Xiang T, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector[J]. Cell Host &Microbe, 2010, 7:290-301.
[37] Feng F, Yang F, Rong W, et al. A Xanthomonas uridine 5’-monophosphate transferase inhibits plant immune kinases[J]. Nature,2012, 485:114-118.
[38] Galletti R, Ferrari S, De Lorenzo G. Arabidopsis MPK3 and MPK6 play different roles in basal and oligogalacturonide- or flagellininduced resistance against Botrytis cinerea[J]. Plant Physiology,2011, 157:804-814.
[39] Genenncher B, Wirthmueller L, Roth C, et al. Nucleoporinregulated MAP kinase signaling in immunity to a necrotrophic fungal pathogen[J]. Plant Physiology, 2016, 172:1293-1305.
[40] Meng X, Xu J, He Y, et al. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance[J]. The Plant Cell, 2013, 25 :1126-1142.
[41] Jiang Y, Yu D. The WRKY57 transcription factor affects the expression of jasmonate ZIM-domain genes transcriptionally to compromise Botrytis cinerea resistance[J]. Plant Physiology,2016, 171:2771-2782.
[42] Birkenbihl RP, Diezel C, Somssich IE. Arabidopsis WRKY33 is a key transcriptional regulator of hormonal and metabolic responses toward Botrytis cinerea infection[J]. Plant Physiology, 2012,159:266-285.
[43] Lai Z, Vinod K, Zheng Z, et al. Roles of Arabidopsis WRKY3 and WRKY4 transcription factors in plant responses to pathogens[J].BMC Plant Biology, 2008, 8:68.
[44] Liu S, Kracher B, Ziegler J, et al. Negative regulation of ABA signaling by WRKY33 is critical for Arabidopsis immunity towards Botrytis cinerea 2100[J]. ELife, 2015, 4:e07295.
[45] Li GJ, Meng XZ, Wang RG, et al. Dual-Level Regulation of ACC Synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis[J]. PLoS Genetics, 2012, 8(6):e1002767.
[46] Liao CJ, Lai ZB, Lee S, et al. Arabidopsis HOOKLESS1 regulates responses to pathogens and abscisic acid through interaction with MED18 and acetylation of WRKY33 and ABI5 chromatin[J].The Plant Cell, 2016, 28:1662-1681.
[47] Liu B, Hong YB, Zhang YF, et al. Tomato WRKY transcriptional factor SlDRW1 is required for disease resistance against Botrytis cinerea and tolerance to oxidative stress[J]. Plant Science:an International Journal of Experimental Plant Biology, 2014, 227:145-156.
[48] Adachi H, Ishihama N, Nakano T, et al. Nicotiana benthamiana MAPK-WRKY pathway confers resistance to a necrotrophic pathogen Botrytis cinerea[J]. Plant Signaling & Behavior, 2016,11:e1183085.
[49] Zhao Y, Wei T, Yin KQ, et al. Arabidopsis RAP2. 2 plays an important role in plant resistance to Botrytis cinerea and ethylene responses[J]. The New Phytologist, 2012, 195 :450-460.
[50] Moffat CS, Ingle RA, Wathugala DL, et al. ERF5 and ERF6 play redundant roles as positive regulators of JA/Et-mediated defense against Botrytis cinerea in Arabidopsis[J]. PLoS One, 2012, 7(4):e3599.
[51] Zhang H, Hong Y, Huang L, et al. Arabidopsis AtERF014 acts as a dual regulator that differentially modulates immunity against Pseudomonas syringae pv. tomato and Botrytis cinerea[J].Scientific Reports, 2016, 6:30251.
[52] Lu X, Jiang W, Zhang L, et al. AaERF1 positively regulates the resistance to Botrytis cinerea in Artemisia annua[J]. PLoS One,2013, 8:e57657.
[53] Lu X, Zhang L, Zhang F, et al. AaORA, a trichome-specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea[J]. The New Phytologist, 2013, 198:1191-1202.
[54] Ramirez V, Agorio A, Coego A, et al. MYB46 modulates disease susceptibility to Botrytis cinerea in Arabidopsis[J]. Plant Physiology, 2011, 155:1920-1935.
[55] Sardesai N, Laluk K, Mengiste T, et al. The Arabidopsis Myb transcription factor MTF1 is a unidirectional regulator of susceptibility to Agrobacterium[J]. Plant Signaling & Behavior,2014, 9. pii:e28983.
[56] Krol E, Mentzel T, Chinchilla D, et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2[J]. The Journal of Biological Chemistry, 2010, 285:13471-13479.
[57] Giri MK, Singh N, Banday ZZ, et al. GBF1 differentially regulates CAT2 and PAD4 transcription to promote pathogen defense in Arabidopsis thaliana[J]. The Plant Journal:for Cell and Molecular Biology, 2017, 91:802-815.
[58] Yang Y, Li L, Qu LJ. Plant Mediator complex and its critical functions in transcription regulation[J]. Journal of Integrative Plant Biology, 2016, 58:106-118.
[59] Kidd BN, Edgar CI, Kumar KK, et al. The mediator complex subunit PFT1 is a key regulator of jasmonate-dependent defense in Arabidopsis[J]. The Plant Cell, 2009, 21 :2237-2252.
[60] Cevik V, Kidd BN, Zhang P, et al. MEDIATOR25 acts as an integrative hub for the regulation of jasmonate-responsive gene expression in Arabidopsis[J]. Plant Physiology, 2012, 160 :541-555.
[61] Ou B, Yin KQ, Liu SN, et al. A high-throughput screening system for Arabidopsis transcription factors and its application to Med25-dependent transcriptional regulation[J]. Molecular Plant, 2011, 4:546-555.
[62] Yang Y, Ou B, Zhang J, et al. The Arabidopsis Mediator subunit MED16 regulates iron homeostasis by associating with EIN3/EIL1 through subunit MED25[J]. The Plant Journal:for Cell and Molecular Biology, 2014, 77:838-851.
[63] Chen R, Jiang H, Li L, et al. The Arabidopsis mediator subunit MED25 differentially regulates jasmonate and abscisic acid signaling through interacting with the MYC2 and ABI5 transcription factors[J]. The Plant Cell, 2012, 24 :2898-2916.
[64] Lai Z, Schluttenhofer CM, Bhide K, et al. MED18 interaction with distinct transcription factors regulates multiple plant functions[J]. Nature Communications, 2014, 5 :3064.
[65] Zhu Y, Schluttenhoffer CM, Wang P, et al. CYCLIN-DEPENDENT KINASE8 differentially regulates plant immunity to fungal pathogens through kinase-dependent and -independent functions in Arabidopsis[J]. The Plant Cell, 2014, 26 :4149-4170.
[66] Wang C, Du X, Mou Z. The mediator complex subunits MED14,MED15, and MED16 are involved in defense signaling crosstalk in Arabidopsis[J]. Frontiers in Plant Science, 2016, 7 :1947.
[67] Wang C, Ding Y, Yao J, Zhang Y, et al. Arabidopsis Elongator subunit 2 positively contributes to resistance to the necrotrophic fungal pathogens Botrytis cinerea and Alternaria brassicicola[J].The Plant Journal, 2015, 83:1019-1033.
[68] Wang C, Zhang X, Li JL, Zhang Y, et al. The Elongator complexassociated protein DRL1 plays a positive role in immune responses against necrotrophic fungal pathogens in Arabidopsis[J].Molecular Plant Pathology, 2016, 19:286-299.
[69] Berr A, McCallum EJ, Alioua A, et al. Arabidopsis histone methyltransferase SET DOMAIN GROUP8 mediates induction of the jasmonate/ethylene pathway genes in plant defense response to necrotrophic fungi[J]. Plant Physiology, 2010, 154 :1403-1414.
[70] Lee S, Fu F, Xu S, et al. Global regulation of plant immunity by histone lysine methyl transferases[J]. The Plant Cell, 2016, 28 :1640-1661.
[71] Lopez A, Ramirez V, Garcia-Andrade J, et al. The RNA silencing enzyme RNA polymerase v is required for plant immunity[J].PLoS Genetics, 2011, 7:e1002434.
[72] Dhawan R, Luo H, Foerster AM, et al. HISTONE MONOUBIQUITINATION1 interacts with a subunit of the mediator complex and regulates defense against necrotrophic fungal pathogens in Arabidopsis[J]. The Plant Cell, 2009, 21 :1000-1019.
[73] Zhang Y, Li D, Zhang H, et al. Tomato histone H2B monoubiquitination enzymes SlHUB1 and SlHUB2 contribute to disease resistance against Botrytis cinerea through modulating the balance between SA- and JA/ET-mediated signaling pathways[J]. BMC Plant Biology, 2015, 15:252.
[74] El Oirdi M, El Rahman TA, Rigano L, et al. Botrytis cinerea manipulates the antagonistic effects between immune pathways to promote disease development in tomato[J]. The Plant Cell,2011, 23:2405-2421.
[75] Asselbergh B, Curvers K, Franca SC, et al. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis[J]. Plant Physiology, 2007, 144 :1863-1877.
[76]Abuqamar S, Luo H, Laluk K, et al. Crosstalk between biotic and abiotic stress responses in tomato is mediated by the AIM1 transcription factor[J]. The Plant Journal:for Cell and Molecular Biology, 2009, 58:347-360.
[77] Ouyang Z, Liu S, Huang L, et al. Tomato SlERF. A1, SlERF. B4,SlERF. C3 and SlERF. A3, members of B3 group of ERF Family,are required for resistance to Botrytis cinerea[J]. Frontiers in Plant Science, 2016, 7:1964.
[78] Van der Does D, Leon-Reyes A, Koornneef A, et al. Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59[J]. The Plant Cell, 2013, 25:744-761.
[79] Caarls L, Elberse J, Awwanah M, et al. Arabidopsis JASMONATEINDUCED OXYGENASES down-regulate plant immunity by hydroxylation and inactivation of the hormone jasmonic acid[J].Proceedings of the National Academy of Sciences of the United States of America, 2017, 114:6388-6393.
[80] Smirnova E, Marquis V, Poirier L, et al. Jasmonic acid oxidase 2 hydroxylates jasmonic acid and represses basal defense and resistance responses against Botrytis cinerea Infection[J].Molecular Plant, 2017, 10:1159-1173.
[81] Du M, Zhao J, Tzeng DTW, et al. MYC2 orchestrates a hierarchical transcriptional cascade that regulates jasmonate-mediated plant immunity in tomato[J]. The Plant Cell, 2017, 29:1883-1906.
[82] Lai Z, Wang F, Zheng Z, et al. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens[J]. The Plant Journal, 2011, 66:953-968.
[83] Kabbage M, Kessens R, Dickman MB. A plant Bcl-2-associated athanogene is proteolytically activated to confer fungal resistance[J]. Microb Cell, 2016, 3 :224-226.
[84] Li Y, Kabbage M, Liu W, et al. Aspartyl protease-mediated cleavage of BAG6 is necessary for autophagy and fungal resistance in plants[J]. The Plant Cell, 2016, 28 :233-247.
[85] Li X, Zhang H, Tian L, et al. Tomato SlRbohB, a member of the NADPH oxidase family, is required for disease resistance against Botrytis cinerea and tolerance to drought stress[J]. Frontiers in Plant Science, 2015, 6:463.
[86] Survila M, Davidsson PR, Pennanen V, et al. Peroxidase-generated apoplastic ROS impair cuticle integrity and contribute to DAMP-elicited defenses[J]. Frontiers in Plant Science, 2016, 7 :1945.
[87] Bi FC, Liu Z, Wu JX, et al. Loss of ceramide kinase in Arabidopsis impairs defenses and promotes ceramide accumulation and mitochondrial H2O2bursts[J]. The Plant Cell, 2014, 26 :3449-3467.
[88] Rossi FR, Krapp AR, Bisaro F, et al. Reactive oxygen species generated in chloroplasts contribute to tobacco leaf infection by the necrotrophic fungus Botrytis cinerea[J]. The Plant Journal:for Cell and Molecular Biology, 2017, 92:761-773.
[89] Mao G, Meng X, Liu Y, et al. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis[J]. The Plant Cell, 2011,23:1639-1653.
[90] Khare D, Choi H, Huh SU, et al. Arabidopsis ABCG34 contributes to defense against necrotrophic pathogens by mediating the secretion of camalexin[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114:E5712-E5720.
[91] Burow M, Halkier BA, Kliebenstein DJ. Regulatory networks of glucosinolates shape Arabidopsis thaliana fitness[J]. Current Opinion in Plant Biology, 2010, 13:348-353.
[92] Fan J, Doerner P. Genetic and molecular basis of nonhost disease resistance:complex, yes;silver bullet, no[J]. Current Opinion in Plant Biology, 2012, 15:400-406.
[93] Zhang Y, Zhang Y, Qiu D, et al. BcGs1, a glycoprotein from Botrytis cinerea, elicits defence response and improves disease resistance in host plants[J]. Biochemical and Biophysical Research Communications, 2015, 457:627-634.
[94] Xu J, Meng J, Meng X, et al. Pathogen-Responsive MPK3 and MPK6 reprogram the biosynthesis of indole glucosinolates and their derivatives in Arabidopsis immunity[J]. The Plant Cell, 2016,28:1144-1162.
[95] Zhang Y, Jin X, Ouyang Z, et al. Vitamin B6 contributes to disease resistance against Pseudomonas syringae pv. tomato DC3000 and Botrytis cinerea in Arabidopsis thaliana[J]. Journal of Plant Physiology, 2015, 175:21-25.
[96] Lemonnier P, Gaillard C, Veillet F, et al. Expression of Arabidopsis sugar transport protein STP13 differentially affects glucose transport activity and basal resistance to Botrytis cinerea[J].Plant Molecular Biology, 2014, 85:473-484.
[97] Rizzi YS, Cecchini NM, Fabro G, et al. Differential control and function of Arabidopsis ProDH1 and ProDH2 genes on infection with biotrophic and necrotrophic pathogens[J]. Molecular Plant Pathology, 2017, 18:1164-1174.
[98] Zhao P, Zhang F, Liu D, et al. Matrix metalloproteinases operate redundantly in Arabidopsis immunity against necrotrophic and biotrophic fungal pathogens[J]. PLoS One, 2017, 12 :e0183577.
[99] Li D, Zhang H, Song Q, et al. Tomato Sl3-MMP, a member of the Matrix metalloproteinase family, is required for disease resistance against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000[J]. BMC Plant Biology, 2015, 15:143.
[100]Camanes G, Scalschi L, Vicedo B, et al. An untargeted global metabolomic analysis reveals the biochemical changes underlying basal resistance and priming in Solanum lycopersicum, and identifies 1-methyltryptophan as a metabolite involved in plant responses to Botrytis cinerea and Pseudomonas syringae[J].The Plant Journal, 2015, 84:125-139.
[101]Zhang H, Hong Y, Huang L, et al. Virus-Induced gene silencingbased functional analyses revealed the involvement of several putative trehalose-6-phosphate synthase/phosphatase genes in disease resistance against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000 in tomato[J]. Frontiers in Plant Science, 2016, 7:1176.
[102]Polturak G, Grossman N, Vela-Corcia D, et al. Engineered gray mold resistance, antioxidant capacity, and pigmentation in betalain-producing crops and ornamentals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114:9062-9067.
[103]Yan X, Qiao H, Zhang X, et al. Analysis of the grape(Vitis vinifera L.)thaumatin-like protein(TLP)gene family and demonstration that TLP29 contributes to disease resistance[J].Scientific Reports, 2017, 7:4269.
[104]Wang Y, Wang D, Wang F, et al. Expression of the grape VaSTS19 Gene in Arabidopsis improves resistance to powdery mildew and Botrytis cinerea but increases susceptibility to Pseudomonas syringe pv tomato DC3000[J]. Int J Mol Sci, 2017, 18(9).pii:E2000.
[105]Huang L, Zhang S, Singer SD, et al. Expression of the Grape VqSTS21 Gene in Arabidopsis confers resistance to osmotic stress and biotrophic pathogens but not Botrytis cinerea[J]. Frontiers in Plant Science, 2016, 7:1379.
[106]Cerrudo I, Keller MM, Cargnel MD, et al. Low red/far-red ratios reduce Arabidopsis resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acidindependent mechanism[J]. Plant Physiology, 2012, 158:2042-2052.
[107]Cargnel MD, Demkura PV, Ballare CL. Linking phytochrome to plant immunity:low red:far-red ratios increase Arabidopsis susceptibility to Botrytis cinerea by reducing the biosynthesis of indolic glucosinolates and camalexin[J]. The New Phytologist,2014, 204:342-354.
[108]Costa A, Barbaro MR, Sicilia F, et al. AIR12, a b-type cytochrome of the plasma membrane of Arabidopsis thaliana is a negative regulator of resistance against Botrytis cinerea[J]. Plant Sci,2015, 233:32-43.
[109]Gommers CM, Keuskamp DH, Buti S, et al. Molecular profiles of contrasting shade response strategies in wild plants:differential control of immunity and shoot elongation[J]. The Plant Cell,2017, 29:331-344.
[110]Mhamdi A, Noctor G. High CO2primes plant biotic stress defences through redox-linked pathways[J]. Plant Physiology, 2016,172:929-942.
[111] Tomas-Grau RH, Requena-Serra FJ, Hael-Conrad V, et al.Soft mechanical stimulation induces a defense response against Botrytis cinerea in strawberry[J]. Plant Cell Reports, 2018,37:239-250.
[112]Garcia T, Gutierrez J, Veloso J, et al. Wounding induces local resistance but systemic susceptibility to Botrytis cinerea in pepper plants[J]. Journal of Plant Physiology, 2015, 176 :202-209.
[113]Tripathi D, Zhang T, Koo AJ, et al. Extracellular ATP acts on jasmonate signaling to reinforce plant defense[J]. Plant Physiology, 2017, 176:511-523.
[114]Segarra G, Santpere G, Elena G, et al. Enhanced Botrytis cinerea resistance of Arabidopsis plants grown in compost may be explained by increased expression of defense-related genes, as revealed by microarray analysis[J]. PLoS One, 2013, 8 :e56075.
[115]Nie P, Li X, Wang S, et al. Induced systemic resistance against Botrytis cinerea by Bacillus cereus AR156 through a JA/ET-and NPR1-dependent signaling pathway and activates PAMP-triggered immunity in Arabidopsis[J]. Frontiers in Plant Science, 2017, 8:238.
[116]Martinez-Hidalgo P, Garcia JM, Pozo MJ. Induced systemic resistance against Botrytis cinerea by Micromonospora strains isolated from root nodules[J]. Frontiers in Microbiology, 2015,6:922.
[117] Sanchez-Bel P, Troncho P, Gamir J, et al. The nitrogen availability interferes with mycorrhiza-induced resistance against Botrytis cinerea in tomato[J]. Front Microbiol, 2016, 7:1598.
[118]Miotto-Vilanova L, Jacquard C, Courteaux B, et al. Burkholderia phytofirmans PsJN confers grapevine resistance against Botrytis cinerea via a direct antimicrobial effect combined with a better resource mobilization[J]. Front Plant Sci, 2016, 7:1236.
[119]Sharifi R, Ryu CM. Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarm signals to Arabidopsis seedlings for eliciting induced resistance, or both?[J]. Frontiers in Microbiology, 2016, 7:196.
[120]Noda J, Brito N, Gonzalez C. The Botrytis cinerea xylanase Xyn11A contributes to virulence with its necrotizing activity, not with its catalytic activity[J]. BMC Plant Biol, 2010, 10:38.
[121]Frias M, Brito N, Gonzalez C. The Botrytis cinerea cerato-platanin BcSpl1 is a potent inducer of systemic acquired resistance(SAR)in tobacco and generates a wave of salicylic acid expanding from the site of application[J]. Molecular Plant Pathology, 2013,14:191-196.
[122]Zhu W, Ronen M, Gur Y, et al. BcXYG1, a Secreted Xyloglucanase from Botrytis cinerea, triggers both cell death and plant immune responses[J]. Plant Physiology, 2017, 175 :438-456.
[123]Ouyang Z, Li X, Huang L, et al. Elicitin-like proteins Oli-D1 and Oli-D2 from Pythium oligandrum trigger hypersensitive response in Nicotiana benthamiana and induce resistance against Botrytis cinerea in tomato[J]. Mol Plant Pathol, 2015, 16:238-250.
[124]Weiberg A, Wang M, Lin FM, et al. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways[J]. Science, 2013, 342 :118-123.
[125]Wang M, Weiberg A, Lin FM, et al. Bidirectional crosskingdom RNAi and fungal uptake of external RNAs confer plant protection[J]. Nature Plants, 2016, 2:16151.

