Research Progress in Catalytic Cracking Reaction of Tetralin and Decalin
Li Xiao; Xie Chaogang; Wei Xiaoli
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
1 Introduction
Due to the increasingly heavy and inferior quality of crude oil, the proportion of heavy oil in the catalytic cracking feed is increasing, and the content of aromatic hydrocarbons in the raw materials also increases accordingly. During the catalytic cracking reaction, polycyclic aromatic hydrocarbons are prone to condensation reaction. When coexisting with olefins, alkanes and cycloalkanes, the polycyclic aromatic hydrocarbons also hinder the cracking of other hydrocarbons and reduce the yield of high-value products[1-2]. Moreover, the emissions of particulate matter in the vehicle exhaust are related to the content of aromatics in the liquid fuel[3]. Catalytic hydrogenation is an important means of deep processing of heavy oil to obtain light oil. The integrated catalytic hydrogenation and catalytic cracking technology has been gradually used by refineries[4-6]. After hydrogenation, the H/C ratio of the feedstock is increased, and the heteroatoms content is reduced with the nature of the raw material being greatly improved. The content of cycloalkanes and naphthenic aromatics in the hydrogenated oil is increased as compared to the conventional catalytic cracking feedstock. Corma, et al.[7]have come to conclusion that the reactivity of the hydrogenated products emanating from catalytic cracking of the tricyclic aromatics is similar to that of the hydrogenated bicyclic aromatics. Therefore,most researchers use naphthalene hydrogenation products—tetralin and decalin as the model compounds to study the catalytic cracking reaction of hydrogenated products obtained through catalytic hydrogenation of polycyclic aromatic hydrocarbons[7-10]. In this paper, the reaction behavior of naphthalene hydrogenation products—tetralin and decalin—have been reviewed from three aspects, viz.:the reaction mechanism, the effect of catalytic material,and the process parameters, so as to provide basic information for the study of catalytic cracking reaction of hydrogenated aromatics.
2 Catalytic Cracking Reaction Mechanism of Tetralin and Decalin
Tetralin and decalin are products obtained by hydrogenation of naphthalene. Tetralin contains a naphthenic ring and an aromatic ring. Decalin has twonaphthenic rings. Judging from the spatial structure of tetralin, the six carbon atoms of benzene ring and two carbon atoms attached to the benzene ring are substantially located in the same plane, leaving two carbon atoms, with one being distinctly downward, and the other being slightly upturned. So the naphthenic ring is similar to the chair structure (with the specific structure shown in Figure 1). The ten carbon atoms of decalin are not in the same plane, showing two-chair structures (with the specific structure shown in Figure 2). It is clear that the differences in functional groups and spatial structure will lead to different reactivity of tetralin and decalin[11].
Figure 1 Three-dimensional structure of tetralin
Figure 2 Three-dimensional structure of decalin
2.1 Mechanism of catalytic cracking of tetralin
The reaction mechanism of tetralin at different temperature is different. Koichi Sato, et al.[12]studied the reaction mechanism of tetralin with hydrogen at 250-350 °C. They believed that the reaction of tetralin could be initiated by two different mechanisms, namely: the unimolecular mechanism and the bimolecular mechanism.The bimolecular mechanism stated that the first step in the reaction was the formation of phenylbutyl tetralin(PBT) from two tetralin molecules adsorbed on the acid sites of USY zeolite. PBT was consecutively decomposed into benzene and the tetrahydronaphthylbutyl cation. The latter, which was not stable, did not yield butyltetralin but was converted into OHA or OHP. OHA and OHP were subsequently isomerized or cracked to alkyltetralin,as shown in Figure 3[13]. Corma, et al.[7]investigated the catalytic cracking of tetralin on the Y zeolite catalyst at 450—550 °C. As a result, almost no phenylbutyl tetralin(PBT) intermediate was found. Therefore, he believed that the catalytic cracking reaction of tetralin followed the unimolecular reaction mechanism under the catalytic cracking reaction conditions. Townsend[14]found a small amount of hydrogen in the tetralin catalytic cracking reaction, which was also consistent with Corma’s results.In conclusion, the catalytic cracking reaction of tetralin follows the unimolecular reaction mechanism. The aromatic rings or the naphthenic rings are adsorbed on the Brønsted sites of the zeolite catalyst. The β-scission and protolytic cracking of the resultant carbenium ions can explain the opening of the naphthenic ring, forming alkenyl and alkylbenzenes,respectively. On the other hand, the isomerization reaction can occur by means of a PCP mechanism, which may be responsible for contraction of the ring and formation of methylindanes and methylindenes. Dealkylation reactions produce gaseous molecules and aromatics with fewer than 10 carbon atoms, while disproportionation reactions between carbenium ions and aliphatic species or between aromatics and olefins can explain the formation of aliphatic and aromatic compounds with more than 4 and 10 carbon atoms,respectively. The formation of naphthalene as a primary product is explained by the fast hydrogen transfer between tetralin and the adsorbed carbenium ions formed during the isomerization and ring-opening of tetralin. The main reaction path is shown in Figure 4[7].
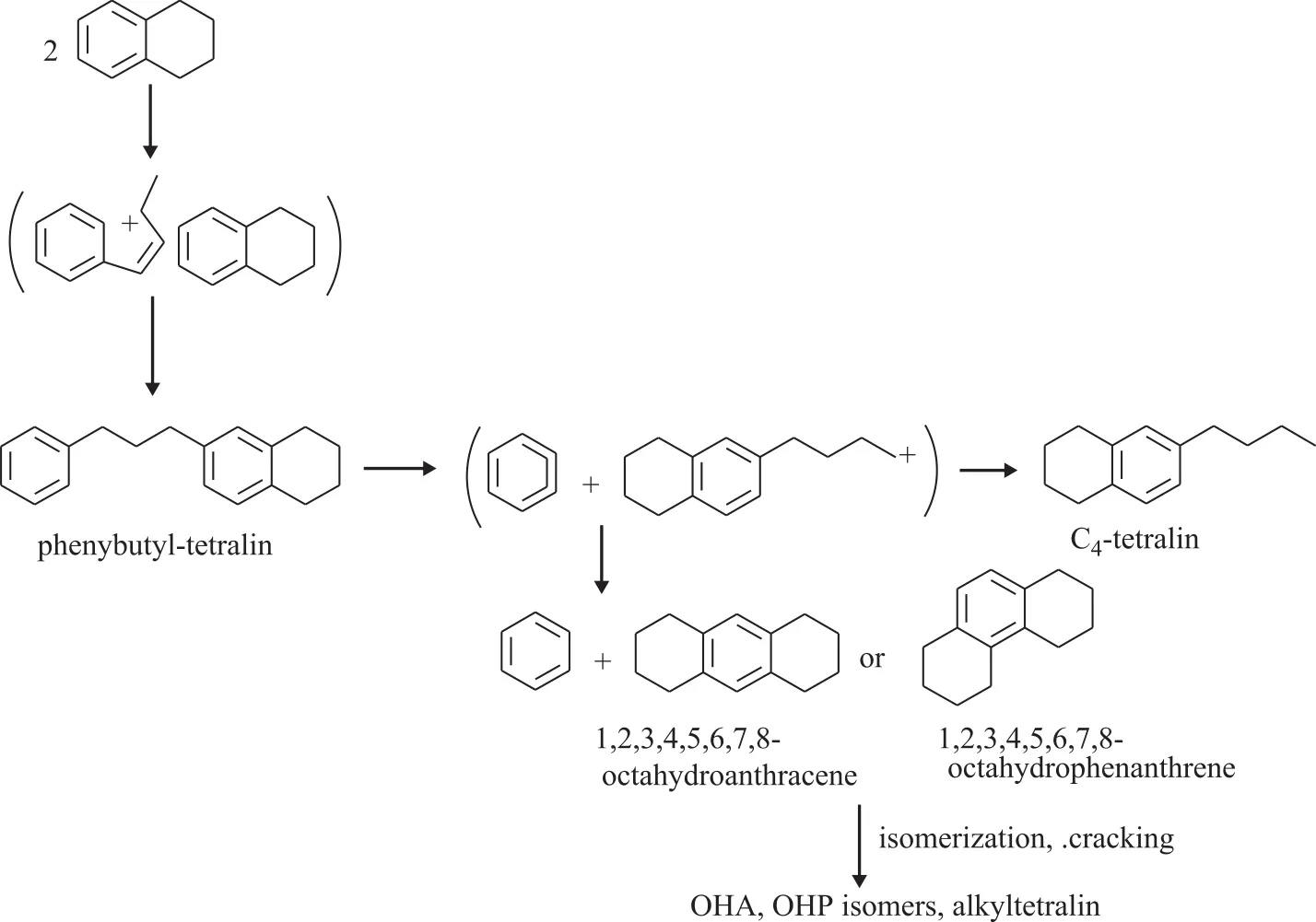
Figure 3 Mechanism of bimolecular reaction of tetralin at low temperature[12]
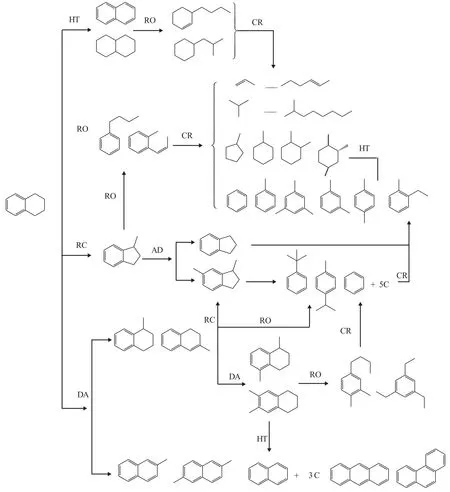
Figure 4 Main reaction pathways for hydrocracking of tetralin
2.2 Mechanism of catalytic cracking of decalin
The reaction mechanism of decalin at different reaction temperatures is different. Kubička, et al.[15-16]investigated the reaction mechanism of decalin with hydrogen, at 200-300 °C. Results showed that the primary reaction of decalin was the skeleton isomerization reaction. The secondary reaction is the ring-opening of the isomerized products. However, Corma, et al.[7]studied the catalytic cracking of decalin at 450-550 °C. He suggests that the naphthenic ring of decalin is attacked at the Brønsted sites to form carbenium ions, followed by β-scission reaction,isomerization reaction, hydrogen transfer, alkyl transfer reaction, etc. Al-Sabawi, et al.[17]demonstrated that the isomerization reaction rate was significantly higher than that of cracking reaction at lower reaction temperature[18].In conclusion, the reaction is initiated by the attack at a
Brønsted site on a carbon–carbon bond of the decalin to form a carbonium ion, which cracks protolytically against an alkylnaphthene carbenium ion. The formation of gaseous molecules and naphthenes that are lighter than the feed can be explained on the theory of unimolecular reactions,involving the isomerization and β-scission reactions of the alkylnaphthene carbenium ions. Meanwhile, the bimolecular hydrogen transfer and hydride transfer can produce C10alkyl, alkenyl, or naphthenic-aromatics.Other bimolecular disproportionation reactions, involving transalkylation, are also responsible for the formation of aromatics with more carbon atoms than the feed. The main reaction path is shown in Figure 5[7].
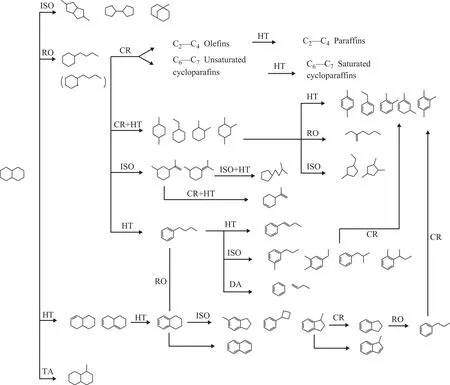
Figure 5 Main reaction pathways for catalytic cracking of decalin
The conversion rate of tetralin is significantly lower than that of decalin under the same reaction conditions. Judging from the view of products distribution, the yields of gasoline, diesel, and coke are higher when tetralin is used as the feed, while the yield of gas is higher when decalin is used as the feed. The yield of aromatics from tetralin is as high as 90%, while that from decalin is less than 40%[19],which indicates that the rate of hydrogen transfer reaction in tetralin is significantly higher than that in decalin. Based on the different reaction performance, Gong, et al.[6,20]have proposed the selective hydrogenation of naphthalene contained in LCO to form tetralin rather than decalin. They then further optimize the reaction path of tetralin in order to maximize the selectivity of ring-opening reaction to attain higher gasoline yield through altering the reaction conditions.Therefore, it is necessary to control the hydrogenation depth of feed oil to be catalytically cracked in an attempt to optimize the properties of catalytic cracking products and maximize the yield of target products.
3 Effect of Catalyst on Catalytic Cracking of Tetralin and Decalin
3.1 Effect of pore structure
The pore structure of zeolite has a selective effect on the reactant molecules and product molecules. If the molecular size is smaller than that of pore structure,molecules can enter the inside of zeolite. If the two sizes are equivalent, molecules can still enter the inside of zeolite, but there is diffusion resistance. If the molecular size is larger than that of pore structure, it is difficult to enter the inside of zeolite[21-22].
Corma, et al.[7]studied the effect of catalyst pore topology on product distribution. A set of zeolites with mediumsized (ZSM-5, MCM-22, ITQ-2), large (USY, beta),and ultra-large pores (UTD-1), as well as a mesoporous MCM-41, are used as catalysts at 450 °C. UTD-1 has higher activity per active site than any of the other zeolites studied and is highly selective for bimolecular reactions(hydrogen transfer) owing to the possibility of fitting two molecules that are in close proximity in the channels. The condensed rings of aromatic naphthenes will be cracked on large pores of the USY or beta zeolites, leading to the opening of naphthenic ring and a minimized dealkylation of the alkylaromatics formed. The author also pointed out if the aim is to crack LCO in the FCC unit and to maximize the amount of propene formed, a “cocktail” of zeolites with large- and medium-pores is desirable. The opening of naphthenic ring occurred over the zeolite with large-pored channels (preferably beta zeolite) and the cracking of the alkyl side-chain of branched naphthenics or alkylaromatics took place over the zeolite with 10 MR main channels such as ZSM-5 or MCM-22 zeolite.
Arribas M A, et al.[23]synthesized ITQ-21 zeolite with six 12-membered-ring windows and three-dimensional macroporous structures. This zeolite can reduce the occurrence of secondary cracking reaction, increase the yield and selectivity of ring-opening products, and is conducive to the diffusion of ring-opening products.Judging from the view of thermal effect, Jiang Shuai[24]showed the difference between reactions of tetralin on the ZSM-5 zeolite and the USY zeolite. The temperature of reactor rises rapidly when the USY zeolite is used. The thermal effect using ZSM-5 zeolite is a stark contrast to the USY zeolite. The dominant reaction type on the ZSM-5 catalyst is an endothermic ring-opening reaction, while the predominant dehydrogenated condensation reaction on the USY catalyst is exothermic. This is why the thermal effects of the two zeolites are so different.
In a word, the three-dimensional zeolite catalyst with 12-membered-ring structure can facilitate the diffusion of decalin and tetralin as well as their products. This also improves the reaction conversion rate and reduces excessive cracking. Although conversion is high when an ultra-large pores zeolite catalyst is used, more hydrogen transfer reaction takes place, which is not conducive to the product distribution. If the desired product is propylene, a “cocktail” of large- (beta) and medium-pore(ZSM-5) zeolites is preferred.
3.2 Effect of catalyst acidity
The acidity of zeolite catalyst directly affects its catalytic performance. It is generally believed that the ring-opening reactions take place at the center of Brønsted acid site,and the effect of Lewis acid on the activity and selectivity of the reaction is negligible[25]. Chen, et al.[26]investigated the catalytic cracking reaction of tetralin on beta zeolite and Y zeolite. It is found that the beta zeolite with strong acid sites and Brønsted acid sites is more favorable for unimolecular cracking reaction. McVicker, et al.[18]showed that the strong acid sites contribute to isomerization of six-membered ring to form five-membered ring, thereby promoting the ring-opening reaction of the cycloalkane ring[27]. Arribas, et al.[23]suggested that the density of Brønsted acid sites should not be too high. The moderate density of Brønsted acid sites is conducive to reducing the secondary cracking and dealkylation reactions, thereby increasing the selectivity of ring-opening reaction. To summarize, the stronger acid sites are conducive to promoting the ring-opening reaction and isomerization reaction, and the moderate acid density is conducive to reducing the occurrence of secondary reactions.
4 Catalytic Cracking Reaction Kinetics of Tetrallin and Decalin
The catalytic cracking reaction of tetralin and decalin is a complex parallel sequential reaction with as many as hundreds of products. In order to provide data for the design and optimization of the catalytic cracking process, researchers have studied the catalytic cracking kinetics of decalin. Al-Sabawi, et al.[17]divided the complex products into five categories, viz.: the CPO—unsaturated C10monocyclic cycloalkanes and olefins with a carbon number of less than 10; the CPP—saturated C10monocyclic cycloalkanes and cycloalkanes and paraffins with a carbon number of less than 10; the ISO—isomerization products with a bicyclic structure and a carbon number of 10; the AP—aromatics and naphthenic aromatics, the carbon number of which is not more than 10; and the HP—all products with a carbon number of greater than 10. Then he established a heterogeneous kinetic model for the catalytic cracking of decalin to describe the thermal effects as well as the adsorption and reaction phenomena which were applicable in the context of CREC riser simulator, as shown in Figure 6.
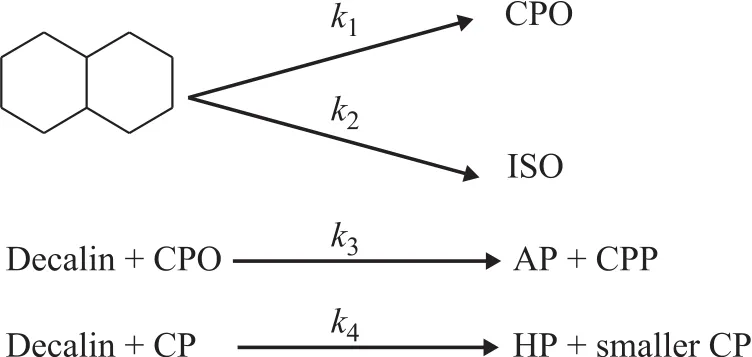
Figure 6 Primary kinetics of catalytic cracking of decalin
The catalytic cracking reaction involves complex processes such as adsorption, reaction, desorption, etc[28].The activation energy of cracking reaction is the sum of the heat of adsorption and the intrinsic activation energy[24]. Corma, et al. have shown that the adsorption heat of alkanes and naphthenes is consistent with their physical heat[29]. The heat of adsorption of decalin is approximately 112.9 kJ/mol, which is similar to that of alkanes with the same number of C—C bonds. The heat of adsorption of olefins increases by about 40 kJ/mol as compared to that of the alkanes with the same carbon number. Similarly, due to the existence of benzene ring,the heat of adsorption of tetralin is 153 kJ/mol, which is higher than that of decalin. Although the apparent activation energy of tetralin and decalin is similar, the difference in the heat of adsorption can result in a great difference in their reactivity.
The process of adsorption not only can affect the reaction rate, but can also have an important effect on the deactivation of catalyst. The deactivation of catalyst is not entirely complete due to the generation of coke that can block the catalyst pores. The products formed during catalytic cracking of tetralin and decalin such as naphthalene, methyl-naphthalene and other heavy components can occupy the acid sites and reduce the reactivity of catalyst, leading to deactivation of catalyst.Forissie[30]and Nevicato[31]also came to the similar conclusions. Therefore, in order to increase the activity of catalysts and reduce the deactivation of catalyst,it is important to optimize the heat of adsorption of hydrogenated aromatics on the catalyst.
5 Process Parameters
The catalytic cracking conversion of tetralin and decalin improves with the increase of reaction temperature[17]. In general, low temperature is conducive to the occurrence of hydrogen transfer reaction, while high temperature is conducive to the occurrence of naphthenic ring-opening reaction[32]. At lower temperature, the hydrogen transfer reaction is dominant in catalytic cracking reaction of decalin, but the ring-opening reaction and cracking reaction of naphthenic ring dominate under higher temperature conditions[19,29]. Tang, et al.[28,33]investigated the ratio of ring-opening/ dehydrogenation condensation reaction of tetralin and decalin at 450-550 °C. The study shows that although catalytic cracking conversion of tetralin and decalin improves with the increase of temperature[17], the ratio of ring-opening reaction/dehydrogenation condensation reaction decreases. The selectivity of non-aromatic components slightly increases,but the selectivity of monocyclic aromatic hydrocarbons greatly decreases. Moreover the selectivity of polycyclic aromatic hydrocarbons and coke greatly increases, which is not conducive to the products distribution.
Tang, et al.[28,33]studied the effects of catalyst/oil ratio on catalytic cracking of tetralin and decalin at a space velocity of 10 h-1and a catalyst/oil ratio of 3—9 at 500 °C on the Y zeolite catalyst. With the increase of the catalyst/oil ratio, although the catalytic cracking conversion of tetralin and decalin is increased, the rate of ring-opening reaction/dehydrogenation condensation reaction is decreased. With the increase of catalyst/oil ratio, the acid density is increased and the degree of cracking is deepened. This fact can lead to the increase of hydrogen transfer reactivity, resulting in the increase of selectivity of polycyclic aromatic hydrocarbons and coke to eventually form less favorable products.
In conlusion, although the improvement in reaction temperature and catalyst/oil ratio is beneficial to enhancing the catalytic cracking reaction conversion of tetralin and decalin, the high temperature and high catalyst/oil ratio will lead to a decreasing ratio of ringopening reaction/ dehydrogenation condensation, which is not conducive to the product distribution.
6 Conclusions
With the deterioration of raw materials, pre-hydrotreating of feedstock is becoming one of the effective methods to improve the selectivity of catalytic cracking process. The catalytic cracking reaction of hydrogenated aromatics follows a positive carbon ion mechanism and consists of a series of complex parallel sequential reactions such as the ring-opening reaction, the β-scission reaction, the isomerization reaction, the hydrogen transfer reaction, and the transalkylation reaction. The three-dimensional zeolite catalyst with 12-membered-ring channels can improve the conversion of tetralin and decalin, but the ultra-largepores zeolite will favor the hydrogen transfer reaction,which is not conducive to the product distribution. The shape-selective zeolite catalyst is beneficial to improving the selectivity of propylene, while the zeolite catalyst with strong Brønsted acid intensity is conducive to the ring-opening reaction. Catalytic cracking conversion of tetralin and decalin is improved with the increase of reaction temperature and catalyst/oil ratio. However, at high temperature and high catalyst/oil ratio, the ratio of ring-opening/dehydrogenation condensation reaction of tetralin and decalin is reduced, which is not conducive to the product distribution. The reactivity of hydrogenated aromatics with different hydrogen saturation rates is quite different. It is necessary to control the hydrogenation depth,optimize the hydrocarbon composition of catalytic cracking feed materials for maximizing the yield of target products.
Reference
[1]Chen Junwu, Xu Youhao. Catalytic Cracking Process and Engineering [M]. Beijing: China Petrochemical Press,2015
[2]Corma A, Mocholi F, Orchilles V, et al. Methylcyclohexane and methylcyclohexene cracking over zeolite Y catalysts[J]. Applied Catalysis, 1990, 67(1): 307–324
[3]Williams M F, Fonfé B, Sievers C. Hydrogenation of tetralin on silica–alumina-supported Pt catalysts I. Physicochemical characterization of the catalytic materials[J]. Journal of Catalysis, 2007, 251(2): 485–496
[4]Niu Chuanfeng, Zhang Ruichi, Dai Lishun, et al. A new integration process of residue hydrotreating combined with catalytic cracking[J]. Petroleum Processing and Petrochemicals, 2002, 33(1): 27–29(in Chinese)
[5]Xu Youhao, Dai Lishun, Long Jun, et al. Integrated Technology (IHCC) of Hydrotreating FCC Gas Oil and Highly Selective Catalytic Cracking for Maximizing Liquid Yield[J]. Petroleum Processing and Petrochemicals,2011, 42(3): 7–12(in Chinese)
[6]Gong Jianhong, Mao Anguo, Liu Xiaoxin, et al. LTAG Technology for Producing High Octane Number Gasoline and Light Aromatics[J]. Petroleum Processing and Petrochemicals, 2016, 47(9): 1–5(in Chinese)
[7]Corma A, González-Alfaro V, Orchillés A V. Decalin and Tetralin as Probe Molecules for Cracking and Hydrotreating the Light Cycle Oil[J]. Journal of Catalysis,2001, 200(1): 34–44
[8]Santikunaporn M, Herrera J E, Jongpatiwut S, et al. Ring opening of decalin and tetralin on HY and Pt/HY zeolite catalysts[J]. Journal of Catalysis, 2004, 228(1): 100–113
[9]Chen Yan, Da Zhijian, Zhuyuxia, et al. Interaction of tetralin and dodecane in the catalytic cracking processing[J]. Acta Petrolei Sinica (Petroleum Processing Section),2016, 32(4): 773–779 (in Chinese)
[10]Sun Wei, Li Shuo, Liu Yibin. Research & development of decalin catalytic cracking [J]. Petroleum Refinery Engineering, 2015, 45(8): 1–5(in Chinese)
[11]Yang Zhe, Zong Shimeng, Long Jun. Quantum mechanical studies on the micro-structure of tatrahydro-naphthalene[J].Computers and Applied Chemistry, 2012(04): 465–468
[12]Sato K, Iwata Y, Miki Y, et al. Hydrocracking of tetralin over NiW/USY zeolite catalysts: For the improvement of heavy-oil upgrading catalysts[J]. Journal of Catalysis,1999, 186(1): 45–56
[13]Townsend A T, Abbot J. Catalytic cracking of tetralin on HY zeolite[J]. Applied Catalysis A General, 1992, 90(2):97–115
[14]Townsend A T, Abbot J. Catalytic reactions of tetralin on HZSM-5 zeolite[J]. Applied Catalysis A General, 1993,95(2): 221–236
[15]Kubička D, Kumar N, Mäki-Arvela P, et al. Ring opening of decalin over zeolites : I. Activity and selectivity of proton-form zeolites[J]. Journal of Catalysis, 2004, 222(1):65–79
[16]Kubička D, Kumar N, Mäki-Arvela P, et al. Ring opening of decalin over zeolites : II. Activity and selectivity of platinum-modi fi ed zeolites[J]. Journal of Catalysis, 2004,227(2): 313–327
[17]Al-Sabawi M, Lasa H D. Modeling thermal and catalytic conversion of decalin under industrial FCC operating conditions[J]. Chemical Engineering Science, 2010, 65(2):626–644
[18]Mcvicker G B, Daage M, Touvelle M S, et al. Selective ring opening of naphthenic molecules[J]. Journal of Catalysis, 2002, 210(1): 137–148
[19]Mostad H B, Riis T U, Ellestad O H. Catalytic cracking of naphthenes and naphtheno-aromatics in fixed bed micro reactors[J]. Applied Catalysis, 1990, 63(1): 345–364
[20]Gong Jianhong, Long Jun, Mao Anguo, et al. Development of the LTAG technology for LCO to produce higher RON naphtha and light aromatics [J]. Acta Petrolei Sinica(Petroleum Processing Section), 2016, 32(5): 867–874 (in Chinese)
[21]Yang Ping, Xin Jing, Li Mingfeng. Research advances in the hydroconversion of tetralin[J]. Petroleum Processing and Petrochemicals, 2011, 43(8): 1–6(in Chinese)
[22]Martinez-Triguero J, Diaz-Cabañas M J, Camblor M A,et al. The Catalytic Performance of 14-Membered Ring Zeolites[J]. Journal of Catalysis, 1999, 182(2): 463–469.
[23]Arribas M A, Corma A, Díaz-Cabañas M J, et al.Hydrogenation and ring opening of tetralin over bifunctional catalysts based on the new ITQ-21 zeolite[J].Applied Catalysis A General, 2004, 273(1): 277–286
[24]Jiang Shuai. Study on Catalytic Cracking Performance of Aromatics from Coker Gas Oil [D]. Dongying: China University of Petroleum
[25]Galperin L B, Bricker J C, Holmgren J R. Effect of support acid–basic properties on activity and selectivity of Pt catalysts in reaction of methylcyclopentane ring opening[J]. Applied Catalysis A General, 2003, 239(1/2):297–304
[26]Chen Yan, Song Haitao, Zhu Yuxia, et al. Comparison of Catalytic Cracking of Tetralin Over Beta and Y Zeolites[J].Acta Petrolei Sinica (Petroleum Processing Section), 2015,31(3): 650–656 (in Chinese)
[27]Du H, Fairbridge C, Yang H, et al. The chemistry of selective ring-opening catalysts[J]. Applied Catalysis A General, 2005, 294(1): 1–21
[28]Tang Jinlian, Xu Youhao, Wang Xieqin, et al. Opening of naphthenic ring in decalin over zeolite catalysts [J].Journal of Fuel Chemistry and Technology, 2012, 40(12):1422–1428
[29]Corma A, Ortega F J. Influence of adsorption parameters on catalytic cracking and catalyst decay[J]. Journal of Catalysis, 2005, 233(2): 257–265
[30]Forissier M, Bernard J R. Deactivation of Cracking Catalysts with Vacuum Gas Oils[J]. Studies in Surface Science & Catalysis, 1991, 68: 359–366
[31]Nevicato D, Pitault I, Forissier M, et al. The activity decay of cracking catalysts: Chemical and structural deactivation by coke[J]. Studies in Surface Science & Catalysis, 1994,88: 249–256
[32]Long Jun. Medium-pore shape selective zeolite and catalytic cracking of hydrocrbons [J]. Petroleum Processing and Petrochemicals, 1997, 38(7): 24–28(in Chinese)
[33]Tang Jinlian, Xu Youhao, Wang Xieqin, et al. Opening of naphthenic ring in tetralin over zeolite catalysts[J].Petroleum Processing and Petrochemicals, 2012, 33(1):20–25(in Chinese)
- 中国炼油与石油化工的其它文章
- Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
- Emission-Reductive and Multi-Objective Coordinative Optimization of Binary Feed for Atmospheric and Vacuum Distillation Unit
- A pH Model for Calculating the pH Value of Mixed–Acid–Base Equilibria of Overhead Condensing Systems in Crude Distillation
- Simultaneous Biological Removal of p-Cresol, Sulfide and Nitrate by Denitrification
- The Effects of Ultrasonic Treatment on the Molecular Structure of Residual Oil
- Degradation of Nitrobenzene Wastewater via Iron/Carbon Micro-electrolysis Enhanced by Ultrasound Coupled with Hydrogen Peroxide

