Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
Wasan Jaruchai, Tidarat Monkham, Sompong Chankaew, Bhalang Suriharn, Jirawat Sanitchon
Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Khon Kaen 40002,Thailand
RESEARCH ARTICLE
Evaluation of stability and yield potential of upland rice genotypes in North and Northeast Thailand
Wasan Jaruchai, Tidarat Monkham, Sompong Chankaew, Bhalang Suriharn, Jirawat Sanitchon
Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Khon Kaen 40002,Thailand
The planting of upland rice is one cropping option in area with limited water availability and low soil fertility in North and Northeast Thailand. The varietal selection was determined by grain yield potential, wide adaptation, and good stability. This study was aimed at evaluation of indigenous upland rice germplasm for yield and yield stability in multi-locations. Thirty-six upland rice genotypes collected from six provinces of the North and Northeast Thailand and one check variety (Sewmaejan)were assessed under five locations in the rainy seasons of 2009 and 2010. The experiment was laid out in a randomized complete block design with three replications. The genotype grain yield was highly affected by location (59.90%), followed by genotypes (G)×location (L) interaction (12.80%) and genotype (6.79%). The most suitable location for the genotype evaluation was L3 (Khon Kaen-KKU10) which associated with stability of grain yield for all genotypes. Furthermore, biplot and regression analysis indicated that genotype numbers 6 (Jaowmong 1), 10 (Neawmong 1), 18 (Neawdum 1), 19 (Leamna),20 (Prayaleamkang), 32 (Kunwang 2), and 33 (Kunwang 3) showed great yield stability over five locations. The genotypes will be applicant for upland rice production area and parental base in breeding program.
upland rice, yield stability, germplasm, multi-location trails, G×L interaction
1. Introduction
Upland rice is one of the most popular cropping options in slope area under rainfed condition, and accounts for about 11% of global rice production (Tuhina-Khatun et al. 2015), of which about 13 million ha were grown in Asia (Acuña et al.2008). In Thailand, upland rice accounted for 11% of the total rice planted area (IRRI 1998), most of which located in North and Northeast Thailand (Bell and Seng 2004). Upland rice in Thailand is normally grown along upland, hilly, slope,and mountainous area which diverse in topography and environment leading to a large diverse in varieties. There is a need for the evaluation of yield potential and yield stability through multi-location testing as the basis for future upland rice production in North and Northeast Thailand, where there are considerable diversity of both genotypes and production environments.
The high water use ef ficiency is one of the most important attributes of upland rice (Price et al. 2002), and the main criteria for its selection in upland areas with low rainfall.Furthermore, upland rice is useful as a donor for breeding improved root systems and improved adaptation to water stress growing environments (Bernier et al. 2008), improved resistance to disease and insect pests (Fukuoka and Okuno 2001), and for intercropping of sugarcane to improve soil fertility and increase income (Vityakon et al. 2000). A large range of upland rice varieties have been used in breeding programs to improve crop adaptation. However, the screening methods for a large number of large genotypes under wide range of environments can be a potential constraint due to the high variation in genotypes, the variability of environments, and genotypes (G)×location (L) interaction(Wade et al. 1999; Xing et al. 2002; Acuña et al. 2008).
Evaluation of grain yield under multi-environments is one of numerous approaches to verify the stability of genotypes(Acuña et al. 2008). However, the interaction of genotypes and environment always contributes to the stability of rice varieties (Bose et al. 2012). The use of the regression coef ficient to explain the response of genotypes is another approach for evaluating the stability (Eberhart and Russell 1966). Currently, the principal component method/analysis is a powerful tool for the evaluation of G×L interaction with a high degree of accuracy, while also allowing easy interpretation from single graphs (Yan et al. 2000, 2007;Gauch et al. 2008; Balestre et al. 2010). Moreover, the bi-plot approach is also helpful in breeding programs for the selection of a suitable environment for crop evaluation,genotypic selection for a speci fic location and/or stability of crop genotypes such as mung bean (Alam et al. 2014),maize (Fan et al. 2007), durum wheat (Mohammadi et al.2009), and rice (Samonte et al. 2005). Therefore, high yielding upland rice varieties with good stability need to be identi fied on the basis of being area speci fic. Available tools do allow the identi fication of appropriate upland rice varieties for growing conditions where there are high G×L interaction. The objective of this study is the evaluation of the stability and yield potential of upland rice genotypes in multi-location growing environments.
These results enable us to identify suitable varieties of diverse upland rice (both glutinous and non-glutinous)with high yield performance and stability for promotion and extension to farmer in numerous upland are in North and Northeastern Thailand.
2. Materials and methods
2.1. Rice genotypes and experimental design
Thirty-six upland rice genotypes were collected from six provinces in North and Northeast Thailand (Phetchabun,Loei, Phitsanulok, Chiang Mai, Khon Kaen, and Mukdahan)provided by the Rice Germplasm Project of Khon Kaen University, Thailand (Table 1). The variety Sewmaejun was used as a standard check variety from Rice Research Department, Thailand, which high cooking quality and upland recommended. Thus, 37 genotypes were evaluated.The comparative study of the varieties was conducted in five locations in North and Northeast Thailand during the 2009 and 2010 wet-seasons, the locations being: Ban Had 2009 (BH09, L1), Ban Had 2010 (BH10, L2), Khon Kaen University Field 2010 (KKU10, L3), and Chum Phae Rice Research Center 2010 (CPA10, L4) were located in northeastern part of Thailand and Mae Hong Son Rice Research Center 2010 (MHS10, L5) was located in northeastern part of Thailand (Table 2 and Appendix A). The experiments were laid in a randomized complete block design with three replications.
Planting date was determined by the rainfall distribution at each site, and usually took place in the period between early June and early July, with harvesting taking place between late October and early November (Table 2). The plots were direct seeded using 3–5 seeds per hill, with subsequent thinning to 1 seedling per hill at 10–15 days after the seedlings had emerged. The plot size was 1.8 m×4.0 m(96 plants per plot) with a row and hill spacing of 30 and 25 cm, respectively. Fertilizer was applied at a rate of 14.6 kg ha–1:14.6 kg ha–1:14.6 kg ha–1(N:P2O5:K2O) at 30 days after seedling emergence, at the same time as weeding, with a top-dressing of 57.5 kg N ha–1at 60 days after seedling emergence. The moisture condition was dependent on the rainfall at each location. Fungicide was applied at location MHS10 at 60 days after-sowing due to an outbreak of leaf blast disease.
2.2. Data collection
Tiller and panicle number per hill and plant height were collected from four hills selected at random in the middle row of each plot. Days to flowering (DTF) were based on counts of flowering plants in each plot every 3 days to determine the time of 50% flowering. Number of seeds per panicle was based on seed counts of four panicles per plot, the panicles being randomly selected. Grain yield was based on the grain harvested from the middle row of each plot (56 plants per plot); the harvested seed was then air-dried to about 14% moisture content and weighed to provide the basis of yield in terms of kg ha–1. 1 000-seed weight was measured.
2.3. Data analysis
The analysis of all parameters was based on a randomized complete block design with three replications in all sites. Stability analysis on a mean genotype was used to determine the genotypic consistency between sites (envi-ronments), and also to determine the factors contributing to grain yield variation in each site. The regression analysis followed the Eberhart and Russell (1966) method, while the program R-language and environment for statistical computing and graphics ver. 3.2.1 were used to determine the genotypic main effect plus genotype-by-environment
interaction (GGE)-bi-plot (Gollob 1968; Onofri and Ciricifolo 2007).

Table 1 Upland rice genotypes, seed location source and mean grain yield in each experiment location of Thailand
3. Results
3.1. Yield and yield components across locations
Grain yield and yield component of the 37 genotypes in five locations were evaluated. The main effect on variation was location, which affected both grain yield and yield components. The 10 highest grain yields were mostly from varieties originating in North Thailand, with most having a higher grain yield than the check cultivar(Sewmaejan). Nevertheless, most northeastern locations had higher grain yields than northern locations, especially at BH10 (Appendix A). The mean grain yield of the 37 genotypes is summarized in Table 1 for the 5 locations.The mean grain yield of genotypes ranged from 1 637.5 to 2 768.8 kg ha–1(Table 1). Grain yield differences among genotypes were highly significant, as also 1 000-seed weight, seeds per panicle, DTF, plant height,and tillers per hill. The highly signi ficant effect of experiment location was re flected in the signi ficance of G×L interaction for most plant parameters, except for panicle number per hill. The average grain yield range from 1 125 kg ha–1(L5)to 3 162.5 kg ha–1(L2), while the mean grain yield across the five locations were 2 062.5 kg ha–1(Table 3). In terms of the contribution of different factors to variability in yield,location accounted for 59.90% of variability, genotype 6.79%, and their interaction 12.80% (Table 4). The results indicated that location had the greatest effect on grain yield of upland rice genotype.
3.2. The yield stability of upland rice genotypes thought five locations in Thailand
The stability of the genotypes in all environments showed that the genotypes on the right hand side in Fig. 1 had a higher grain yield than those on the left hand side. Genotypes numbers 6, 10, 18, 19, 20, and 33 showed higher yields than others, even though genotypes 6 and 19 showed negative principal component 1 (PC 1) scores (Fig. 1). Thegrowing locations L3 and L5 had positive PC 1 scores near zero, indicated that there was only a small G×L interaction.All of the genotypes showed good performance in these locations (L3 and L5). However, genotypes numbers 18,20, and 33 showed high levels of stability and less affected by the growing environment, with PC 1 scores close to zero and the most favorable location being L3. The best locations for genotype selection were L3 and L5, due to the high stability of upland rice grain yield. The genotypes with high grain yield and stability were genotypes numbers 18 and 33. A summary of G and L performance was illustrated in Fig. 2. Genotype number 10 showed the highest grain yield at three locations (L1, L3 and L5). Genotypes numbers 23 and 21 showed the highest grain yield at locations L1 and L4. Genotypes numbers 12, 19, and 20 showed the highest grain yields at locations L4 and L2. L2 and L5 were the best locations for genotypes numbers 28 and 35. Genotype number 6 showed high stability in grain yield, as re flected low PC 2 and being close to the point of origin in the Fig. 2.The results showed that genotypes with the greatest yield stability across the different locations were not those which gave the highest grain yields.
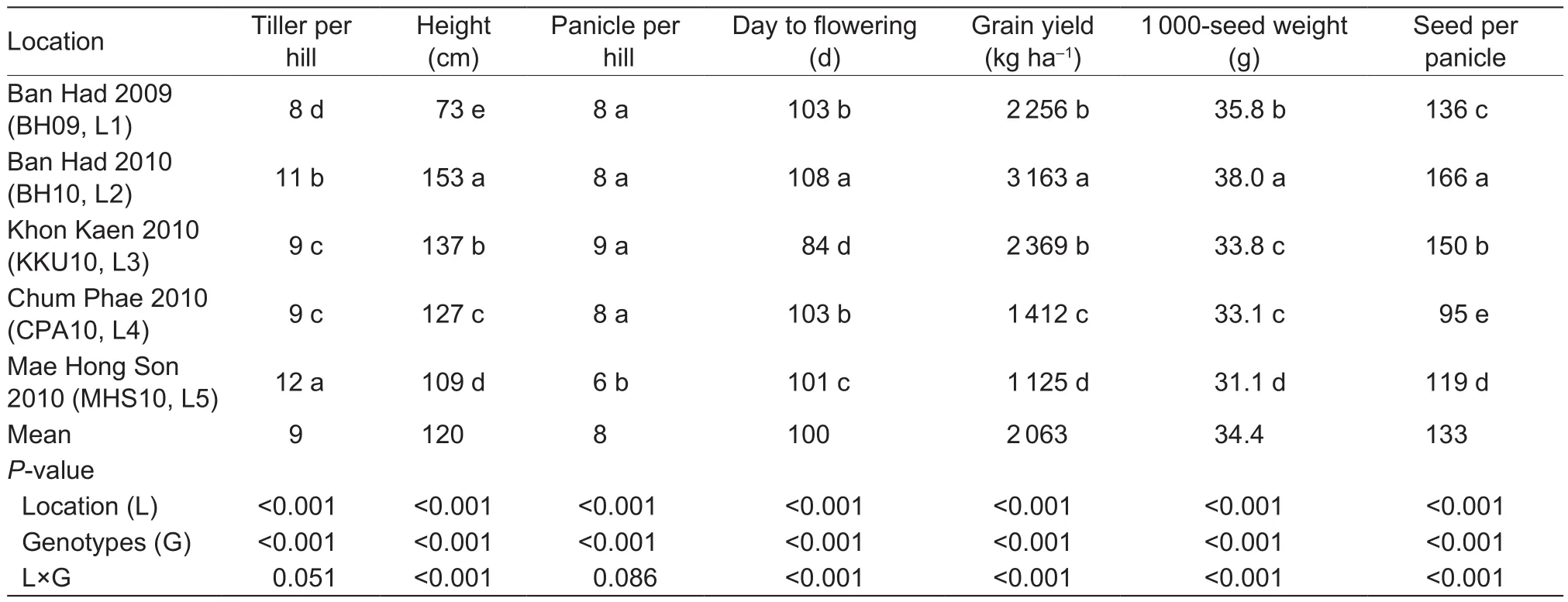
Table 3 Grain yield (GY), 1 000-seed weight, number of seeds per panicle, panicle number per hill, days to flowering (DTF), plant height, and tiller number per hill for the five locations of Thailand

Table 4 Combined analysis of variance of grain yield for 37 upland rice genotypes evaluated at five locations of Thailand in 2009 and 20101)

Fig. 1 Genotypic main effect plus genotype-by-environment interaction (GGE)-bi-plot of grain yield (kg ha–1) of 37 rice genotypes (G) and five locations (L) of Thailand. L1, Ban Had 2009; L2, Ban Had 2010; L3, Khon Kaen 2010; L4, Chum Phae 2010; L5, Mae Hong Son 2010. The average environment axis (AEA) is the straight line that passes though the origin(0), principle component 1 (PC 1), and the mean grain yield(kg ha–1) is vertical dot line.
In reference to the PC 1 and PC 2 scores, 70.1% were accounted for by G and G×L interaction. Genotypes numbers 19 and 21 showed high levels of interactions. Location 2 was highly different from the other locations. The bi-plot showed the highest grain yield in genotypes numbers 19, 20, 33, 6,18, 10, and 32, which were grouped with locations and were close to the graph origin (0,0). Another group consisted of low grain yielding genotypes in the 2rd quadrant (genotypes numbers 16, 17, 3, 5, and 2) (Fig. 3). The genotypes which located nearer to the X-axis showed greater stability. Thus,genotypes numbers 19, 20, 6, and 18 had high stability and grain yields. The regression coef ficient (b) according to the method of Eberhart and Russell (1966), showed that three groups of genotypes; group 1 were b>1.0, group 2 were b=1.0, and group 3 were b<1.0. The regression coef ficient of genotypes which have b=1.0 indicated that high stability.In this experiment, the high grain yield and b-value close to 1.0 were the criteria for genotype selection. The genotypes in group 2 with high grain yield were genotypes numbers 4,6, 10, 18, 19, 20, 28, 32, and 33 (Fig. 4). The combination of the two methods of stability analysis showed that most of genotypes with high grain yield, e.g., 6, 10, 18, 19, 20,32, and 33 were also stable in all conditions.

Fig. 2 Polygon view of the genotypic main effect plus genotypeby-environment interaction (GGE)-bi-plot based on grain yield pattern for genotypes (1–37) and environments in five locations of Thailand. L1, Ban Had 2009; L2, Ban Had 2010; L3, Khon Kaen 2010; L4, Chum Phae 2010; L5, Mae Hong Son 2010.PC 1, 44.3% and PC 2, 25.6%.
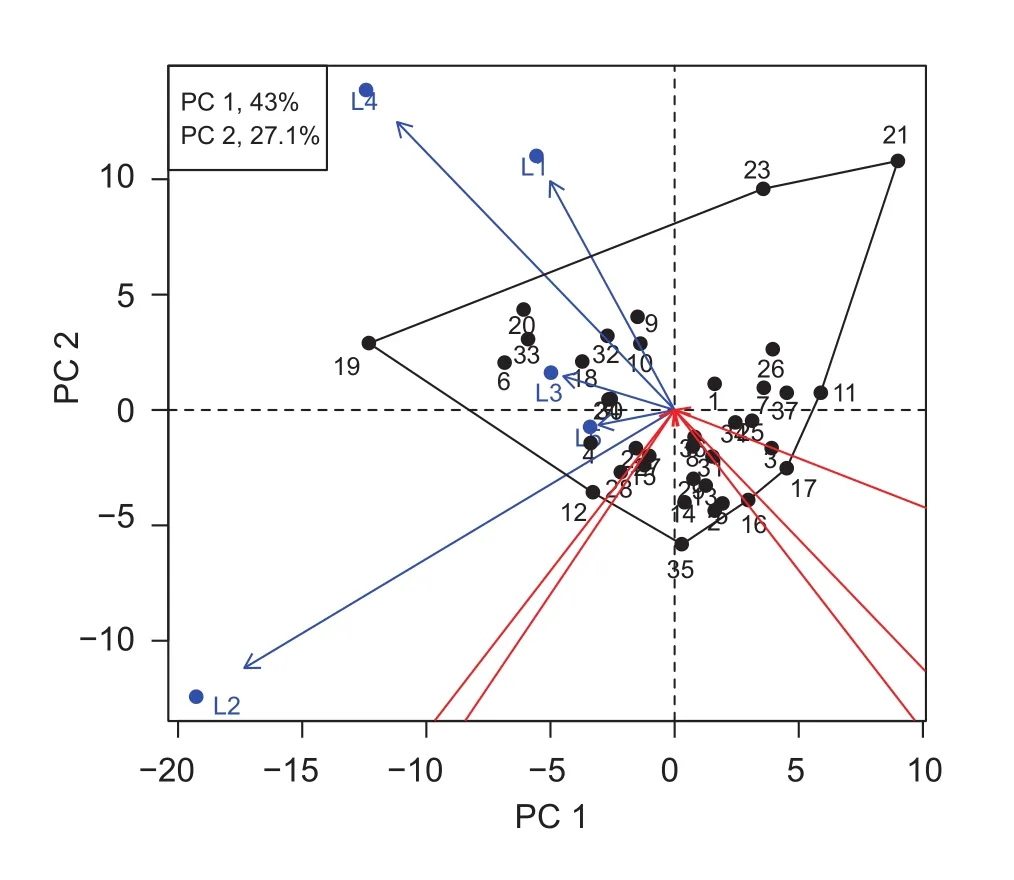
Fig. 3 Genotypic main effect plus genotype-by-environment interaction (GGE)-bi-plot based on grain yield of 37 upland rice genotypes in five locations of Thailand. L1, Ban Had 2009; L2,Ban Had 2010; L3, Khon Kaen 2010; L4, Chum Phae 2010; L5,Mae Hong Son 2010. PC, principle component.
4. Discussion
4.1. The effected of location of upland rice performance

Fig. 4 The relationship between regression coef ficient (b)and grain yields of 37 upland rice genotypes in five locations of Thailand.
The upland areas of North and Northeast Thailand were characterized by sloping land with low/poor water storage(Crews-Meyer 2004; Mongkolsawat et al. 2006; Panomtarinichigul 2006; Tingting et al. 2008). The impact of differences in the geographic environment were the main factor in variety selection in each area. As the lowland and upland rice growing environments require good yielding rice varieties,multi-location testing of varieties should be done. The percentage of sum square (SS) is explained by the effects of different factors. In this study, location showed the highest explained SS which was affected by interactions. The effect of genotype by location interactions was mainly in response to the environment rather than genotypes (Saito et al. 2010;Tariku et al. 2013). The location which gave the highest average grain yield of genotypes (BH10) did not receive the highest amount of rainfall, while the lowest grain yield location (MHS10) showed the lowest rainfall. High rainfall was not the main determination of final grain yield. Upland rice crops were less water requirement than lowland rice as aerobic rice which suitable for water scarce area (Bouman et al. 2005). This was re flected in the breeding strategy for water de ficit areas (Courtois et al. 2000; Bernier et al. 2007,2008) and there are widely used in water saving systems(Tuong and Bouman 2003). Upland rice generally needs between 14 to 20 mm of rainfall in five-day cycles during the growing cycle (Oikeh et al. 2008). All five locations in this study had suf ficiency rainfall for upland rice cropping.The differences of genotypes performance between locations were overlapped due to the variations in the growing environment. The best growing environment in terms of grain yield was L2 (BH10), but in terms of stability as a test location site L3 (KKU) was a suitable location (Fig. 1). The small interaction effect of the environment was close to zero in the PC 1 score, and this had only a small impact on variation of genotype performance (Akter et al. 2014). L3, L1,and L5 were suitable locations for assessing the potential of genotypes, with the low interaction being re flected in shorter spokes (Fig. 2). Based on the report of Akter et al. (2014),the shorter line of location from the graph origin referred to the lower the genotype (G)×location (L) interaction.
4.2. Genotype performance and stability of upland rice
Yield components were the main effect on rice production(Yoshida and Parao 1976; Tao et al. 2006). The high grain yield of genotypes was due to a combination of tiller number(r=0.607**), panicle number (r=0.643**), 1 000-seed weight(r=–0.421**), and days to flowering (DTF) (r=0.403*) (data not shown). The grain yield of rice was mainly affected by environment (60%) follow by the G×L and L (13%) and genotypes (7%), which similar to that reported by Lakew et al. (2014), who suggested that the different responses of G across L was determined more by G×L interaction,which was nearly thrice larger than genotypes effect as also reported by Naveed et al. (2007), Saito et al. (2010),and Shrestha et al. (2012). Due to the large effect of the G×L interaction in the rice evaluation program, the ranking of genotypes was different in each location. Thus, speci fic genotypes were selected for diverse environments (Bose et al. 2012; Akter et al. 2014; Liang et al. 2015). Some of the genotypes re flected the diverse hydrological environments,even though they had similar backcrossing lines (Acuña et al. 2008). The G×L interaction was mostly due to the variation in the growing environment affected to grain yield and genotypes stability (Eberhart and Russell 1966; Asenjo et al. 2003). Yan et al. (2007) reported the comparison of the genotypic main effect plus genotype-by-environment interaction (GGE) and additive main effects and multiplicative interaction (AMMI) methods were useful for the evaluation of genotypes (G) and G×L interactions under mega-environments, rather than separate G and G×L interactions for individual environments. Furthermore,the genotypes with a regression coef ficient (b) close to 1 were identi fied as having more stability than b>1 and b<1,according to Eberhart and Russell (1966) method. The combination of high stability and high grain yield were of greatest importance for variety recommendations to farmers.Grain yield of genotypes numbers 6 (Jaowmong 1) and 19(Leamna) showed better in favorable condition, numbers 18(Neawdum 1) and 32 (Kunwang 2) showed good in adverse condition. For the stability following Eberhart and Russell(1966) method, genotypes numbers 10 (Neawmong 1), 20(Prayaleamkang), and 33 (Kunwang 3) showed the highest compared with other genotypes, and showed greater stability following GGE-bi-plot method. All of these selected genotypes were originated from upland regions (300–800 m above sea level) in Phetchabun and Chiang Mai provinces.The genotypes with the highest grain yield in each location were not always the genotypes with the greatest stability,e.g., genotypes numbers 21 (Sewtong 1) and 23 (Sewgreng) gave the highest grain yields at the L1 (BH09) and L4 (CPA10), respectively. Some of the test locations were subjected to abiotic (e.g., drought) or biotic (e.g., disease)stress. At the MHS10 (L5), genotypes were exposed to leaf blast disease during the vegetative stage, so grain yields in this site were the lowest, despite abundant rainfall and an altitude suitable for upland rice. Moreover, high amount of rainfall in the slope area was created the problems such as the reaching of pesticide application and soil erosion. Blast disease caused by Pyricularia oryzae Cav. was a potentially important production constraint in temperate, sub-topical and especially tropical upland rice growing areas (Bonman 1992). Genotype number 28 gave the best performance in location 5 (MHS10), and was classi fied as having high stability (b close to 1) and deposited at top ten grain yield group. For special environments, selection needs to be based on the performance of individual genotypes which also useful in parental selection for breeding improvement in the future, because of high G×E interaction.
5. Conclusion
The evaluation of the stability of upland rice genotypes were necessary for the large areas upland in North and Northeast Thailand. The results of this study indicated that the most suitable location for the performance evaluation of rice genotype was L3 (KKU10). Among the upland rice genotypes evaluated, genotype numbers 6 (Jaowmong 1), 10(Neawmong 1), 18 (Neawdum 1), 19 (Leamna), 20(Prayaleamkang), 32 (Kunwang 2), and 33 (Kunwang 3)gave the highest grain yields and had the great stability across the five locations. All seven genotypes should be applicant for upland rice production area and parental base for breeding program in the future.
Acknowledgements
This research was supported by the Plant Breeding Research Centre for Sustainable Agriculture and Research Center of Agricultural Biotechnology for Sustainable Economy, Khon Kaen University, Thailand. The authors thank Dr.John Schiller of the University of Queensland, Australia, for proofreading the manuscript. Thanks also extended to the Faculty of Agriculture at Khon Kaen University for providing financial support for manuscript preparation activities.
Appendixassociated with this paper can be available on http://www.ChinaAgriSci.com/V2/En/appendix.htm
Acuña T L B, La fitte H R, Wade L J. 2008. Genotype×environment interactions for grain yield of upland rice backcross lines in diverse hydrological environments. Field Crops Research,108, 117–125.
Akter A, Hassan J, Kulsum U, Islam M R, Hossain K, Rahman M. 2014. AMMI biplot analysis for stability of grain yield in hybrid rice (Oryza sativa L.). Rice Research Journals,2, 126.
Alam A M, Somta P, Jompuk C, Chatwachirawong P, Srinives P. 2014. Evaluation of mungbean genotypes based on yield stability and reaction to mungbean yellow mosaic virus disease. The Plant Pathology Journal,30, 261–268.
Asenjo C A, Bezus R, Acciaresi H A. 2003. Genotypeenvironment interactions in rice (Oryza sativa L.) in temperate region using the Joint Regression Analysis and AMMI methods. Cereal Research Communications,31,97–104.
Balestre M, dos Santos V B, Soares A A, Reis M S. 2010.Stability and adaptability of upland rice genotypes. Crop Breeding and Applied Biotechnology,10, 357–363.
Bell R W, Seng V. 2004. Rainfed lowland rice-growing soils of Cambodia, Laos, and Northeast Thailand. In: Seng V,Craswell E, Fukai S, Fischer K, eds., Water in Agriculture.ACIAR Proceedings No. 116. Australian Centre for International Agricultural Research, Canberra, Australia.pp. 161–173.
Bernier J, Atlin G N, Serraj R, Kumar A, Spaner D. 2008.Breeding upland rice for drought resistance. Journal of the Science of Food and Agriculture,88, 927–939.
Bernier J, Kumar A, Ramaiah V, Spaner D, Atlin G. 2007. A large-effect QTL for grain yield under reproductive-stage drought stress in upland rice. Crop Science,47, 507–516.
Bonman J M. 1992. Durable resistance to rice blast disease -Environmental in fluences. Breeding for Disease Resistance,1, 115–123.
Bose L K, Nagaraju M, Singh O N. 2012. Genotype×environment interaction and stability analysis of lowland rice genotypes.Journal of Agricultural Sciences,57, 1–8.
Bouman B A M, Peng S, Castañeda A R, Visperas R M.2005. Yield and water use of irrigated tropical aerobic rice systems. Agricultural Water Management,74, 87–105.
Courtois B, McLaren G, Sinha P K, Prasad K, Yadav R, Shen L. 2000. Mapping QTLs associated with drought avoidance in upland rice. Molecular Breeding,6, 55–66.
Crews-Meyer K A. 2004. Agricultural landscape change and stability in Northeast Thailand: Historical patch-level analysis. Agriculture, Ecosystems and Environment,101,155–169.
Eberhart S A, Russell W A. 1966. Stability parameter for comparing varieties. Crop Science,6, 36–40.
Fan X, Kang M S, Chen H, Zhang Y, Tan J, Xu C. 2007. Yield stability of maize hybrids evaluated in multi-environment trials in Yunnan, China. Agronomy Journal,99, 220–228.
Fukuoka S, Okuno K. 2001. QTL analysis and mapping of pi21, a recessive gene for field resistance to rice blast in Japanese upland rice. Theoretical and Applied Genetics,103, 185–190.
Gauch H G, Piepho H P, Annicchiarico P. 2008. Statistical analysis of yield trials by AMMI and GGE: Further considerations. Crop Science,48, 866–889.
Gollob H F. 1968. A statistical model which combine features of factor analytic and analysis of variance techniques.Psychometrika,33, 73–114.
IRRI (International Rice Research Institute). 1998. World Rice Statistics. Los Baños, Laguna, Philippines.
Lakew T, Tariku S, Alem T, Bitew M. 2014. Agronomic performances and stability analysis of upland rice genotypes in North West Ethiopia. International Journal of Scienti fic and Research Publications,4, 1–9.
Liang S, Ren G, Liu J, Zhao X, Zhou M, McNeil D, Ye G. 2015.Genotype-by-environment interaction is important for grain yield in irrigated lowland rice. Field Crops Research,180,90–99.
Lv T T, Sun X, Zhang D, Xue Z, Gong J. 2008. Assessment of soil erosion risk in Northern Thailand. In: The International Archives of The Photogrammetry, Remote Sensing and Spatial Information Sciences. Part B8, 3. ISPRS(International Society for Photogrammetry and Remote Sensing), Beijing. pp. 703–708.
Mohammadi R, Haghparast R, Amri A, Ceccarelli S. 2009. Yield stability of rainfed durum wheat and GGE biplot analysis of multi-environment trials. Crop and Pasture Science,61,92–101.
Mongkolsawat C, Paiboonsak S, Chanket U. 2006. Soil erosion in northeast Thailand: A spatial modeling. In: The Proceedings of International 93 Conference on Space Technology and Geo-Informatics. Pattaya City, Chonburi province, Thailand. pp. 5–8.
Naveed M, Nadeem M, Islam N. 2007. AMMI analysis of some upland cotton genotypes for yield stability in different milieus. World Journal of Agricultural Sciences,3, 39–44.
Oikeh S O, Nwilene F E, Agunbiade T A, Oladimeji O, Ajayi O, Mande S, Tsunematsu H, Samejima H. 2008. Growing Upland Rice: A Production Handbook. Africa Rice Center,Benin. p. 40.
Onofri A, Ciricifolo E. 2007. Using R to perform the AMMI analysis on agriculture variety trials. The Newsletter of the R Project for Statistical Computing,7, 1.
Panomtarinichigul M. 2006. Research on sustainable hill farming in Northern Thailand. In: International Conference on Challenges to Interdisciplinary Collaborative Research Institute of Ethnology. Academia Sinica, Taipei, Taiwan of China.
Price A H, Steele K A, Gorham J, Bridges J M, Moore B J,Evans J L, Richardson P, Jones R G W. 2002. Upland rice grown in soil- filled chambers and exposed to contrasting water-de ficit regimes I. Root distribution, water use and plant water status. Field Crops Research,76, 11–24.
Saito K, Azoma K, Sokei Y. 2010. Genotypic adaptation of rice to lowland hydrology in West Africa. Field Crops Research,119, 290–298.
Samonte S O P B, Wilson L T, McClung A M, Medley J C. 2005.Targeting cultivars onto rice growing environments using AMMI and SREG GGE biplot analyses. Crop Science,45,2414–2424.
Shrestha S, Asch F, Dusserre J, Ramanantsoanirina A, Brueck H. 2012. Climate effects on yield components as affected by genotypic responses to variable environmental conditions in upland rice systems at different altitudes. Field Crops Research,134, 216–228.
Tao H, Brueck H, Dittert K, Kreye C, Lin S, Sattelmacher B.2006. Growth and yield formation of ricez (Oryza sativa L.)in the water-saving ground cover rice production system(GCRPS). Field Crops Research,95, 1–12.
Tariku S, Lakew T, Bitew M, Asfaw M. 2013. Genotype by environment interaction and grain yield stability analysis of rice (Oryza sativa L.) genotypes evaluated in north western Ethiopia. Netherlands Journal of Agricultural Science,1,10–16.
Tuhina-Khatun M, Hana fiM M, Yusop M R, Wong M Y, Salleh F M, Ferdous J. 2015. Genetic variation, heritability, and diversity analysis of upland rice (Oryza sativa L.) genotypes based on quantitative traits. BioMed Research International,2015, 1–7.
Tuong T P, Bouman B A M. 2003. Rice production in water scarce environments. In: Kijne J W, Barker R, Molden D, eds., Water Productivity in Agriculture: Limits and Opportunities for Improvement. CAB International,Wallingford, UK.
Vityakon P, Meepech S, Cadisch G, Toomsan B. 2000. Soil organic matter and nitrogen transformation mediated by plant residues of different qualities in sandy acid upland and paddy soils. Netherlands Journal of Agricultural Science,48, 75–90.
Wade L J, McLaren C G, Quintana L, Harnpichitvitaya D,Rajatasereekul S, Sarawgi A K, Kumar A, Ahmed H U,Sarwoto, Singh A K, Rodriguez R, Siopongco J, Sarkarung S. 1999. Genotype by environment interactions across diverse rainfed lowland rice environments. Field Crops Research,64, 35–50.
Xing Y, Tan Y, Hua J, Sun X, Xu C, Zhang Q. 2002.Characterization of the main effects, epistatic effects and their environmental interactions of QTLs on the genetic basis of yield traits in rice. Theoretical and Applied Genetics,105, 248–257.
Yan W, Hunt L A, Sheng Q, Szlavnics Z. 2000. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Science,40, 597–605.
Yan W, Kang M S, Ma B, Woods S, Cornelius P L. 2007. GGE biplot vs. AMMI analysis of genotype-by-environment data.Crop Science,47, 641–653.
Yoshida S, Parao F T. 1976. Climatic in fluence on yield and yield components of lowland rice in the topics. In: Climate and Rice. International Rice Research Institute, Philippines.pp. 471–794.
6 December, 2016 Accepted 20 February, 2017
Correspondence Jirawat Sanitchon, Mobile: +66-81-5674364,Tel: +66-43-202360, Fax: +66-43-202361, E-mail: jirawat@kku.ac.th
© 2018 CAAS. Publishing services by Elsevier B.V. All rights reserved.
10.1016/S2095-3119(16)61609-X
Managing editor WANG Ning
 Journal of Integrative Agriculture2018年1期
Journal of Integrative Agriculture2018年1期
- Journal of Integrative Agriculture的其它文章
- Climate change and agriculture: Impacts and adaptive responses in Iran
- Development of elite restoring lines by integrating blast resistance and low amylose content using MAS
- ldenti fication of the resistance gene to powdery mildew in Chinese wheat landrace Baiyouyantiao
- New clues concerning pigment biosynthesis in green colored fiber provided by proteomics-based analysis
- Constitutive expression of feedback-insensitive cystathionine γ-synthase increases methionine levels in soybean leaves and seeds
- Characterization of groundnut (Arachis hypogaea L.) collection using quantitative and qualitative traits in the Mediterranean Basin
