萘嘧啶镝和铈稀土配合物的单分子磁性
邹晓艳,董艳萍,李光明
(黑龙江大学 a. 功能无机材料化学省部共建教育部重点实验室;b. 化学化工与材料学院;c.《黑龙江大学工程学报》编辑部, 哈尔滨 150080)
Single-molecule magnet of the homo-multinuclear lanthanide complexes continue to be attractive owing to their potential applications for the uses of high-density magnetic memories, molecular spintronics and quantum computing devices.[1-3]Particular attention has been devoted to DyIIIion, attributed to the inherently large magnetic moment with a Kramers ground state of6H15/2and a large Ising-type magnetic anisotropy. It has indisputably led to the largest number of pure 4f SMMs with various nuclear.[4-5]M. L. Tong, et al. presented a pentagonal bipyramidal DyIIIwhich the effective energy barrier (Ueff=1 025 K) for relaxation of magnetization reached a breakthrough among the SMMs[6].
More efforts for lanthanide complexes have focused mainly on the construction and preparation of versatile coordination structures, as well as the structure-property relationships. The large ionic radius of lanthanide ions affords high and variable coordination numbers, providing more difficulty in controlling the synthetic reaction than transition-metal ones[7]. Among the strategies, the rational selection of organic ligands or co-ligands according to their length, rigidity and functional groups is important for the assembly of structurally controllable lanthanide complexes with magnetic behaviors. From benzimidazole base ligands (HL1=2-(1H-benzoimidazol-2-yl)-4-bromo-6-methoxy-phenol, Scheme 1a), the lanthanide complexes with various structures were prepared by Jones, Yang and coworkers[8]. We suppose that replacement of phenyl with larger conjugated aromatic system, such as naphthyl group, would enlargement of the fused ring structure might provide different coordination modes. Herein, synthesized 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole (HL, scheme 1b) and two mononuclear lanthanide complexes have been isolated with crystal structures determined by X-ray crystallographic analysis. Magnetic properties of DyIIIcomplex has been investigated which has scarcely been reported.
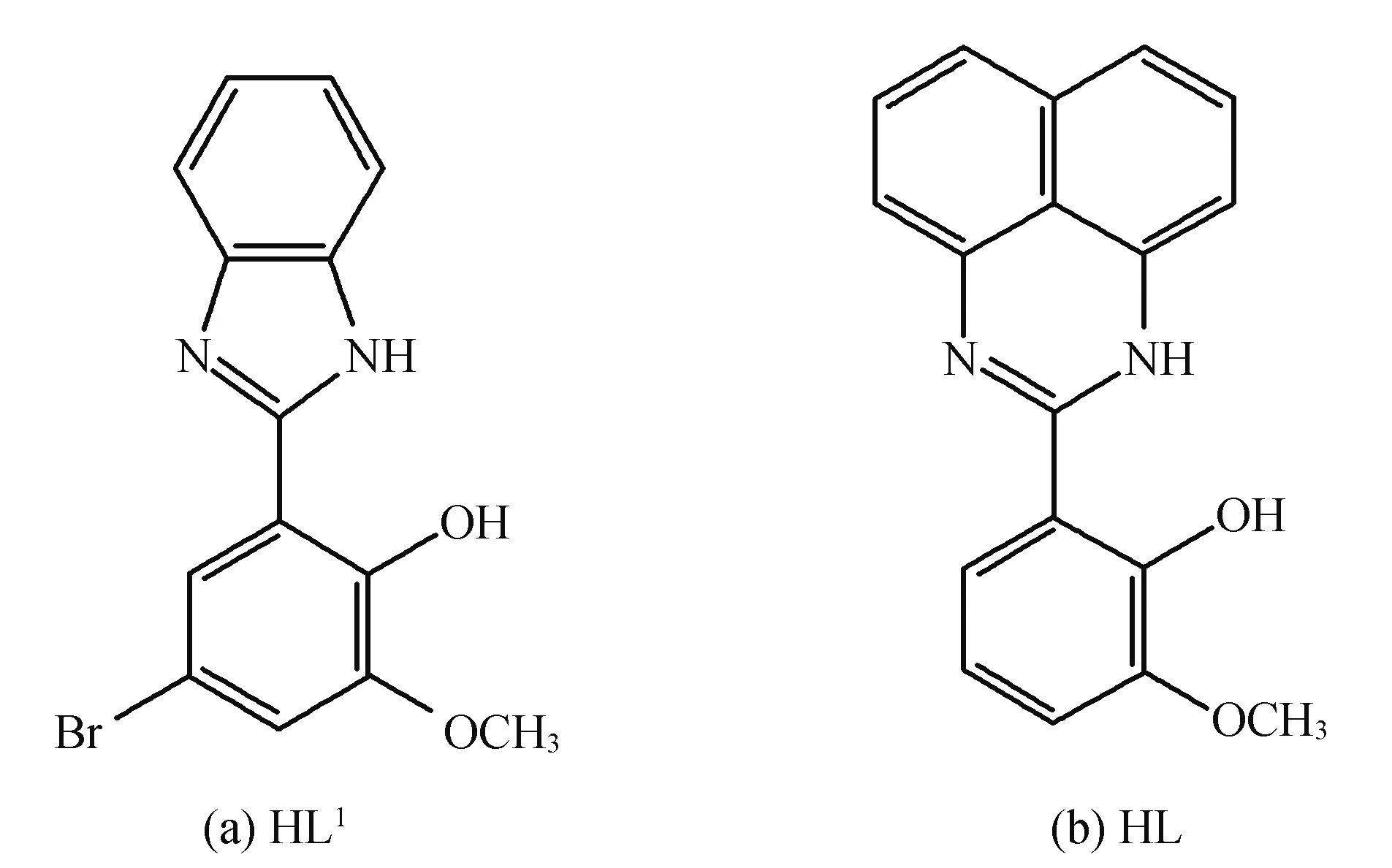
Scheme 1 Structures of HL1 (a) and HL (b)
1 Experimental
1.1 Materials and general methods
Ln(NO)3·6H2O were prepared by the reactions of Ln2O3with nitric acid in the aqueous solution. All the other chemicals were obtained from commercial sources and used without further purification. Fourier transform infrared (FT-IR) data were recorded on a PerkinElmer 100 spectrophotometer in the range of 4 000~500 cm-1using KBr disks. UV-vis spectra (in CH3CN) were recorded on a PerkinElmer Lambda 35 spectrometer. Elemental (C, N, and H) analysis were carried out on a PerkinElmer 2 400 analyzer. Thermal analyses were carried out on a PerkinElmer STA 6000 in the temperature range of 30~800 ℃ with a heating rate of 10 ℃ min-1under atmosphere. Powder X-ray diffraction (PXRD) data were recorded on a Rigaku D/Max-3B X-ray diffractometer with Cu Kα radiation, the scanning rate was 4°/s, 2θ ranging from 5~50°. The magnetic susceptibility for complex1was conducted with a Quantum Design MPMS-XL-7 SQUID-VSM magnetometer. The magnetic corrections were made by using Pascal’s constants.The detailed information of samples is shown in Fig.1-4.

Fig.1 TG-DSC curves of complex 1

Fig.2 TG-DSC curves of complex 2

Fig.3 PXRD patterns of simulated (black) and experimental (red) for complex 1
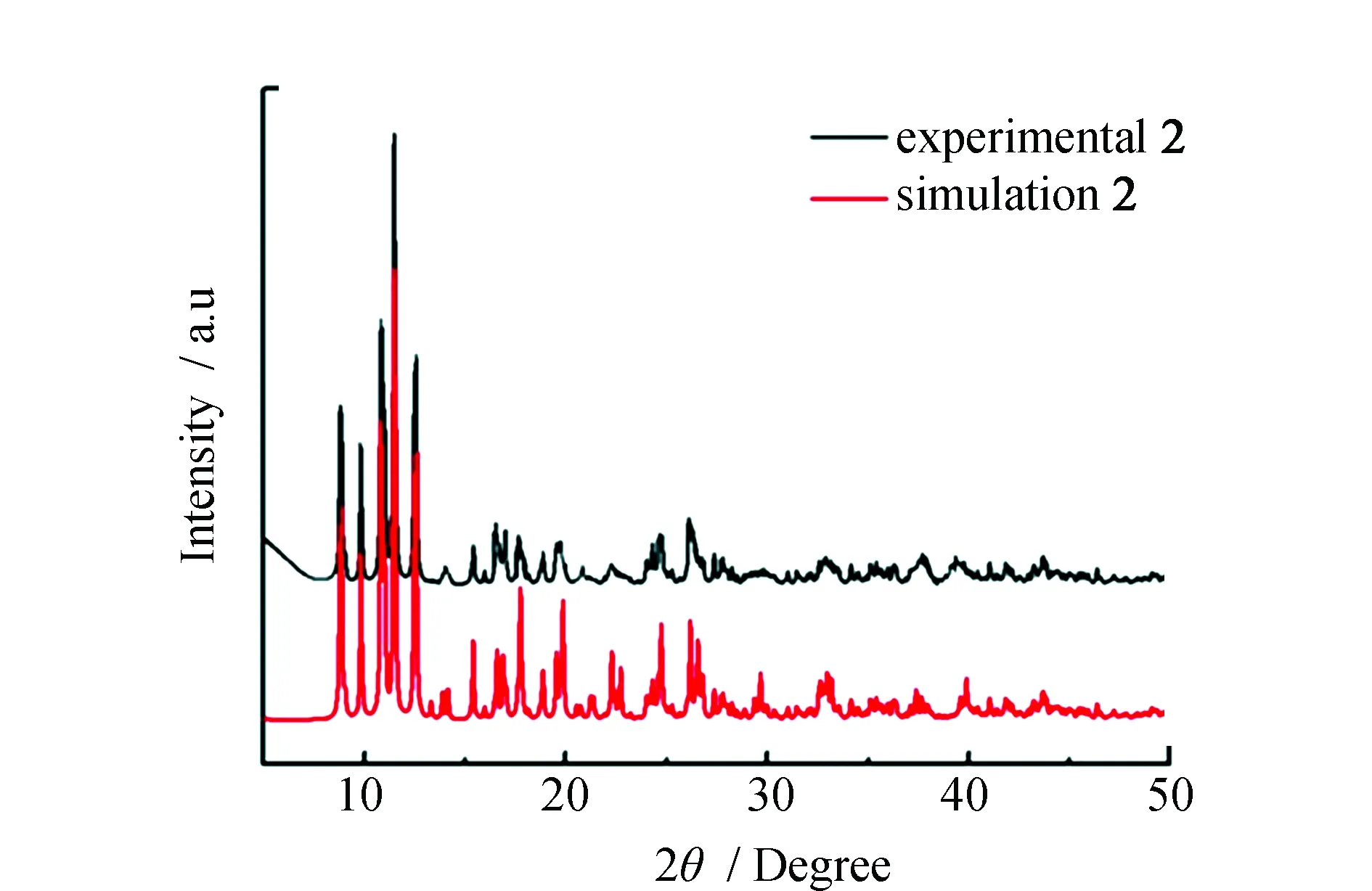
Fig.4 PXRD patterns of simulated (black) and experimental (red) for complex 2
1.2 Syntheses of HL, complexes 1 and 2
1.2.1 Syntheses of 2-(2′-hydroxyphenyl-3′-methox-yl)naphthoimidazole (HL)
2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole (HL) have been prepared by refluxing the mixtures of 1,8-diaminonaphthalene (1.58 g, 0.01 mol) and o-Vanillin (3.04 g, 0.02 mol) in EtOH (30 mL) for 4~5 h. The precipitates formed were filtered, washed with cold EtOH, and recrystallized from hot EtOH. Anal. Calcd for C18H14N2O2(290.32): C, 74.47; H, 4.86; N, 9.65 wt%. Found: C, 74.39; H, 4.83; N, 9.69 wt%. Yield, 70 wt%.
1.2.2 [Dy(HL)2(NO3)3] ]·CH2Cl2(1)
Dy(NO)3·6H2O (0.1 mmol, 0.045 7 g) and 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole (HL) (0.2 mmol, 0.085 2 g) were dissolved in a mixture solution of CH3OH/CH2Cl2, (20 mL, v/v, 1/1). The mixture was consequently allowed to stir at room temperature for 4 h. Then hexane (60 mL) was allowed to diffuse slowly into the filtrate. Crystals suitable for single crystal X-ray analysis were obtained in 7 days.Anal. Calcd for C37H30Cl2DyN7O13(1 014.06): C, 43.82; H, 2.98; N, 9.67 wt%. Found: C, 43.70; H, 2.63; N, 9.80 wt%. Yield, 72 wt%. IR (KBr, cm-1): 3 056, 2 939, 2 840, 1 623, 1 508, 1 384, 1 220, 1 065, 1 029, 819. UV-vis [MeOH, λ]: 203, 229, 270, 279, 342 nm.
1.2.3 [Ce(HL)2(NO3)3] ]·CH2Cl2(2)
Ce(NO)3·6H2O (0.1 mmol, 0.043 4 g) and 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole (HL) (0.2 mmol, 0.085 2 g) were dissolved in a mixture solution of CH3OH/CH2Cl2, (20 mL, v/v, 1/1). The mixture was consequently allowed to stir at room temperature for 4 h. Then hexane (60 mL) was allowed to diffuse slowly into the filtrate. Crystals suitable for single crystal X-ray analysis were obtained in 7 days. Anal. Calcd for C37H30Cl2CeN7O13(1 024.08): C, 44.53; H, 3.34; N, 9.67 wt%. Found: C, 44.52;H, 3.32; N, 9.69 wt%. Yield, 80 wt%. IR (KBr, cm-1): 3 060, 2 940, 2 845, 1 623, 1 508, 1 383, 1 285, 1 066, 819, 736. UV-vis [MeOH, λ]: 202, 229, 271, 279, 344 nm.
1.3 Determination of the crystal structures
Single crystals of complexes1and2were selected at 293±2 K for X-ray diffraction analysis on an Oxford Xcalibur Gemini Ultra diffractometer using graphite-monochromated Mo-Kα radiation (λ=0.071 073 nm) at room temperature. The structures of complexes1and2were solved by direct methods, and all non-hydrogen atoms were anisotropically refined by full-matrix least squares methods on F2 using SHELXS-97 crystallographic software package[9]. There was one CH2Cl2molecule in the unit cells of complexes1and2, respectively, which has been treated as diffuse contributions to the overall scattering without specific atom positions using the SQUEEZE/PLATON tools, respectively. The SQUEEZE results were consistent with TG-DSC and elemental analysis. The crystal data and structure refinement details were summarized in table 1 for complexes1and2. CCDC No.1441074 and 1441078 contain the supplementary crystallographic data for complexes1and2, respectively. These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.
2 Results and discussions
2.1 Description of crystal structures
Structural analysis shows that complexes1and2are isostructural crystallized in the monoclinic P21/cspace group. In a typical structure of complex1(Fig.5a), the DyIIIion is ten-coordinated to four phenolate O atoms from two ligands and six O atoms from three bidentate nitrate groups adopting sphenocorona geometry (Fig.5b). The Dy-O bond lengths are in the range of 0.227 3(90)-0.269 0(107) nm in accordance with the reported values[10]. The N atoms of the ligands remain exclusively uncoordinated. Notably, the two adjacent ligands attached to the DyIIIions pack in an offset head-to-head fashion.

Table 1 Crystal data and structure refinement for complexes 1 and 2
2.2 Magnetic Properties
The temperature dependence curves of the direct current (dc) magnetic susceptibilities under an applied dc field of 100 Oe in the temperature range of 2~300 K (Fig.6) show that the χMT of complex1at 300 K is 14.13 cm3K mol-1, which is close to the expected values for an isolated DyIIIion (S=5/2, L=5, J=15/2,6H15/2, g=4/3). Upon cooling, the χMT product gradually decreased to reach a minimum value of 8.70 cm3K mol-1at 2.0 K for complex1, which is mostly due to the thermal depopulation of the Stark sublevels and/or significant magnetic anisotropy in DyIIIion systems. The field dependence of the magnetization for complex1rises slowly before reaching 4.13 N μBat 2.0 K. Moreover, the absence of a superposition of the M versus H data at higher field indicates the presence of significant magnetic anisotropy in complex1.
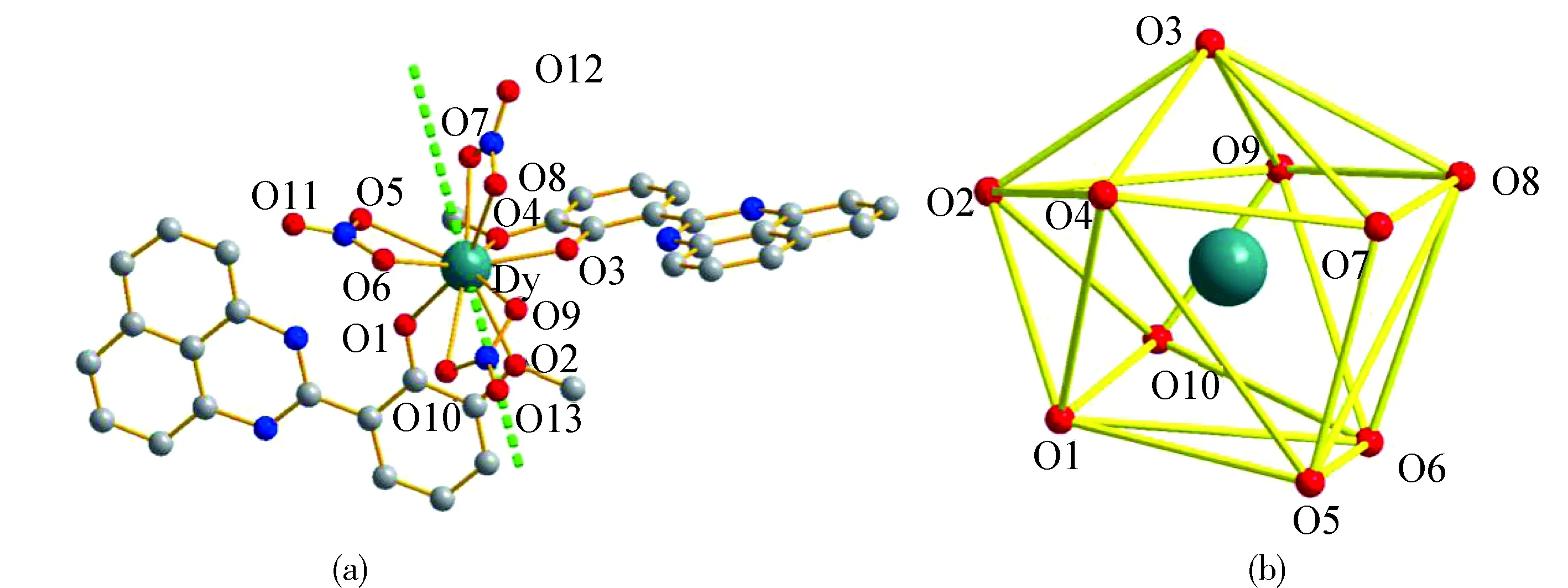
Fig.5 (a) The molecular structures of complex 1 (hydrogen atoms have been omitted for clarity). Orientation of the main magnetic anisotropy axes in the ground-state for the DyIII ion center;(b) Polyhedron view of the coordination geometry

Fig.6 Outset: Temperature dependence of χmT at 1 kOe field for complex 1Inset: The field dependence of magnetizationfor complex 1 at 2.0~8.0 K
Alternating current (ac) susceptibility curves at zero external field show no in-phase signal (χ′) and out-of-phase signal (χ″) of the ac susceptibility at frequencies up to 1 000 Hz and the temperatures down to 1.8 K for complex1. It suggests that the magnetization relaxation time (τ) is much shorter than 1/2 πν and that the quantum tunnelling of the magnetization (QTM) plays an important role[11]. However, when a static direct current (dc) field of 2 kOe is applied, theχ′ andχ″ component is strongly enhanced indicating that the presence of QTM can be significantly reduced by the applied field (Fig.7)[12]. In the temperature dependent ac magnetic susceptibility data, the clear frequency-dependent full peaks can be observed both inχ′ andχ″ plots in the frequency region of 1~1 000 Hz over the testing temperature range (Fig.7a and Fig.7c). Moreover, theχ″ signals shift to a lowertemperature with decreasing frequency, indicating a thermally activated relaxation process. In the frequency dependent ac magnetic susceptibility data (Fig.7b and Fig.7d), the relaxation times (τ) are calculated from the frequencies (τ-1=2 πν) of the peak maxima at the corresponding temperatures.
Frequency dependence ofχ″ peaks is analysed through Arrhenius law (τ=τ0exp(Ueff/kT), where T is the temperature of the maximumχ″ at different frequencies,τ=1/2 πν, and Ueffis the anisotropic energy barrier) affords the anisotropic energy barrier of 28 K and the pre-exponential factor τ0of 1.36 ×10-6s (Fig.8a), which are comparable to 50 K (Ueff/ kB) and 6.80 × 10-7s (τ0) for [Dy(L2)(NO3)2]·CH3OH (H2L2=N,N′-bis-pyridin-2-yl-methelene-1,8-diamino-3,6-dioxaoctane)[13]. According to the generalized Debye model for the high frequency region. The Cole-Cole plots at 2~5 K of complex1(Fig.8b) can be fitted well, withαvalues in the range of 0.236~0.409 for complex1(Table 2), indicating a relatively narrow distribution of the relaxation processes.
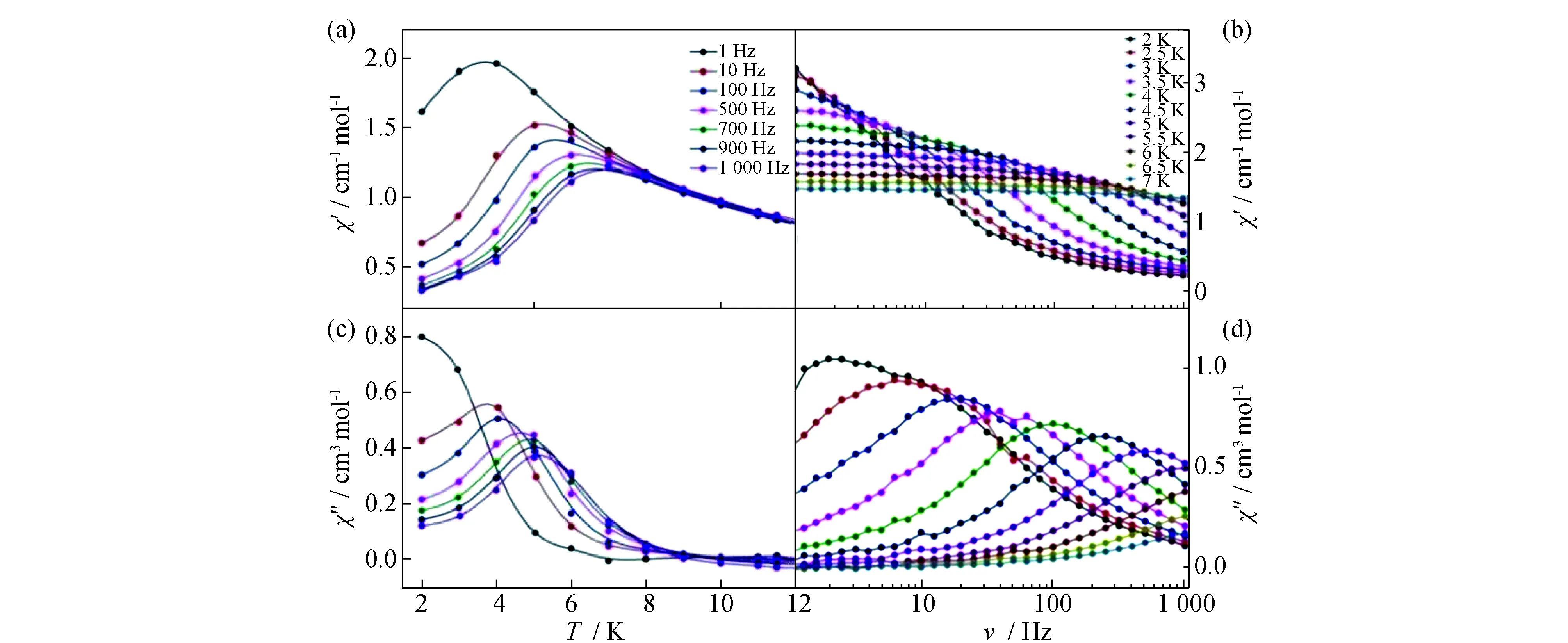
Fig.7 Temperature dependence of the in-phase (a) and out-of-phase (c) ac susceptibility and frequency dependence of in-phase (b) and out-of phase (d) ac susceptibilities for complex 1 under 2 kOe dc field

Fig.8 (a) The τ versus T-1 plot for complex 1 under 2 000 Oe dc fields, the red solid line represent the best fits using the Arrhenius law; (b) The Cole-Cole plots at 2~5 K of complex 1 measured under 2 000 Oe dc field, and the red solid lines are the best fitting according to the generalized Debye model

Table 2 Relaxation Fitting Parameters from the Least-Square Fitting of the Cole-Cole plots of complex 1 according to the CC-FIT
To further study the magnetism for complex1, the anisotropy axis for DyIIIion was calculated on the basis of the electrostatic model recently reported by Chilton et al[14]. Following the program MAGELLAN, the ground-state magnetic anisotropy axes for complex1and reported [Dy(L2)(NO3)2]·CH3OH were found (Fig.9). Since the overall shape of free-ion electron density for DyIIIion is oblate, it can increase the energy barrier by longitudinal tensioning the electron density of DyIIIion[15]. The two nitrate groups with electron deficient from [Dy(L2)(NO3)2]·CH3OH are better to longitudinal tension the electron density of DyIIIion in the magnetic anisotropy axis direction. Therefore, the energy barrier of [Dy(L2)(NO3)2]·CH3OH is much higher than that for complex1(Fig.10).

Fig.9 Orthogonal configurations for the magnetic anisotropy axis in complex 1 (left) and [Dy(L2)(NO3)2]·CH3OH (right) [S1]

Fig.10 Molecular structure of complex 1 (a) and [Dy(L2)(NO3)2]·CH3OH (b) (hydrogen atoms have been omitted for clarity). Orientation of the main magnetic anisotropy axes in the ground-state for the DyIII ion center
3 Conclusions
Isolation of two complexes1and2demonstrates that 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole is able to coordinate to the lanthanide ions affording mononuclear lanthanide complexes, and the structures of the ligand dominate the structures of the complexes and the coordination geometries of the DyIIIions. Static and dynamitic magnetic analysis suggests that the nature of the DyIIIions produces the single molecule magnetism for complex1. Notably, the ligand 2-(2′-hydroxyphenyl-3′-methoxyl)naphthoimidazole induce the magnetic relaxations of the DyIIIions strengthen the magnetic anisotropy of the DyIIIions, resulting in a 28 K energy barrier. The anions along the magnetic anisotropy axis dominate the magnetism of complex1.
[1] Sorace L, Nelli C, Tteschi D. Lanthanides in molecular magnetism: old tools in a new field [J]. Chem. Soc. Rev.,2011, 40:3092-3104.
[2] Woodruff D N, Winpenny R E P, Layfield R A. Lanthanide single molecule magnets [J]. Chem. Rev.,2013, 113:5110-5148.
[3] Yang P P, Gao X F, Song H B, et al. Slow magnetic relaxation in novel Dy4 and Dy8 compounds [J], Inorg. Chem.,2011, 50:720-722.
[4] Habib F, Lin P H, Long J, et al. The use of magnetic dilution to elucidate the slow magnetic relaxation effects of a Dy2 single-molecule magnet [J]. J. Am. Chem. Soc.,2011, 133:8830-8833.
[5] Chen Y C, Liu J L, Ungur L, et al. Symmetry-supported magnetic blocking at 20 K in pentagonal bipyramidal Dy(III) single-ion magnets [J].J. Am. Chem. Soc.,2016, 138:2829-2837.
[6] Liu J, Chen Y C, Liu J L, et al. A stable pentagonal-bipyramidal Dy(III) single-ion magnet with a record magnetization reversal barrier over 1000 K [J]. J. Am. Chem. Soc.,2016, 138:5441-5450.
[7] Feng X, Ling X L, Liu L, et al. A series of 3D lanthanide frameworks constructed from aromatic multi-carboxylate ligand: structural diversity, luminescence and magnet [J]. Dalton Trans.,2013, 42:10292-10303.
[8] Yang X, Jones R A, Oye M M, et al. Transformation of a luminescent benzimidazole-based Yb3 cluster into a one-dimensional coordination polymer [J].New J. Chem.,2011, 35:310-312.
[9] Sheldrick G M. SHELXL-97, a crystallographic program for structure refinement, Germany:University of Göttingen,1997.
[10] Ma Y, Xu G F, Yang X, et al. Pyrazine-bridged Dy2 single-molecule magnet with a large anisotropic barrier [J]. Chem. Commun.,2010, 46:8264-8266.
[11] Liu J L, Yuan K, Leng J D, et al. Anion-induced self-assembly of luminescent and magnetic homoleptic cyclic tetranuclear Ln4(salen)4 and Ln4(salen)2 complexes (Ln = Nd, Yb, Er, or Gd) [J]. Inorg. Chem.,2012, 51:8538-8542.
[12] Poneti G, Bernot K, Bogani L, et al.A rational approach to the modulation of the dynamics of the magnetisation in a dysprosium-nitronyl-nitroxide radical complex[J].Chem. Commun,2007:1807-1809.
[13] Campbell V E, Guillot R G, Riviere E, et al. Subcomponent self-assembly of rare-earth single-molecule magnets [J]. Inorg. Chem.,2013, 52:5194-5200.
[14] Chilton N F, Collison D, McInnes E J L, et al. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes [J]. Nat. Commun.,2013, 4:2551-2557.
[15] Rinehart J D, Long J R, Exploiting single-ion anisotropy in the design of f-element single-molecule magnets [J].Chem. Sci.,2011, 2:2078-2085.

