Synthesis and Tribological Properties of Graphene-Copper Nanoparticle Composites as Lithium Grease Additive
Wang Jing; Guo Xiaochuan; He Yan; Jiang Mingjun; Zhou Weigui
(1. Army Logistical University of PLA, Chongqing 401311; 2. POL Research Institute of PLA, Beijing 102300)
1 Introduction
Graphene, as a new material, has been attracting lots of attention in the field of tribology thanks to its nanolayered structure, large surface area, ultrahigh mechanical strength, and excellent mechanical properties[1-2]. The tribological properties of graphene and the development of graphene composite as a type of lubricating material have thus become a central research topic. It is found that graphene[3-9]and its metal composites[10-13]are capable of not only reducing the friction coefficient by acting like nanobearings but also improving the loading capacity of the lubricant via the formation of a friction adsorption film.
Nano-copper is also an excellent lubricating additive, and by forming a ductile metal deposition film on the surface of the frictional pair, nano-copper can significantly improve the tribological properties of the lubricant by simultaneously reducing the friction and wear loss[14-15]and repairing the friction surface[16-17].
Meanwhile, researchers have found that the large surface area and unique three-dimensional folded structure of graphene make itself a superior carrier for copper nanoparticles (Cu NPs)[18]. The adsorption of Cu NPs onto the surface of the graphene can prevent the aggregation and oxidation of Cu NPs[19-20]. And the adsorption of Cu nanoparticles onto the surface of graphene can weaken the π-π interaction between the graphene sheets and consequently reduce the stacking of graphene. The GN/Cu NPs composites not only can preserve the original properties of the individual components, but also exhibit a synergistic effect from its components that provide the lubricating oil with the novel tribological property[21-22].However, there are few study about the effect of GN/Cu NPs composites on the tribological properties of grease, which, as one of the main lubricants, is widely used in many occasions, the requirements of which the conventional lubricating oil cannot meet.
The current processes for the preparation of graphene/metal composites mainly involve the chemical reduction method[23-24], the chemical precipitation method[25-26],the hydrothermal method[27], the sol-gel method[28],the electrochemical method[29], and the microwave method[30-31]. The most frequently used and studied,as well as the easiest to be commercialized method is the chemical reduction, which involves the addition of reductants to a mixture of metal salts and graphene oxide.And both the metal salts and graphene oxide are reducedsimultaneously to give rise to the formation of graphene/metal composite. However, the graphene produced by this way has many defects, which would result in an incompetent performance of the graphene[32]. In order to ensure the integrity of the graphene sheets, researchers have tried to exploit alternatives that can reduce graphene oxides for the preparation of graphene carriers[33-35].
In our study, the graphene prepared by liquid exfoliation can reduce the structural defects caused by chemical reduction of graphene oxide and maintain the integrity of the structure. And the GN/Cu NPs composites are prepared via the in situ chemical reduction in the aqueous solution.The factors that might affect the preparation process are analyzed and the corresponding mechanism is proposed.Finally, the effects of the GN/Cu NPs composites on the tribological properties of grease as well as its mechanism of action are included in this study to provide a theoretical guidance for the subsequent application of GN/Cu NPs composites in the field of tribology.
2 Experimental
2.1 Materials
Copper sulfate pentahyd rate (CuSO4·5H2O), ascorbic acid (C6H8O6), polyvinylpyrrolidone (PVP), NaOH, and ethanol were purchased from the Kelong Chemical Co.Ltd., Chengdu. All chemicals were of the analytical reagent grade and used without further purification. The water used throughout this work was the reagent grade water produced by a Milli-Q SP ultrapure water purification system made by the Nihon Millipore Ltd., Tokyo.
2.2 Preparation and characterization of the GN/Cu NPs composites
A typical preparation procedure is described as follows.0.24968 g of CuSO4·5H2O were dissolved in 50 mL of deionized water under magnetic stirring until the solution turned into a transparent light blue color, which was then marked as A. To prepare solution B, 0.66 g of PVP were dissolved in 50 mL of deionized water under constant stirring followed by addition of 1.9373 g of ascorbic acid under continuous stirring until the solution turned clear. Then, A was slowly added dropwise into B using a constant pressure dropping funnel at a rate of 5 mL/min when the temperature of B reached 85 °C. The pH value of the solution was adjusted to 10 by adding aqueous NaOH when A was completely added to B. The solution was continuously mixed by magnetic stirring and was subjected to reaction for 2 hours at 85 °C. Within the 2-hour period, the solution gradually turned from light blue to orange, and finally became reddish-brown. Once the reaction was completed, the resulting liquid mixture was centrifuged, with the precipitate collected. The precipitate was washed by centrifuging with deionized water and ethanol to remove the unreacted copper ions,excessive PVP, and other impurities. The resulting product was freeze-dried to prevent aggregation of the nanomaterials obtained by the above procedures.
Graphene prepared by the liquid-phase exfoliation method was ultrasonically dispersed in the deionized water (with a power of 200 W in 2 h). The dispersion was centrifuged at a rotary speed of 4000 r/min for 10 min to remove the non-exfoliated graphene. The concentration of the dispersed graphene in the supernatant was about 0.1 mg/mL. Then, 0.24968 g of CuSO4·5H2O were dissolved under magnetic stirring in 50 mL of graphene solution just obtained thereby. The resulting system was marked as C. By substituting A with C, the reddish-brown powder of the GN/Cu NPs composites was finally obtained according to the same synthesis method as that used for the preparation of Cu NPs described above.
The microstructure and morphology of the sample powder were investigated by the scanning electron microscopy(SEM, JEOL JSM-7800F). The size and structural characteristics were analyzed by the transmission electron microscopy (TEM, FEI Tecnai F20) with an acceleration voltage of 200 kV. The X-ray diffractometer (XRD,Shimadzu XRD-6100) was applied to study the structure of the synthetic nanoplates using Cu-Kα (λ= 0.15406 nm)radiation with an accelerating voltage of 40 kV and an applied current of 30 mA. The XRD patterns were recorded from 10°—80° with a scanning speed of 2(°)/min.
2.3 Tribological measurements
Grease samples with additives are prepared according to the procedures mentioned in the literature[36]. According to the ASTM D5707-11 method, tests on friction and wear were performed with an SRV-IV friction and wear tester purchased from OPTIMOL AG, Germany. A ball-disk with a point contact mode was chosen for conducting this experiment, and the test conditions covered a load of 200 N,a frequency of 50 Hz, an amplitude of 1 mm, a temperature of 80°C, and a test duration of 2 hours. The ball and the disk were both standardized specimens obtained from OPTIMOL. The white-light interferometry purchased from Bruker was then applied to quantify the wear loss of disk in order to characterize the anti-wear property of grease samples, which was inversely correlated with the amount of wear loss. Also, a lower friction coefficient usually corresponded to better friction reducing property.
3 Results and Discussion
3.1 The effect of pH value on particle size and structure of GN/Cu NPs composites
The effects of pH value on the particle size and the structure of materials were investigated at a PVP concentration of 0.06 mol/L. Figure 1 presents the scanning electron microscopy (SEM) images showing the morphology of the materials prepared at different pH values. It can be seen that when pH=4 (Figure 1(a)), the particles prepared thereby have porous surface with their size being in the micron range. When pH=6 (Figure 1(c)),the particles have their size still remaining in the micron range but the surface is free from pores. When pH=8(Figure 1(e)), there is a drop in the mean particle size to be under 50 nm, with some large particles remaining.When the pH value continues to increase to 10 (Figure 1(g)), the diameters of the nanoparticles produced are uniformly equal to around 25 nm, and no obvious aggregation is observed which can meet the needs of the experiment. Nevertheless, when the pH value reaches 12(Figure 1(i)), the micron-sized particles start to reappear among the nanoparticles. It can be seen that nanoparticles can be obtained only in the alkaline solution. And an appropriate concentration of OH-ions is beneficial to the formation of spherical nanoparticles.
A similar phenomenon has been observed in the preparation of GN/Cu NPs composites. The particles are relatively large when the pH value is less than or equal to 8, and they cannot perfectly combine with the graphene,resulting in serious stacking of the graphene. When pH=10 (Figure 1(h)), Cu NPs that are uniform in size are evenly adsorbed onto the surface of the graphene sheets,which can effectively prevent the stacking of graphene.

Figure 1 The SEM morphology of materials prepared at different pH values
Figure 2 (a) shows the XRD spectra of the materials prepared at different pH values. As shown in Figure 2 (a),the product is prepared when pH≤10, and strong crystal plane diffraction peaks can be seen around diffraction angle(2θ) of 43.4°, 50.54° and 74.2°, which correspond to the face-centered cubic (FCC) crystal planes of copper (111),(200), and (220), respectively (JCPDS No. 04–0836). No peaks of impurities are detected. For the product prepared at pH=12, there are crystal plane diffraction peaks appearing at diffraction angle (2θ) of 36.46°, 42.34°, 61.42°, and 73.84°, which match with FCC crystal planes of Cu2O (111),(200), (220) and (311), respectively (JCPDS No.05-0667).Cu2O is formed by the decomposition of Cu(OH)2, which is an unstable intermediate due to the presence of high concentration of OH-ions. A weak and widened diffraction peak appears at 24.9° in the XRD spectra of GN/Cu NPs composites (Figure 2 (b)), suggesting that the graphene has been fully exfoliated due to the adsorption of Cu NPs on the surface of the graphene that can prevent the stacking of graphene sheets.

Figure 2 The XRD spectra of the nanomaterials prepared at different pH values (a), and GN/Cu NPs composites prepared at pH=10 (b)◆—Cu; ●—Cu2O
The above experimental results show that the solution pH value has a great in fl uence on the size and purity of the Cu NPs. This phenomenon can occur, because the reductivity of ascorbic acid depends heavily on the pH value of the solution. When the solution is acidic, the reductivity of ascorbic acid is very weak so that only a small amount of nuclei is produced in the initial stage of the reaction.In this situation, large particles are formed because the growth of the particles is possible only on these nucleuses as the reduction goes on. Also, the bonding between big particles and the graphene is not strong enough to prevent the graphene stacking. The reductivity of ascorbic acid is stronger when pH = 8, as compared with that in the acidic condition. More nucleuses are formed in the early stage of the reaction and thus the majority of particles are obtained in the nano range, when the reaction is complete. At pH= 10, the reductivity of the ascorbic acid is significantly enhanced so that a large quantity of nucleuses are formed in the early stage of the reaction, which can result in a dramatic reduction of soluble Cu2+ions from the solution.The growth of the particles is therefore limited due to the low concentration of Cu2+ions, leading to an ultimate mean particle size of 25 nm. Nevertheless, when the pH value is further increased, the high concentration of OH-ions leads to the formation of Cu(OH)2, which may complicate the process of particles growth, resulting in the appearance of particles in various shapes.
3.2 The effect of PVP concentration on the structure of copper nanoparticles
It is generally accepted that PVP functions as a non-covalent bond modifier or a surfactant, which enables the graphene to be fully dispersed in the aqueous medium for avoiding the stacking and aggregation. To further understand the role of PVP in the preparation of GN/Cu NPs composites, the effects of PVP concentration on the structure and purity of the Cu NPs are investigated in detail.
As shown in the SEM images of the copper nanoparticles,prepared under different PVP concentrations, the diameter of the copper nanoparticles depends largely on the concentration of the PVP (Figure 3). Without PVP, the size distribution of Cu NPs is in the range of 50 — 80 nm.When the PVP concentration is 0.03 mol/L, the distribution of the particle size is not uniform, namely, a majority of the particles is reduced to 20—50 nm coupled with a small portion of larger particles. When the PVP concentration is increased to 0.06 mol/L, the size of Cu NPs prepared is around 25 nm and the size distribution is quite uniform.However, a further increase of the PVP concentration would result in a sharply increase in the size of the Cu NPs.XRD is used to further analyze the effects of PVP concentration on the structure of the Cu NPs. As shown in Figure 4, the XRD pattern indicates that the product is a mixture of Cu and Cu2O without using PVP. When the PVP concentration is 0.03 mol/L, the intensity of diffraction peak of Cu2O (FCC) clearly decreased. When the PVP concentration is 0.06 mol/L, the XRD pattern shows the clear and sharp diffraction peaks that are assigned to the FCC crystal planes of Cu (111), (200), and(220), and no other peaks are detected.

Figure 3 SEM images of nanoparticles prepared at different PVP concentration
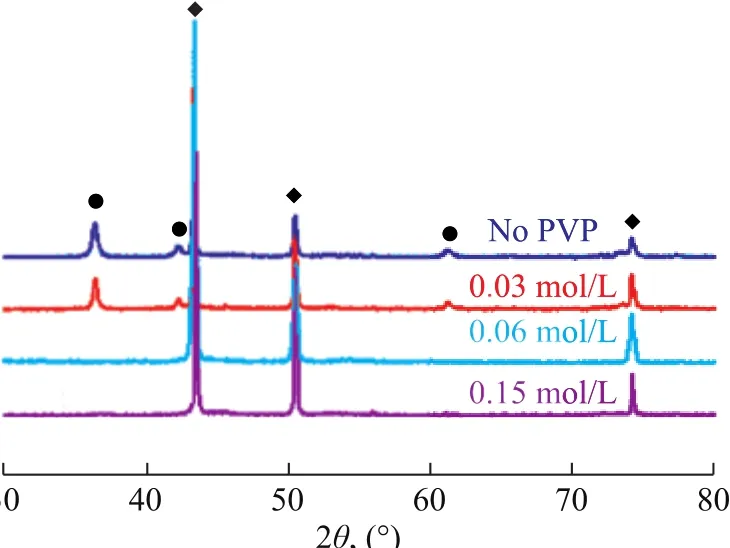
Figure 4 XRD spectra of nanoparticles prepared at different PVP concentration◆—Cu; ●—BCu2O
As shown in the EDS of the Cu NPs prepared under different PVP concentrations (Figure 5), the main components of the nano-products, when no PVP is added,are Cu and O elements (with Si elements emanating from the monocrystalline silicon substrate), and the relative quantity of elemental oxygen goes down when the PVP concentration increases. When the PVP concentration reaches 0.06 mol/L, a small amount of C and O elements are found, which are presumably contaminates originated from CO2and O2in the air.
Meanwhile, the GN/Cu NPs composites prepared at a PVP concentration of 0.06 mol/L and pH = 10 are analyzed with EDS, and the C, Cu, and O elements are found in the composites. The elemental surface scanning analysis of GN/Cu NPs composites (Figure 5(d)) shows that the elemental O is uniformly distributed throughout the selected area, which indicates that the elemental O may come from the O2or CO2contaminants in the air during sample preparation, and are not originated from CuO or Cu2O. The supporting evidence from both EDS and XRD can confirm that the material obtained thereby is Cu NPs with a high purity.
In summary, the PVP concentration has a great influence on the purity of the Cu NPs. To be specific, if reaction (1)went on irreversibly to the right, the resulting Cu would not remain stable. However the equilibrium constant K of the reaction is quite big (K =[Cu2+]/[Cu+]=1.2×106), so the reaction equilibrium would shift to the left to form the stable Cu powder.

Nevertheless, a small amount of the remaining Cu+ions can still form Cu2O. To be more explicit, in the presence of PVP,the lone pair electrons on the O atom of the polar group in PVP may join with the sp orbit of Cu2+ions to form a Cu-O bond, which lowers the Cu+concentration by depleting the Cu2+ions and thus hinders the reaction (1) from shifting to the right effectively to reduce the formation of Cu2O. On the other hand, PVP can inhibit reaction (2), and therefore no Cu2O is produced from the decomposition of Cu(OH)2.

It can be seen that except for serving as surfactant to promote the dispersion and to prevent the stacking of graphene, the more important role of PVP is to inhibit the formation of copper oxide and copper hydroxide. Also,with the assistance of PVP, Cu NPs can be effectively attached onto the surface of graphene to promote the formation of GN/Cu NPs composites.
Figure 6 shows the TEM and HRTEM images of GN/Cu NPs composites prepared at a pH value of 10 and a PVP concentration of 0.06 mol/L. It can be seen that the Cu NPs have a diameter of 25 nm, and they display regular lattice fringes. In addition, the Cu NPs are evenly distributed onto the surface of the graphene and are strongly combined with each other, which is in agreement with the SEM results.The electron diffraction patterns reveal the polycrystalline structures of Cu NPs.
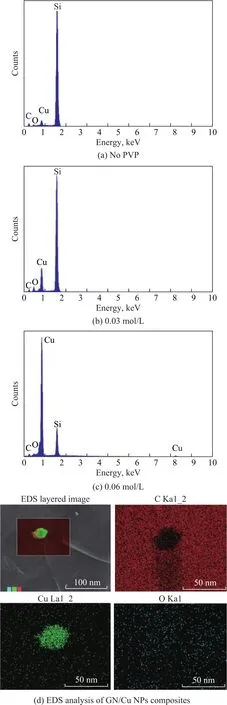
Figure 5 EDS spectra of nanoparticles prepared at different PVP concentration
3.3 Tribological properties
In order to make a comparative analysis, the graphene,Cu NPs, the simple mixture of graphene and Cu NPs,as well as the graphene-copper nanoparticle composite are separately added into the base grease (abbreviated as base grease+GN, base grease+Cu, base grease+GN+Cu and base grease+GN/Cu, respectively) to test their tribological properties. It can be seen that (Figure 7(a)) all four additives can to a certain extent reduce the wear loss of the disk, which means that all four additives are able to improve the tribological properties of the grease. Among these four samples, the grease with 0.5% of GN/Cu NPs composites shows the best tribological performance with an 82.5% drop in the wear loss to 0.8×105μm3from the original value of 4.7×105μm3, as compared to that of the base grease, while reflecting a synergistic effect of graphene and copper nanoparticles. When a mixture of Cu NPs and graphene is added to the grease, the wear loss of the disk is higher as compared to the case when only a single additive is used. This may be attributed to the malign competition of the two mixed additives that are in contact with the friction disk. Figure 7(b) shows the average friction coefficient of the four grease samples plotted against the amount of additives. Similar to the curve of the wear loss, the GN/Cu NPs composites can effectively improve the tribological properties of the lubricating grease and the average coefficient of friction is reduced by 15.5% with the addition of 0.5% of GN/Cu NPs composites. Figure 7(c) demonstrates the curves of friction coefficient plotted against time, and it shows that the GN/Cu NPs composites not only can reduce but also can stabilize the friction coefficient over time.
The white light interferometry images of the worn surfaces of disks are shown in Figure 8. It suggests that upon being lubricated with the base grease, the disk reflects primarily abrasive wear due to the plowing effect of the hard particles. When graphene is added, it can deposit onto the surface of the disk to form a protective film layer[4]. This layer is effective in reducing the friction on the surface of the disk, and thus reducing the wear loss caused by the abrasive wear. When Cu NPs are added,they can be transformed into a molten deposition film on the worn surface under constant friction[17]. This film covers the disk surface through absorbing most of the friction, and can thereby protect the disk. When a mixture of GN and Cu NPs is added to the grease, the abrasive wear and the adhesive wear are both observed on the surface of the disk, leading to peeling effects that can occupy a large area of the disk surface. This phenomenon might be a result of the competitive deposition between the two types of materials that can eventually lead to a more serious wear loss, even though the graphene and Cu NPs can individually reduce the friction.
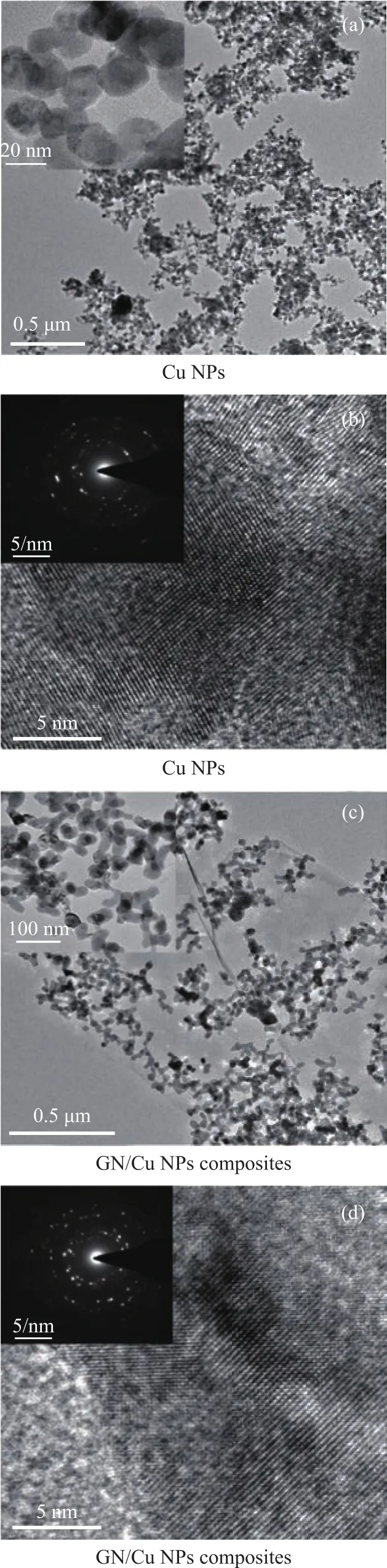
Figure 6 TEM images of the products synthesized at pH=10 and a PVP concentration of 0.06 mol/L

Figure 7 Wear loss (a) and friction coefficient (b) as the function of concentration of additives (200 N, 50 Hz, 2 h);and (c) variation of friction coefficient of base grease and grease doped with 0.5 wt% of additives during the friction tests (200N, 50Hz, 2h)
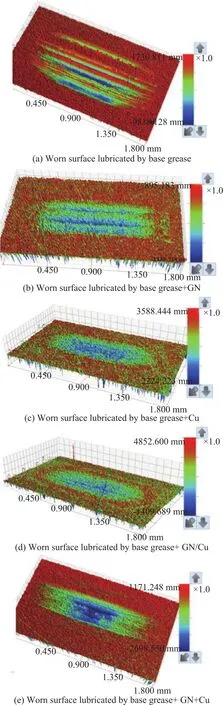
Figure 8 White light interferometry images of the disk worn surfaces
The supreme tribological properties GN/Cu NPs composites are primarily attributed to the following points. Firstly, the extremely thin lamellar structure of graphene offers an excellent self-lubricating property.Meanwhile, its exceptional mechanical strength helps to further protect the surface of the disk. Secondly, the Cu NPs on the surface of the graphene sheet can serve as nano-bearings that can convert the sliding friction into the rolling friction, and hence can lower the friction coefficient. At the same time, the Cu NPs are melted into elemental Cu owing to the high pressure and high temperature induced locally by friction. The molten Cu species can fill in the micro-pits to appreciably reduce the surface roughness and consequently lower the friction coefficient. Finally, the Cu NPs can be turned into a tribochemical film during friction, which can further protect the disk surface from wear loss. The combined effect of these factors contributes to the supreme tribological properties of the GN/Cu NPs composites.For future researches, we will focus on the contents and chemical states of elements on the worn surfaces of the disk.
4 Conclusions
This study has mainly described the synthesis of the GN/Cu NPs composites by chemical reduction in liquid along with analyses on the effects of pH value and PVP concentration on the particle size and structures of the composites. As a key factor during synthesis, the pH value can affect the particle size of the Cu NPs via controlling the reductivity of the ascorbic acid while PVP can effectively protect the Cu NPs from oxidation. The GN/Cu NPs composites are successfully produced at pH=10 and a PVP concentration of 0.06 mol/L. The tribological tests have shown that the base grease added with the GN/Cu NPs composites has significantly reduced not only the wear loss of the disk but also the friction coefficient of the grease. This excellent tribological property is primarily attributed to the synergistic effect of the GN and Cu NPs.Acknowledgements: This work was financially supported by the program of Chongqing Postgraduate Research and Innovation Project (CYB16130) and the Chongqing Science and Technology Nova Plan (KJXX2017023).
[1]Berman D, Erdemir A, Sumant A V. Graphene: a new emerging lubricant[J]. Materials Today, 2014, 17(1):31-42
[2]Penkov O, Kim H J, Kim H J, et al. Tribology of graphene:a review[J]. International Journal of Precision Engineering& Manufacturing, 2014, 15(3): 577-585
[3]Song H J, Li N. Frictional behaviour of oxide graphene nanosheets as water-base lubricant additive[J]. Applied Physics A, 2011, 105(4): 827-832
[4]Berman D, Erdemir A, Sumant A V. Few layer graphene to reduce wear and friction on sliding steel surfaces[J].Carbon, 2013, (54): 454-459
[5]Ota J, Hait S K, Sastry M I S, et al. Graphene dispersion in hydrocarbon medium and its application in lubricant technology[J]. RSC Advances, 2015, 5(66): 53326-53332
[6]Kinoshita H, Nishina Y, Alias A A, et al. Tribological properties of monolayer graphene oxide sheets as waterbased lubricant additives[J]. Carbon, 2014, 66(1): 720-723
[7]Fan X, Wang L. High-performance lubricant additives based on modified graphene oxide by ionic liquids[J].Journal of Colloid & Interface Science, 2015, 452: 98-108
[8]Mao F, Wiklund U, Andersson A M, et al. Graphene as a lubricant on Ag for electrical contact applications[J].Journal of Materials Science, 2015, 50(19): 6518-6525
[9]Azman S S N, Zulki fl i N W M, Masjuki H, et al. Study of tribological properties of lubricating oil blend added with graphene nanoplatelets[J]. Journal of Materials Research,2016, 31(13): 1932-1938
[10]Zhang Y, Tang H, Ji X, et al. Synthesis of reduced graphene oxide/Cu nanoparticle composites and their tribological properties[J]. RSC Advances, 2013, 3(48): 26086-26093
[11]Li H, Chen L, Zhang Y, et al. Synthesis of MoSe2/reduced graphene oxide composites with improved tribological properties for oil-based additives[J]. Crystal Research &Technology, 2014, 49(4): 204–211
[12]Meng Y, Su F, Chen Y. A novel nanomaterial of graphene oxide dotted with Ni nanoparticles produced by supercritical CO2-assisted deposition for reducing friction and wear [J]. ACS Applied Materials & Interfaces, 2015,7(21): 11604–11612
[13]Zhao J, He Y, Wang Y, et al. An investigation on the tribological properties of multilayer graphene and MoS2nanosheets as additives used in hydraulic applications[J].Tribology International, 2016, 97: 14-20
[14]Xiong X, Kang Y, Yang G, et al. Preparation and evaluation of tribological properties of Cu nanoparticles surface modified by tetradecyl hydroxamic acid[J]. Tribology Letters, 2012, 46(3): 211-220
[15]Zhang C, Zhang S, Song S, et al. Preparation and tribological properties of surface-capped copper nanoparticle as a water-based lubricant additive[J].Tribology Letters, 2014, 54(1): 25-33
[16]Zhang Y, Xu Y, Yang Y, et al. Synthesis and tribological properties of oil-soluble copper nanoparticles as environmentally friendly lubricating oil additives[J].Industrial Lubrication & Tribology, 2015, 67(3): 227-232
[17]Nan F, Xu Y, Xu B, et al. Effect of Cu nanoparticles on the tribological performance of attapulgite base grease[J].Tribology Transactions, 2015, 58(6): 1031-1038
[18]Chunder A, Pal T, Khondaker S I, et al. Reduced graphene oxide/copper phthalocyanine composite and its optoelectrical properties[J]. Journal of Physical Chemistry C, 2010, 114(35): 5470-5474
[19]Song E H, Wen Z, Jiang Q. CO catalytic oxidation on copper-embedded graphene[J]. Journal of Physical Chemistry C, 2014, 115(9): 3678-3683
[20]Mondal P, Sinha A, Salam N, et al. Enhanced catalytic performance by copper nanoparticle-graphene based composite[J]. RSC Advances, 2013, 3(16): 5615-5623
[21]Li X, Zhao Y, Wu W, et al. Synthesis and characterizations of graphene–copper nanocomposites and their antifriction application[J]. Journal of Industrial & Engineering Chemistry, 2014, 20(4): 2043-2049
[22]Jia Z, Chen T, Wang J, et al. Synthesis, characterization and tribological properties of Cu/reduced graphene oxide composites[J]. Tribology International, 2015, 88: 17-24
[23]Liu M, Chen W. Graphene nanosheets-supported Ag nanoparticles for ultrasensitive detection of TNT by surface-enhanced Raman spectroscopy[J]. Biosensors &Bioelectronics, 2013, 46(15): 68-73
[24]Hong W, Bai H, Xu Y, et al. Preparation of gold nanoparticle/graphene composites with controlled weight contents and their application in biosensors[J]. Journal of Physical Chemistry C, 2010, 114(4): 1822-1826
[25]Ding Y, Jiang Y, Xu F, et al. Preparation of nano-structured LiFePO4/graphene composites by co-precipitation method[J]. Electrochemistry Communications, 2010,12(1): 10-13
[26]Yan J, Sun W, Wei T, et al. Fabrication and electrochemical performance of hierarchical porous Ni(OH)2nanoflakes anchored on graphene sheets[J]. Journal of Materials Chemistry, 2012, 22(23): 11494-11502
[27]Lin Q, Li Y, Yang M. Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature[J]. Sensors & Actuators B:Chemical, 2012, 173(10): 139-147
[28]Xiang H, Tian B, Lian P, et al. Sol–gel synthesis and electrochemical performance of Li4Ti5O12/graphene composite anode for lithium-ion batteries[J]. Journal of Alloys & Compounds, 2011, 509(26): 7205-7209
[29]Jagannadham K. Electrical conductivity of copper–graphene composite films synthesized by electrochemical deposition with exfoliated graphene platelets[J].Metallurgical & Materials Transactions B, 2012, 43(2):316-324
[30]Lu T, Pan L, Li H, et al. Microwave-assisted synthesis of graphene–ZnO nanocomposite for electrochemical supercapacitors[J]. Journal of Alloys and Compounds,2011, 509(18): 5488-5492
[31]Zhong C, Wang J, Chen Z, et al. SnO2–graphene composite synthesized via an ultrafast and environmentally friendly microwave autoclave method and its use as a superior anode for lithium-ion batteries[J]. J. Phys. Chem. C, 2014,115(50): 25115-25120
[32]Hernandez Y, Nicolosi V, Lotya M, et al. High yield production of graphene by liquid phase exfoliation of graphite[J]. Nature Nanotechnology, 2008, 3(9): 563-568.
[33]Bajpai R, Roy S, Kumar P, et al. Graphene supported platinum nanoparticle counter-electrode for enhanced performance of dye-sensitized solar cells[J]. ACS Applied Materials & Interfaces, 2011, 3(10): 3884-3889
[34]Su C Y, Lu A Y, Xu Y, et al. High-quality thin graphene films from fast electrochemical exfoliation.[J]. ACS Nano,2011, 5(3): 2332-2339
[35]Du W, Jiang X, Zhu L. From graphite to graphene: direct liquid-phase exfoliation of graphite to produce singleand few-layered pristine graphene[J]. Journal of Materials Chemistry A, 2013, 1(36): 10592-10606
[36]Wang Jing, Guo Xiaochuan, He Yan, et al. Tribological characteristics of graphene as lithium grease additive[J].China Petroleum Processing & Petrochemical Technology,2017, 19(1): 46-54
- 中国炼油与石油化工的其它文章
- Influence of Initial Water Content on Synthesis of Silicalite-1 Zeolite
- Study on Catalytic Alkylation of Benzene with Methanol over ZSM-22 and ZSM-35
- Preparation of Novel Dechlorination Adsorbent and Study on Its Adsorption Mechanism
- The Function of β Zeolite for Enhancing the Propylene Yield in FCC Process
- Emission-Reductive and Multi-Objective Coordinative Optimization of Binary Feed for Atmospheric and Vacuum Distillation Unit
- Preparation of AgCeY Zeolite Using Microwave Irradiation and Its Adsorptive Desulfurization Performance

