多巴胺检测方法研究进展
黄启同,林小凤,胡世荣,祝杰记,佟庆笑*,
(1.汕头大学化学系,汕头 515063;2.漳州职业技术学院食品与生物工程系,漳州 363000;3.闽南师范大学化学与环境学院,漳州 363000)
作为儿茶酚胺类神经递质的一种,多巴胺(Dopamine,DA,3,4-二羟基-β-苯乙胺)在中枢神经、心血管、肾脏和内分泌系统中扮演着重要的角色[1-3].2000年诺贝尔医学奖获得者——Carlsson曾指出:DA不只是甲状腺素和去甲肾上腺素的前驱,而且还是脑内信息传递者[4].因此,多巴胺含量的大小影响着人们的思考、工作、运动等行为[5-6].人体血液中多巴胺的正常质量浓度0.2-0.4 μg·mL-1,人体血液中DA的质量浓度过低时将会引发许多疾病如精神分裂症、心脏衰竭、帕金森症、神经肌肉失调等[7-8].反之,体内DA含量增多时,会使人感到开心、兴奋,容易让人上瘾,喝酒、吸烟、吸毒等都可以促进DA的分泌,因此,酒鬼,烟民和瘾君子等一般都受DA的控制,与其体内的DA含量有关[9-10].除此之外,作为药物的一种,DA可以作用于交感神经系统,对其产生一些影响,如心率加快、排血量增加、血压升高和心肌收缩力增强等,因此,DA常常被用于治疗抑郁症、心肌梗死、肾功能衰竭、休克以及内毒素败血症等疾病[11-12].因此,DA的含量与一些疾病有着密切相关,高灵敏、简单地检测DA的浓度对于生理功能研究和临床疾病诊断具有重要应用价值.
随着生命科学、生物工程及药物工程的快速发展,对生物分子、药物小分子的快速、灵敏、准确检测成为现代生物学、医学、化学等领域的重要研究课题.由于DA含量的测定在临床应用和生理功能研究方面都具有重要的意义,截至目前,有许多的研究方法和手段为DA的检测做出了巨大的贡献.
1 荧光光谱法
荧光光谱法具有操作简单且快速、灵敏等优点,在分析领域得到了广泛地应用[13-14].Lin等[15]开发了以一个基于DNA-Ag纳米粒子的荧光探针,该探针可以高灵敏性、高选择性地检测DA(图1),该方法是在DNA-Ag材料中加入基因染料Genefinder(GF)和DA分子,DA分子与银纳米粒子结合,破坏了DNA-Ag结构,使DNA得以与GF作用,从而使整个体系的荧光增强,而没有DA存在时,DNA-Ag体系的荧光信号很弱.该探针的检出限达到了6.0 nmol/L.Zhang等[16]通过间苯二酚与DA之间的快速反应(5 min),且其反应产物具有很强的荧光信号,从而实现对DA的测定.检测范围为10 nmol/L-20 μmol/L,检出限达到了1.8 nmol/L,该荧光传感器已成功应用于对尿液中DA含量的测定.众所周知,零维的量子点因其量子尺寸效应,导致其具有优异的荧光发光性能[17-18],所以作为零维量子点的代表碳量子点(CDs)和石墨烯量子点(GQDs)已被广泛地应用于DA的测定.Liu等[19]通过3-氨基苯硼酸制备出了量子产率达到67%的B、N掺杂的CDs(B-N-CDs),通过B-N-CDs表面的-NH2以及-B(OH)2与DA表面的-OH作用,成功实现对DA的测定,该方法的检出限达到了0.1 pmol/L.Zhou等[20]以聚吡咯/石墨烯量子点(ppy/GQDs)核壳材料为发光剂,相比于普通的GQDs,该复合材料荧光强度是其3倍多,通过DA和GQDs间的静电和堆积作用,实现对DA的测定,其检测范围可达5-8000 nmol/L,检出限达到了10 pmol/L.表1总结了近年来部分通过荧光法测定DA的报道[15,16,19-27].
荧光光谱法的检测灵敏度较高,但仍存在荧光淬灭效应、散射光的干扰等问题.同时,用于复杂体系中的DA测定是比较困难.
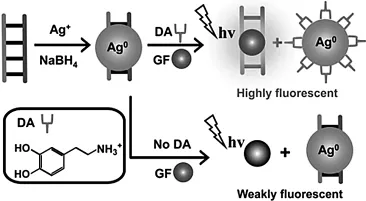
图1 DNA-Ag纳米粒子检测DA的反应机理[15]
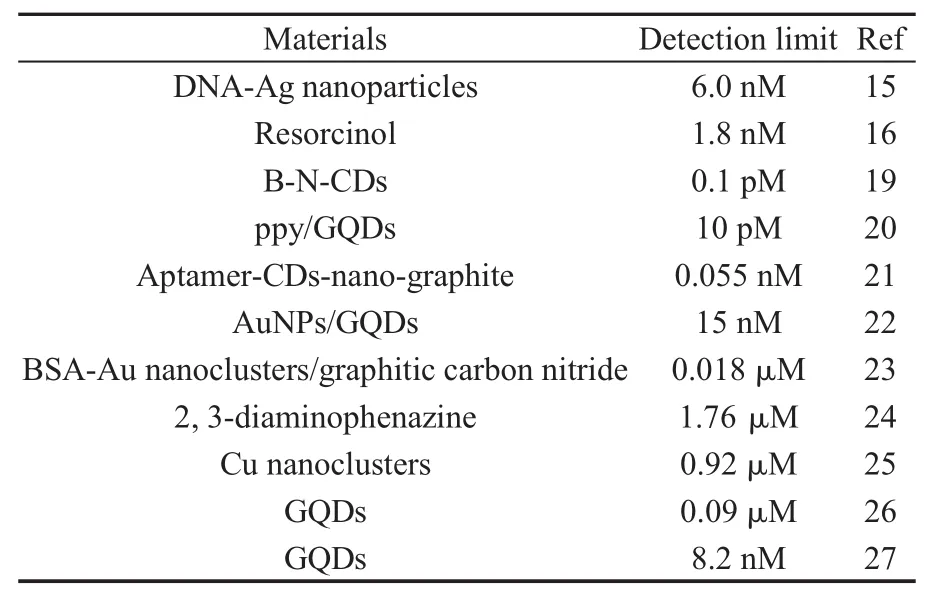
表1 荧光传感器测定DA
2 高效液相色谱法
高效液相色谱法(HPLC)是集分离、定性、定量为一体的分析方法,该方法可以用于分离分析多种共存物质,因此也常被用于DA及其类似物的检测[28-30].Tsunoda等[28]采用HPLC分离分析DA和3,4-二羟基苯乙酸(DOPAC)的浓度,并成功地测出小老鼠体内的DA和DOPAC的浓度分别为(4.98±0.66)μmol/L和(1.00±0.11)μmol/L.Chen等[29]在HPLC上加上一个光电二极管阵列,用来分离检测我国国内的草本植物马齿苋中的去甲肾上腺素(NA)和DA的含量,与标准液对比结果良好.同时,二者的检测范围分别为0.004-6.00 μg和0.011-8.25 μg,检出限分别达到了0.40 ng和0.55 ng.Ribeiro等[30]使用HPLC-质谱/质谱联用仪,成功实现了对左旋多巴、甲基多巴肼、恩他卡朋、托卡朋、3-甲氧酪氨酸和DA的同时测定,该方法具有很高的选择性和灵敏性(检出限达7.0 ng·mL-1).
虽然HPLC在混合物的分离、分析中体现了明显的优势,但是HPLC方法的分析成本高,液相色谱仪价格及日常维护费用高,分析时间一般比较长.
3 毛细管电泳法
毛细管电泳法(CE)对于混合物质的检测与分离具有分离时间短、分离效率高、系统体积小且易实现不同操作单元的集成等优点,因此,该方法也得到了广大研究人员的青睐[31].马健等[32]开发了用CE法测定猪尿中DA残留量的方法.在波长为214 nm处,分离电压为15 kV,进样时间为20 s,分离时间为12 min,pH=5.04的醋酸—醋酸钠缓冲液下运行实现DA的完全分离.同时其最低检测限为0.05 μg·mL-1.Thabano等[33]以Si纳米-异丁烯酸作为填充柱材料来提高对DA等的离子交换能力,从而提高CE法检测DA的灵敏度.Fang等[34]将Pd纳米粒子修饰于碳纤维微盘电极表面,并通过CE法成功测定单个大鼠嗜铬细胞瘤细胞中DA的含量.Zhao等[35]通过CE与电化学发光联用法(CE-CL)同时检测DA和肾上腺素.在这项研究中,CdTe量子点加入到CE的电泳缓冲溶液中来促建CL中鲁米诺和H2O2的反应,以增强CE的信号,达到检测的目的,该方法对于DA和肾上腺素的检出限分别达到23 nmol/L和9.3 nmol/L.
CE法中的毛细管柱效高,成本低,操作较为简便,但是分离能力较弱,对pH值要求较高,而且更为关键的是该方法的重现性差.
4 比色法
比色法无需通过精密的设备,可直接用肉眼观察颜色变化并结合其它仪器如紫外可见分光光度计、荧光光谱仪或者颜色处理软件来测定相关组分浓度,具有经济、快速等优点[36].Liu等[8]开发出一种新型的AuNRs-Ag+非聚集比色传感器检测DA的新方法,该方法的检测范围为6.5-65 μmol/L,检出限为0.3 μmol/L,而且该方法已经成功被应用于血液中DA含量的测定,其反应的机理为:DA与Ag+作用释放出Ag0纳米粒子,之后与AuNRs反应形成Au@Ag纳米材料,使得体系的颜色发生变化.Leng等[37]将DA修饰于金纳米粒子表面(DA-AuNPs),在碱性溶液中,通过巯基乙酸的水解产物作为交联剂,使得DA-AuNPs发生聚集导致体系颜色变化,从而实现对DA的测定,该方法在水中、尿液中和血液中的检出限分别达到33 nmol/L、0.1 μmol/L和94 nmol/L.Tao等[38]基于BSA-金纳米簇(BSA-AuNCs)复合材料,制备出一个简单的荧光-比色双通道检测器,该检测器可以高灵敏地检测DA的含量.其机理如图2所示,BSA-AuNCs溶液荧光发射很强,而当DA加入时,DA通过静电吸附于BSA-AuNCs表面,使体系的荧光强度减弱,从而实现荧光光谱检测;此外,DA与BSA-AuNCs作用后,体系的颜色也随着DA的浓度而发生改变,因此,也可以通过视觉观察来确定其浓度,检出限为10 nmol/L.Wen等[39]使用β-环糊精-Au纳米粒子作为光信号源,制备出DA紫外-比色传感器,该方法的检出限达到20 nmol/L.
比色法虽然方便、快捷,但是其准确度不高且灵敏度不好,并且提高准确度还需要借助其它仪器进一步分析.
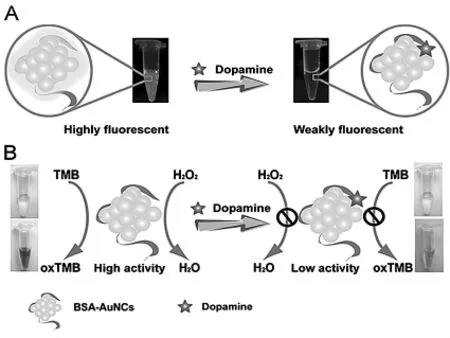
图2 BSA-AuNCs与DA的作用机理[38]
5 电化学分析方法
电化学分析方法具有操作简便、灵敏度高、选择性好等优点,同时,其还可以对活体进行分析,这一优势是其它方法都无法比拟的[40-45].由于DA具有良好的电化学响应性能,在电流作用下,其分子中苯环上的羟基被氧化生成醌,之后醌会被还原回去,从而实现电化学方法检测.但是,由于抗坏血酸(AA)和尿酸(UA)与DA共存于大脑和体液中,在裸电极上三者的氧化电位相近,容易对DA的检测造成干扰[46-48].因此,在直接电化学检测时,必须考虑AA与UA对DA检测的影响.同时,电化学分析方法虽然快速简单但其稳定性还不够好,因此,在过去的几十年时间里,大量的研究人员通过不断开发与改进电极修饰材料,以进一步更加准确地、灵敏地、稳定地检测DA的含量.目前,常见的用于DA检测的电极修饰材料有有机膜材料和纳米材料两种.
5.1 有机膜材料
5.1.1 有机聚合物膜
聚合物膜修饰电极具有良好的选择性、稳定性和重现性[49-51],同时,具备导电性能好、化学性能稳定等优势,此类修饰电极在DA的电化学检测中也得到广泛的应用.
Wu等[52]用β-环糊精-聚(N-异丙基丙烯酰胺)(β-CD-PNIPAM)修饰电极来检测DA的含量,由于β-CD-PNIPAM具有包容特性,其与DA之间能够通过氢键作用,提高了对DA检测的灵敏度、选择性和稳定性;通过用微分脉冲伏安法(DPV),对于DA浓度检测范围为0.1-60 μmol/L,检测极限为3.34 nmol/L.该课题组也使用了聚乙二醇单甲醚[53]检测DA,结果表明,该聚合物对于DA的检测具有很好的灵敏度和稳定性.Zhang等[54]在石墨烯表面修饰上聚(四苯基卟啉亚铁)和聚(4-苯乙烯磺酸钠)实现了在高浓度的UA和AA的溶液中对于DA的测定,且检出限达到了5.73 nnmol/L.Silva等[55]将聚烯丙胺盐酸盐与金纳米粒子作为平台固定漆酶,通过该复合材料的特定催化作用,使用方波伏安法(SWV)实现对DA的测定,其测定范围为0.49-23.0 μmol/L,检出限达0.26 μmol/L.Vasantha等[56]将聚3,4-乙撑二氧噻吩(PEDOT)聚合物修饰于玻碳电极上,并用于DA的检测.在PEDOT中,由于S原子与AA的阴离子之间存在静电吸引作用,使得AA的氧化峰电位向低电位移动,虽然其与DA阳离子之间的作用为静电排斥,但是由于DA与PEDOT间存在着作用而部分抵消,其氧化峰并无明显移动,这样通过利用AA与DA两者的氧化峰电位不同,来实现二者的同时检测.Sheng等[57]通过一种低成本的电沉积方法,成功制备了PEDOT掺杂非水溶性离子液体1-乙基-3-甲基咪唑双三氟甲磺酰亚胺盐的复合材料.直接电聚合沉积法,大大减少了实验中所需的昂贵的离子液体的用量,大大降低了实验成本.实验结果显示该复合材料的导电率高、性能稳定,而且呈现出高度的纳米微孔结构,对DA具有良好的电化学催化活性,其检测限低至51 nmol/L.
虽然聚合物膜修饰电极为检测DA浓度的发展中起了重要的推动作用,但是有些聚合物的合成过程复杂,有些聚合物还具有一定的毒性,对环境会造成一定的污染.
5.1.2 Nafion膜
Nafion膜(全氟磺酸质子交换膜)是典型的阳离子交换膜,其具有优良的离子交换特性,同时其具备优异的电化学性能、良好的化学性质和机械稳定性[58-60].由于Nafion膜存在阳离子传递通道,有利于DA分子的扩散,同时可以抵消部分中性分子和阴离子,这样就大大缩短了体系的响应时间.同时,由Nafion膜修饰电极之后,传感器对AA和UA的响应灵敏度随着吸附时间的增加而逐渐降低,而对于DA的响应则逐渐增大,因此,Nafion修饰电极对DA的检测具备良好的选择性和高的灵敏度[61].Zhou等[62]直接将Nafion涂于微电极表面,使得微电极表面产生屏障,这样就可以抑制干扰物质扩散到电极表面,而对于DA,其在亲水区域可以通过阳离子通道富集于电极表面,从而达到选择性地富集DA的作用,提高对DA浓度检测的灵敏性和选择性.Hou等[63]通过EDTA与石墨烯键合之后,再跟Nafion作用,并修饰于电极表面(EDTA-rGO-Nafion),由于Nafion膜具有阳离子通道,且EDTA-rGO复合材料表面带有负电荷及其与DA之间能够形成作用,这样就有利于选择性地检测DA,而避免了AA的干扰.其检测范围为0.2-25 μmol/L,检出限达到0.01 μmol/L.Quan等[64]使用Nafion/单壁碳纳米管/聚3-甲基噻吩修饰波碳电极(NF/SWCNT/PMT/GCE)在高浓度的AA和UA存在的情况下实现对DA进行检测,结果表明,在1.0 mmol/L AA和1.0 mmol/L UA存在时,使用DPV对DA浓度进行检测,其浓度范围分别在1.5-20 μmol/L和20-120 μmol/L之间呈现出良好的线性关系,同时该方法可用于实际血液的检测.Hsieh等[65]将立方Pd纳米粒子沉积于还原石墨烯表面(rGO-Pd-NCs),之后将该材料与Nafion混合作用,制备出rGO-Pd-NCs/Nafion纳米复合材料,并修饰于玻碳电极表面,基于该材料对DA的优异选择性,实现对DA的测定,检出限达到了7.0 μmol/L.Tyszczuk-Rotko等[66]制备硼掺杂金刚石纳米粒子,并通过Nafion和Pb2+作用,制备出性能良好的纳米电极膜,该电极可以同时测定人体尿液和血液中的DA和对乙酰氨基酚,对于DA和乙酰氨基酚的检出限达到54 nmol/L和140 nmol/L.
总之,Nafion膜作为电极修饰材料在DA检测方面体现出明显的优势,不过Nafion的价格会相对比较昂贵.
5.2 纳米材料
5.2.1 碳纳米材料
由于碳纳米材料具有多种多样的形态和独特的性质(良好的导电导热性、高的比表面积、稳定的化学和机械性质等),使得其在电、磁、光、热、力学、机械等方面得到广泛的应用,同时碳纳米材料在材料科学、生命科学以及化学分析等领域的应用研究中.也起着非常重要的作用[67-73].如今,碳纳米材料广泛地被用于电极表面修饰,表2显示了一些碳纳米材料所修饰的电极在DA的检测方面所做出的许多贡献[74-97].
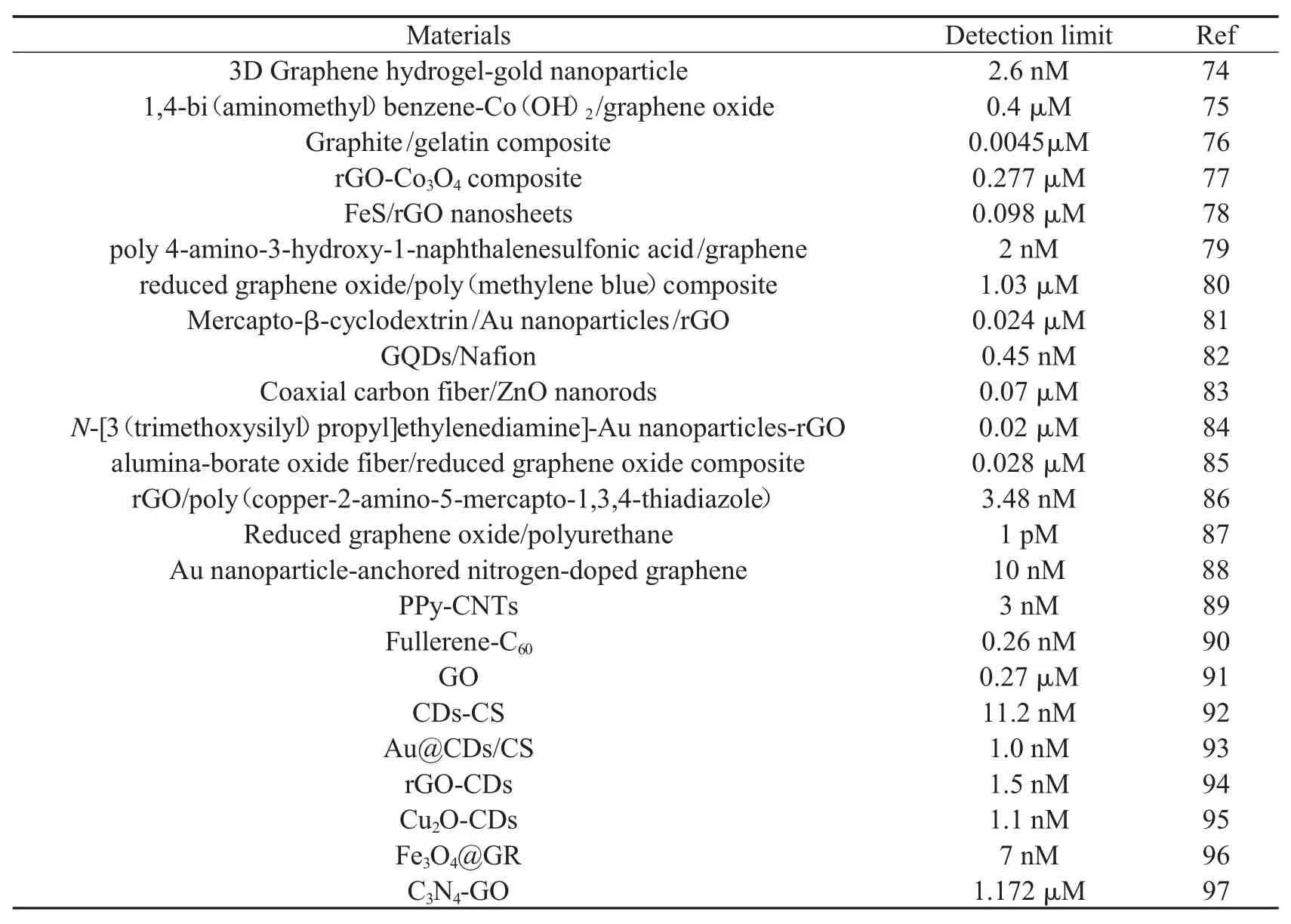
表2 基于碳纳米材料电化学传感器测定DA
Britto等[98]利用溴仿作为粘合剂首次将碳纳米管修饰于电极表面,并用该电极研究DA的氧化行为,通过循环伏安曲线表明了DA在碳纳米管电极上可以进行可逆的氧化还原反应.Li等[89]使用碳纳米管(CNTs)与钛铁试剂掺杂的聚吡咯(PPy)层层组装与电极表面,制备出PPy-CNTs电极.由于掺杂了负离子,该修饰电极对DA的检测具备灵敏度高、选择性好的优点.在最优化条件下用方波伏安法检测多巴胺,该修饰电极检测范围为0.02-100.0 μmol/L,检测限为3 nmol/L.该修饰电极具备背景电流小,重现性好的特点,对多巴胺的检测具有良好的抗干扰能力.Gao等[91]将氧化石墨烯(GO)通过共价固定法固定于玻碳电极(GCE)表面,该电极在大浓度AA的环境下实现对DA的检测,主要是由于DA分子较易与GO通过堆积或者静电作用提高电化学响应.由于AA不能与GO产生作用,同时二者之间存在电子排斥作用,从而导致AA电信号消失,因此,GO/GCE能在AA存在的情况下对DA实现高选择性检测.
本课题组基于CDs-壳聚糖(CDs-CS)复合材料,制备了灵敏、稳定的新型DA电化学传感器,因为CDs表面带有-COOH官能团,其更易与带正电荷的DA作用,同时可以排斥带负电荷AA和UA的干扰,该传感器的检测范围为0.1-30 μmol/L,检出限达到11.2 nmol/L[92].之后我们又制备了Au@CDs[93]纳米材料,由于Au纳米粒子具有良好的导电性能,从而提高了DA传感器的灵敏度.同时,通过制备基于rGO-CDs纳米复合材料的DA传感器[94],利用rGO对DA良好的催化作用,提升了DA传感器的灵敏度和选择性.最近课题组制备出Cu2O-CDs纳米复合材料,利用Cu2O优异的催化性能,结合CDs的良好特性,成功实现了对血液中DA含量的测定(图3)[95].本课题组也制备了核壳的Fe3O4@石墨烯(GR)复合材料用于DA的测定[96],其检测范围为0.020 μmol/L-130.0 μmol/L,检出限达到了7 nmol/L.同时我们也制备了氮化碳-GO纳米复合材料,实现了对DA、AA和UA的同时测定[97].
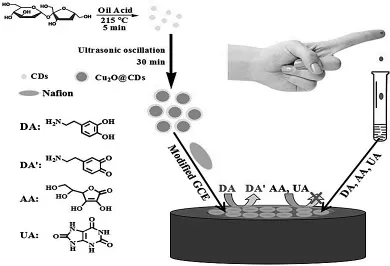
图3 Cu2O-CDs测定DA的机理[95]
5.2.2 其它纳米材料
与碳纳米材料一样,目前还有许多纳米材料如纳米金、纳米氧化铜、纳米氧化锌等,它们具有导电性好、比表面积大等优点,可以提高现有分析方法的灵敏度.近年来越来越多的纳米材料修饰电极被用于DA含量的检测.
Liu等[99]合成二茂铁硫醇盐-Au@Fe3O4纳米材料,并与石墨烯片/壳聚糖耦合(Fc-S-Au@Fe3O4/GS-CS),之后修饰于玻碳电极表面,该修饰电极中Fc-S-Au@Fe3O4和GS-CS都能使体系的信号得到增强,双重信号放大作用使得体系的灵敏度大大地增加(图4),其可以实现对DA、AA、UA以及对乙酰氨基酚(AC)的同时检测.四种物质的检测范围分别为 0.5-50 μmol/L、4.0-400 μmol/L、1.0-300 μmol/L以及 0.3-250 μmol/L.检出限分别为 0.1 μmol/L、1.0 μmol/L、0.2 μmol/L和 0.05 μmol/L.Xia 等[100]通过化学湿选法合成了花瓣状的纳米ZnO材料(f-ZnO),与传统的纳米ZnO相比,f-ZnO具有更大的比表面积以及更好的导电性.将f-ZnO修饰与电极表面,在AA高浓度存在的情况下,使用DPV检测法实现了对DA的灵敏性检测.其检测范围为0.11-180 μmol/L,检出限达0.06 μmol/L.Reddy等[101]通过十六烷基三甲基溴化铵(CTAB)和十二烷基硫酸钠(SDS)共同沉淀的方法合成了不同形状的CuO纳米粒子.之后制备SDS/聚甘氨酸/CuO纳米薄片对电极进行修饰(MCPE),结果显示,MCPE电极在高浓度的AA(250倍)存在下,对于DA的检测具有高的选择性和灵敏性.He等[102]制备了镍铜纳米合金,基于该材料的修饰电极可对DA、AA、UA、鸟嘌呤(G)和腺嘌呤同时测定,其检测范围分别为0.25-40 μmol/L、20-2500 μmol/L、0.5-110 μmol/L、0.5-480 μmol/L 和 0.5-450 μmol/L,检出限分别达到了 0.01 μmol/L、5 μmol/L、0.05 μmol/L、0.1 μmol/L和 0.1 μmol/L.该传感器用于维生素C、多巴胺注入、尿液和DNA中相关分子的测定,结果显示镍铜纳米合金在复杂的生物系统里,具备了优异的测试性能.
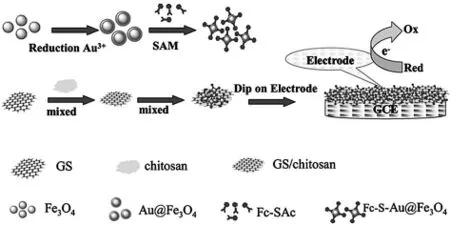
图4 Fc-S-Au@Fe3O4/GS-CS检测DA的机理[99]
6 结论与展望
当然,除了以上介绍的几种方法外,还有电化学发光法[103-104],气相色谱法[105],高效液相色谱-荧光联用法[106]等方法可用于多巴胺含量的检测.随着临床医学的不断发展,对DA分析方法的要求也将会更加苛刻,高灵敏度、高通量、高选择性以及经济环保的分析方法仍然有待进一步研究,因此,目前对DA传感器的研究仍存在以下一些问题亟待解决:
(1)如何开发探讨性能更高效、稳定性更强、更加经济环保的方法用于DA的测定.
(2)合成新型的功能化材料,提升DA传感器的灵敏性和选择性.
(3)测定DA的机理研究还处于研究基础阶段,还有待深入.
(4)扩大DA传感器的应用范围(如:制成微电极,对活体内的DA含量进行实时检测).
[1]CARR F.Dual role for dopamine[J].Nature Reviews Neuroscience,2016,17(1):2-3.
[2]SULZER D.How addictive drugs disrupt presynaptic dopamine neurotransmission[J].Neuron, 2011,69(4):628-649.
[3]XING F,HU X,JIANG J,et al.A meta-analysis of low-dose dopamine in heart failure[J].International Journal of Cardiology,2016,222:1003-1011.
[4]BENES F M.Carlsson and the discovery of dopamine[J].Trends in Pharmacological Sciences,2001,22(1):46-47.
[5]HAMID A A,PETTIBONE J R,MABROUK O S,et al.Mesolimbic dopamine signals the value of work[J].Nature Neuroscience,2016,19(1):117.
[6]LIANG L,WANG R,ZHANG Z.The effect of dopamine on working memory[J].Neural Processing Letters,2012,35(3):257-263.
[7]董钰明,陈晓峰,李春兰,等.微乳液电动毛细管色谱-激光诱导荧光法测定中药和风湿性心脏病患者血浆中的肾上腺素和多巴胺[J].兰州大学学报(自然科学版),2009,45(3):77-81.
[8]LIU J M,WANG X X,CUI M L,et al.A promising non-aggregation colorimetric sensor of AuNRs-Ag+for determination of dopamine[J].Sensors and Actuators B:Chemical,2013,176:97-102.
[9]VOLKOW N D,WANG G J,FOWLER J S,et al.Addiction:beyond dopamine reward circuitry[J].Proceedings of the National Academy of Sciences,2011,108(37):15037-15042.
[10]GEORGE O,LE M M,KOOB G F.Allostasis and addiction:role of the dopamine and corticotropinreleasing factor systems[J].Physiology and Behavior,2012,106(1):58-64.
[11]GRACE A A.Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression[J].Nature Reviews.Neuroscience,2016,17(8):524.
[12]BEAULIEU J M,GAINETDINOV R R.The physiology,signaling,and pharmacology of dopamine receptors[J].Pharmacological Reviews,2011,63(1):182-217.
[13]ZHANG H,HUANG Y,HU S,et al.Fluorescent probes for“off-on”sensitive and selective detection of mercury ions and L-cysteine based on graphitic carbon nitride nanosheets[J].Journal of Materials Chemistry C,2015,3(9):2093-2100.
[14]王永刚,杨光瑞,马雪青,等.荧光光谱法和分子模拟技术研究考马斯亮蓝G-250与牛血清白蛋白的相互作用[J].光谱学与光谱分析,2017,37(8):2474-2479.
[15]LIN Y,YIN M,PU F,et al.DNA-templated silver nanoparticles as a platform for highly sensitive and selective fluorescence turn-on detection of dopamine[J].Small,2011,7(11):1557-1561.
[16]ZHANG X,ZHU Y,LI X,et al.A simple,fast and low-cost turn-on fluorescence method for dopamine detection using in situ reaction[J].Analytica Chimica Acta,2016,944:51-56.
[17]DU Y,GUO S.Chemically doped fluorescent carbon and graphene quantum dots for bioimaging,sensor, catalytic and photoelectronic applications[J].Nanoscale,2016,8(5):2532-2543.
[18]HOU Y,LU Q,DENG J,et al.One-pot electrochemical synthesis of functionalized fluorescent carbon dots and their selective sensing for mercury ion[J].Analytica Chimica Acta,2015,866:69-74.
[19]LIU X,HU X,XIE Z,et al.In situ bifunctionalized carbon dots with boronic acid and amino groups for ultrasensitive dopamine detection[J].Analytical Methods,2016,8(15):3236-3241.
[20]ZHOU X,MA P,WANG A,et al.Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids[J].Biosensors and Bioelectronics,2015,64:404-410.
[21]ZHU L,XU G,SONG Q,et al.Highly sensitive determination of dopamine by a turn-on fluorescent biosensor based on aptamer labeled carbon dots and nano-graphite[J].Sensors and Actuators B:Chemical,2016,231:506-512.
[22]LIN F,GUI C,WEN W,et al.Dopamine assay based on an aggregation-induced reversed inner filter effect of gold nanoparticles on the fluorescence of graphene quantum dots[J].Talanta,2016,158:292-298.
[23]GUO X,WU F,NI Y,et al.Synthesizing a nano-composite of BSA-capped Au nanoclusters/graphitic carbon nitride nanosheets as a new fluorescent probe for dopamine detection[J].Analytica Chimica Acta,2016,942:112-120.
[24]NIU S,FANG Y,ZHANG K,et al.Determination of dopamine using the fluorescence quenching of 2,3-diaminophenazine[J].Instrumentation Science&Technology,2017,45(1):101-110.
[25]WANG L,MIAO H,ZHONG D,et al.Synthesis of dopamine-mediated Cu nanoclusters for sensing and fluorescent coding[J].Analytical Methods,2016,8(1):40-44.
[26]ZHAO J, ZHAO L, LAN C, et al.Graphene quantum dots as effective probes for label-free fluorescence detection of dopamine[J].Sensors and Actuators B:Chemical,2016,223:246-251.
[27]TASHKHOURIAN J,DEHBOZORGI A.Determination of dopamine in the presence of ascorbic and uric acids by fluorometric method using graphene quantum dots[J].Spectroscopy Letters,2016,49(5):319-325.
[28]TSUNODA M,AOYAMA C,NOMURA H,et al.Simultaneous determination of dopamine and 3,4-dihydroxyphenylacetic acid in mouse striatum using mixed-mode reversed-phase and cation-exchange high-performance liquid chromatography[J].Journal of Pharmaceutical and Biomedical Analysis,2010,51(3):712-715.
[29]CHEN J,SHI Y P,LIU J Y.Determination of noradrenaline and dopamine in Chinese herbal extracts from Portulaca oleracea L.by high-performance liquid chromatography[J].Journal of Chromatography A,2003,1003(1):127-132.
[30]RIBEIRO R P,GASPARETTO J C,DE O V R,et al.Simultaneous determination of levodopa,carbidopa,entacapone,tolcapone,3-O-methyldopa and dopamine in human plasma by an HPLC MS/MS method[J].Bioanalysis,2015,7(2):207-220.
[31]AMINI A.Recent developments in chiral capillary electrophoresis and applications of this technique to pharmaceutical and biomedical analysis[J].Electrophoresis,2001,22(15):3107-3130.
[32]马健,张明洲,李晓,等.高效毛细管电泳法用于猪尿中莱克多巴胺含量的测定[J].浙江农业学报,2006,18(5):333-336.
[33]THABANO J R E,BREADMORE M C,HUTCHINSON J P,et al.Silica nanoparticle-templated methacrylic acid monoliths for in-line solid-phase extraction-capillary electrophoresis of basic analytes[J].Journal of Chromatography A,2009,1216(25):4933-4940.
[34]FANG H,PAJSKI M L,ROSS A E,et al.Quantitation of dopamine,serotonin and adenosine content in a tissue punch from a brain slice using capillary electrophoresis with fast-scan cyclic voltammetry detection[J].Analytical Methods,2013,5(11):2704-2711.
[35]ZHAO Y,ZHAO S,HUANG J,et al.Quantum dot-enhanced chemiluminescence detection for simultaneous determination of dopamine and epinephrine by capillary electrophoresis[J].Talanta,2011,85(5):2650-2654.
[36]LI S X,LIN X,ZHENG F Y,et al.Constituting fully integrated visual analysis system for Cu(II)on TiO2/cellulose paper[J].Analytical Chemistry,2014,86(14):7079-7083.
[37]LENG Y,XIE K,YE L,et al.Gold-nanoparticle-based colorimetric array for detection of dopamine in urine and serum[J].Talanta,2015,139:89-95.
[38]TAO Y,LIN Y,REN J,et al.A dual fluorometric and colorimetric sensor for dopamine based on BSA-stabilized Aunanoclusters[J].Biosensors and Bioelectronics,2013,42:41-46.
[39]WEN D,LIU W,HERRMANN A K,et al.Simple and sensitive colorimetric detection of dopamine based on assembly of cyclodextrin-modified Au nanoparticles[J].Small,2016,12(18):2439-2442.
[40]LU P,LIU Q,XIONG Y,et al.Nanosheets-assembled hierarchical microstructured Ni(OH)2hollow spheresforhighlysensitive enzyme-free glucose sensors[J].Electrochimica Acta,2015,168:148-156.
[41]LU P,LEI Y,LU S,et al.Three-dimensional roselike α-Ni(OH)2assembled from nanosheet building blocks for non-enzymatic glucose detection[J].Analytica chimica acta,2015,880:42-51.
[42]LIU J,MORRIS M D,MACAZO F C,et al.The current and future role of aptamers in electroanalysis[J].Journal of the Electrochemical Society,2014,161(5):H301-H313.
[43]LIUJ,WAGANS,DÁVILA-MORRISM,etal.Achievingreproducibleperformanceofelectrochemical,folding aptamer-based sensors on microelectrodes:challenges and prospects[J].Analytical chemistry,2014,86(22):11417-11424.
[44]JETT S E,BONHAM A J.Reusable electrochemical DNA biosensor for the detection of waterborne uranium[J].Chem Electro Chem,2017,4(4):843-845.
[45]WANG Y H,YU C M,GU H Y,et al.The hemoglobin-modified electrode with chitosan/Fe3O4nanocomposite for the detection of trichloroacetic acid[J].Journal of Solid State Electrochemistry,2016,20(5):1337-1344.
[46]CHANDRA U,SWAMY B E K,KUMAR M,et al.Simple flame etching of pencil electrode for dopamine oxidation in presence of ascorbic acid and uric acid[J].International Journal of Nanotechnology,2017,14(9/10/11):739-751.
[47]ZHANG H,DAI P,HUANG L,et al.A nitrogen-doped carbon dot/ferrocene@ β-cyclodextrin composite as an enhanced material for sensitive and selective determination of uric acid[J].Analytical Methods,2014,6(8):2687-2691.
[48]SAJID M,NAZAL M K,MANSHA M,et al.Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid:a review[J].TrAC Trends in Analytical Chemistry,2016,76:15-29.
[49]YU Y,YU C,YIN T,et al.Functionalized poly(ionic liquid)as the support to construct a ratiometric electrochemical biosensor for the selective determination of copper ions in AD rats[J].Biosensors and Bioelectronics,2017,87:278-284.
[50]BAGHAYERIM,ROUHIM,LAKOURAJ M M,et al.Bioelectrocatalysis of hydrogen peroxide based on immobilized hemoglobin onto glassy carbon electrode modified with magnetic poly(indole-co-thiophene)nanocomposite[J].Journal of Electroanalytical Chemistry,2017,784:69-76.
[51]KARIM-NEZHAD G,KHORABLOU Z,DORRAJI P S.Modification of glassy carbon electrode with a bilayer of multiwalled carbon nanotube/poly(l-arginine)in the presence of surfactant:application to discrimination and simultaneous electrochemical determination of dihydroxybenzene isomers[J].Journal of The Electrochemical Society,2016,163(7):B358-B365.
[52]WU Y,DOU Z,LIU Y,et al.Dopamine sensor development based on the modification of glassy carbon electrode with β-cyclodextrin-poly(N-isopropylacrylamide)[J].Rsc Advances,2013,3(31):12726-12734.
[53]WU Y,CUI L,LIU Y,et al.A dopamine sensor based on a methoxypolyethylene glycol polymer covalently modified glassy carbon electrode[J].Analyst,2013,138(4):1204-1211.
[54]ZHANG B,WANG Y,LI M,et al.Graphene-supported poly[iron(II)tetraphenylporphyrin]hybrid fabricated by a solvothermally assistedassembly method and its application for the detection of dopamine[J].Journal of Electroanalytical Chemistry,2015,743:10-17.
[55]SILVA T R,VIEIRA I C.A biosensor based on gold nanoparticles stabilized in poly(allylamine hydrochloride)and decorated with laccase for determination of dopamine[J].Analyst,2016,141(1):216-224.
[56]VASANTHA V S,CHEN S M.Electrocatalysis and simultaneous detection of dopamine and ascorbic acid using poly(3,4-ethylenedioxy)thiophene film modified electrodes[J].Journal of Electroanalytical Chemistry,2006,592(1):77-87.
[57]SHENG G,XU G,XU S,et al.Cost-effective preparation and sensing application of conducting polymerPEDOT/ionic liquid nanocomposite with excellentelectrochemicalproperties[J].RSC Advances,2015,5(27):20741-20746.
[58]WEI C,HUANG Q,HU S,et al.Simultaneous electrochemical determination of hydroquinone,catechol and resorcinol at Nafion/multi-walled carbon nanotubes/carbon dots/multi-walled carbon nanotubes modified glassy carbon electrode[J].Electrochimica Acta,2014,149:237-244.
[59]ER E, ÇELIKKAN H, ERK N.Highly sensitive and selective electrochemical sensor based on high-quality graphene/nafion nanocomposite for voltammetric determination of nebivolol[J].Sensors and Actuators B:Chemical,2016,224:170-177.
[60]YANY,HUANGQ,WEI C,et al.Microwave-assisted synthesis of carbon dots-zinc oxide/multi-walled carbon nanotubes and their application in electrochemical sensors for the simultaneous determination of hydroquinone and catechol[J].RSC Advances,2016,6(116):115317-115325.
[61]BOULKROUNE M,CHIBANI A,GENESTE F.Monocopper complex based on N-tripodal ligand immobilized in a Nafion@film for biomimetic detection of catechols:application to dopamine[J].Electrochimica Acta,2016,221:80-85.
[62]ZHOU S,LIU C,SONG Y,et al.Highly selective and sensitive determination of dopamine using nafion coated microelectrode arrays[J].Journal of Nanoscience and Nanotechnology,2013,13(2):1598-1601.
[63]HOU S,KASNER M L,SU S,et al.Highly sensitive and selective dopamine biosensor fabricated with silanized graphene[J].The Journal of Physical Chemistry C,2010,114(35):14915-14921.
[64]DAI L T,TRAM P T N,BINH N H,et al.Electrochemically selective determination of dopamine in the presence of ascorbic and uric acids on the surface of the modified Nafion/single wall carbon nanotube/poly(3-methylthiophene)glassy carbon electrodes[J].Colloids and Surfaces B:Biointerfaces,2011,88(2):764-770.
[65]HSIEH Y S,HONG B D,LEE C L.Non-enzymatic sensing of dopamine using a glassy carbon electrode modified with a nanocomposite consisting of palladium nanocubes supported on reduced graphene oxide in a nafion matrix[J].Microchimica Acta,2016,183(2):905-910.
[66]TYSZCZUK-ROTKO K,SADOK I.The new application of boron doped diamond electrode modified with nafion and lead films for simultaneous voltammetric determination of dopamine and paracetamol[J].Electroanalysis,2016,28(9):2178-2187.
[67]黄启同,林小凤,李飞明,等.碳量子点的合成与应用[J].化学进展,2015,27(11):1604-1614.
[68]郝玉翠,李艾.Pt-Fe(Ⅲ)/多壁碳纳米管修饰电极测定亚硫酸根[J].中国测试,2015(7):41-45.
[69]HUANG Q,LIN X,ZHU J J,et al.Pd-Au@carbon dots nanocomposite:Facile synthesis and application as an ultrasensitive electrochemical biosensor for determination of colitoxin DNA in human serum[J].Biosensors and Bioelectronics,2017,94:507-512.
[70]YANG H,LI F,ZOU C,et al.Sulfur-doped carbon quantum dots and derived 3D carbon nanoflowers are effective visible to near infrared fluorescent probes for hydrogen peroxide[J].Microchimica Acta,2017,184(7):2055-2062.
[71]HUANG Q,LIN X,LIN C,et al.Ultrasensitive-electrochemical sensor for the detection of luteolin in chrysanthemums and peanut shells using an Au/Pd/reduced graphene oxide nanofilm[J].Analytical Methods,2016,8(33):6347-6352.
[72]LI Q,HUANG Q,ZHU J J,et al.Carbon dots-quinoline derivative nanocomposite:facile synthesis and application as a“turn-off”fluorescent chemosensor for detection of Cu2+ions in tap water[J].RSC Advances,2016,6(90):87230-87236.
[73]胡小蔚,池凌飞,冼志科.锡掺杂氧化铟纳米线的制备及场发射性能研究[J].汕头大学学报(自然科学版),2015,30(3):76-80.
[74]ZHU Q,BAO J,HUO D,et al.3D Graphene hydrogel-gold nanoparticles nanocomposite modified glassy carbon electrode for the simultaneous determination of ascorbic acid,dopamine and uric acid[J].Sensors and Actuators B:Chemical,2017,238:1316-1323.
[75]EJAZ A,JOO Y,JEON S.Fabrication of 1,4-bis(aminomethyl)benzene and cobalt hydroxide@graphene oxide for selective detection of dopamine in the presence of ascorbic acid and serotonin[J].Sensors and Actuators B:Chemical,2017,240:297-307.
[76]RAJKUMAR C,THIRUMALRAJ B,CHEN S M,et al.A simple preparation of graphite/gelatin composite for electrochemical detection of dopamine[J].Journal of Colloid and Interface Science,2017,487:149-155.
[77]NUMAN A,SHAHID M M,OMAR F S,et al.Facile fabrication of cobalt oxide nanograin-decorated reduced graphene oxide composite as ultrasensitive platform for dopamine detection[J].Sensors and Actuators B:Chemical,2017,238:1043-1051.
[78]LIU X,SHANGGUAN E,LI J,et al.A novel electrochemical sensor based on FeS anchored reduced graphene oxide nanosheets for simultaneous determination of dopamine and acetaminophen[J].Materials Science and Engineering:C,2017,70:628-636.
[79]RAJ M,GUPTA P,GOYAL R N,et al.Graphene/conducting polymer nano-composite loaded screen printed carbon sensor for simultaneous determination of dopamine and 5-hydroxytryptamine[J].Sensors and Actuators B:Chemical,2017,239:993-1002.
[80]GORLE D B,KULANDAINATHAN M A.Electrochemical sensing of dopamine at the surface of a dopamine grafted graphene oxide/poly(methylene blue)composite modified electrode[J].RSC Advances,2016,6(24):19982-19991.
[81]CHANG Z,ZHOU Y, HAO L,et al.Simultaneous determination of dopamine and ascorbic acid using β-cyclodextrin/Au nanoparticles/graphene-modified electrodes[J].Analytical Methods,2017,9(4):664-671.
[82]PANG P,YAN F,LI H,et al.Graphene quantum dots and Nafion composite as an ultrasensitive electrochemicalsensorforthe detection ofdopamine[J].AnalyticalMethods,2016,8(24):4912-4918.
[83]YANG C,GU B,ZHANG D,et al.Coaxial carbon fiber/ZnO nanorods as electrodes for the electrochemical determination of dopamine[J].Analytical Methods,2016,8(3):650-655.
[84]VINOTH V,WU J J,ANANDAN S.Sensitive electrochemical determination of dopamine and uric acid using AuNPs(EDAS)-rGO nanocomposites[J].Analytical Methods,2016,8(22):4379-4390.
[85]BABAEI A,SOHRABI M.Selective simultaneous determination of levodopa and acetaminophen in the presence of ascorbic acid using a novel TiO2hollow sphere/multi-walled carbon nanotube/poly-aspartic acid composite modified carbon paste electrode[J].Analytical Methods,2016,8(5):1135-1144.
[86]LI Y,GU Y,ZHENG B,et al.A novel electrochemical biomimetic sensor based on poly(Cu-AMT)with reduced graphene oxide for ultrasensitive detection of dopamine[J].Talanta,2017,162:80-89.
[87]VILIAN A T E,AN S,CHOE S R,et al.Fabrication of 3D honeycomb-like porous polyurethanefunctionalized reduced graphene oxide for detection of dopamine[J].Biosensors and Bioelectronics,2016,86:122-128.
[88]THANH T D,BALAMURUGAN J,LEE S H,et al.Effective seed-assisted synthesis of gold nanoparticles anchored nitrogen-doped graphene for electrochemical detection of glucose and dopamine[J].Biosensors and Bioelectronics,2016,81:259-267.
[89]LI J,LIU Y,WEI W,et al.Fabrication of tiron doped poly-pyrrole/carbon nanotubes on low resistance monolayer-modified glassy carbon electrode for selective determination of dopamine[J].Analytical Letters,2011,44(7):1226-1240.
[90]GOYAL R N,GUPTA V K,BACHHETI N,et al.Electrochemical sensor for the determination of dopamine in presence of high concentration of ascorbic acid using a Fullerene-C60 coated gold electrode[J].Electroanalysis,2008,20(7):757-764.
[91]GAO F,CAI X,WANG X,et al.Highly sensitive and selective detection of dopamine in the presence of ascorbic acid at graphene oxide modified electrode[J].Sensors and Actuators B:Chemical,2013,186:380-387.
[92]HUANG Q,HU S,ZHANG H,et al.Carbon dots and chitosan composite film based biosensor for the sensitive and selective determination of dopamine[J].Analyst,2013,138(18):5417-5423.
[93]HUANG Q,ZHANG H,HU S,et al.A sensitive and reliable dopamine biosensor was developed based on the Au@carbon dots-chitosan composite film[J].Biosensors and Bioelectronics,2014,52:277-280.
[94]HU S,HUANG Q,LIN Y,et al.Reduced graphene oxide-carbon dots composite as an enhanced material forelectrochemicaldetermination ofdopamine[J].Electrochimica Acta,2014,130:805-809.
[95]HUANG Q,LIN X,LIN C,et al.A high performance electrochemical biosensor based on Cu2O-carbon dots for selective and sensitive determination of dopamine in human serum[J].RSC Advances,2015,5(67):54102-54108.
[96]ZHANG W, ZHENG J, SHI J, et al.Nafion covered core-shell structured Fe3O4@graphene nanospheres modified electrode for highly selective detection of dopamine[J].Analytica Chimica Acta,2015,853:285-290.
[97]ZHANG H,HUANG Q,HUANG Y,et al.Graphitic carbon nitride nanosheets doped graphene oxide for electrochemical simultaneous determination of ascorbic acid,dopamine and uric acid[J].Electrochimica Acta,2014,142:125-131.
[98]BRITTO P J,SANTHANAM K S V,AJAYAN P M.Carbon nanotube electrode for oxidation of dopamine[J].Bioelectrochemistry and Bioenergetics,1996,41(1):121-125.
[99]LIU M,CHEN Q,LAI C,et al.A double signal amplification platform for ultrasensitive and simultaneous detection of ascorbic acid,dopamine,uric acid and acetaminophen based on a nanocompositeof ferrocene thiolate stabilized Fe3O4@Au nanoparticles with graphene sheet[J].Biosensors and Bioelectronics,2013,48:75-81.
[100]XIA C,WANG N,WANG L,et al.Synthesis of nanochain-assembled ZnO flowers and their application to dopamine sensing[J].Sensors and Actuators B:Chemical,2010,147(2):629-634.
[101]REDDY S,SWAMY B E K,JAYADEVAPPA H.CuO nanoparticle sensor for the electrochemical determination of dopamine[J].Electrochimica Acta,2012,61:78-86.
[102]HE W,DING Y,JI L,et al.A high performance sensor based on bimetallic NiCu nanoparticles for the simultaneous determination of five species of biomolecules[J].Sensors and Actuators B:Chemical,2017,241:949-956.
[103]FU X,TAN X,YUAN R, et al.A dual-potential electrochemiluminescence ratiometric sensor for sensitive detection of dopamine based on graphene-CdTe quantum dots and self-enhanced Ru(II)complex[J].Biosensors and Bioelectronics,2017,90:61-68.
[104]XIN Y,LI Z,WU W,et al.Recognition unit-free and self-cleaning photoelectrochemical sensing platform on TiO2nanotube photonic crystals for sensitive and selective detection of dopamine release from mouse brain[J].Biosensors and Bioelectronics,2017,87:396-403.
[105]TIAN C Y,XU J J,CHEN H Y.Enhanced electrochemiluminescence of TiO2nanoparticles modified electrode by Nafion film and its application in selective detection of dopamine[J].Electroanalysis,2013,25(5):1294-1300.
[106]SARAJI M,SHAHVAR A.Selective micro solid-phase extraction of epinephrine,norepinephrine and dopamine from human urine and plasma using aminophenylboronic acid covalently immobilized on magnetic nanoparticles followed by high-performance liquid chromatography-fluorescence detection[J].Analytical Methods,2016,8(4):830-839.

