Effects of reduced glutathione on Boer goat semen freezability
Rawash ZM, Ebtihal A Ibrahim, El-Raey M
1Artificial Insemination and Embryo Transfer Department, Animal Reproduction Research Institute, Al Haram
2Biology of Reproduction Department, Animal Reproduction Research Institute, Al Haram
3Department of Theriogenology, Faculty of Veterinary Medicine, Benha University, P.O: 13736, Tokh, Kaliobia, Egypt
Effects of reduced glutathione on Boer goat semen freezability
Rawash ZM1, Ebtihal A Ibrahim2, El-Raey M3✉
1Artificial Insemination and Embryo Transfer Department, Animal Reproduction Research Institute, Al Haram
2Biology of Reproduction Department, Animal Reproduction Research Institute, Al Haram
3Department of Theriogenology, Faculty of Veterinary Medicine, Benha University, P.O: 13736, Tokh, Kaliobia, Egypt
Glutathione Cryopreservation Buck DNA integrity Antioxidant
Objective:To evaluate the effects of reduced glutathione on the quality of cryopreserved Boer buck spermatozoa.Methods:The current study was carried out on five Boer bucks from which semen samples were collected by artificial vagina. After microscopical evaluation at 37 ℃,semen samples that fulfill the ideal requirements for extension were diluted in a tris-based extender including different concentrations of reduced glutathione (2, 5, 7 and 10 mM) and those without glutathione served as a control. Sperm motility, viability, acrosome integrity,DNA integrity, total antioxidant capacity and lipid peroxidation were assessed post-thawing.Results:The current results revealed that post-thawing motility, viability and acrosomal integrity were significantly improved [(66.67±5.50)%, 168.30±18.59 and (12.75±2.45)%,respectively] when 5 mM glutathione was added to semen extender; especially as compared with the control [(40.00 ±2.88)%, 95.00±8.90 and (25.75±3.46)%, respectively]. Similarly, at this concentration (5 mM) sperm DNA damage, tail length and tail moment of cryopreserved semen were significantly (P<0.05) reduced [(2.32±0.27)%, (1.64±0.49) µm and 3.55±0.63,respectively] compared with the control extender [(6.66±0.84)%, (4.09±0.47) µm and 26.47±0.51, respectively]. Moreover, addition of 5 mM glutathione to buck semen extender significantly (P<0.05) increased total antioxidant capacity [0.51±0.07) mµ/mL] and decreased lipid peroxidation of cryopreserved spermatozoa [(8.68±2.72) nmol/mL] compared with the control [(0.18±0.02) mµ/mL and (24.92±5.80) nmol/mL, respectively].Conclusions:The addition of 5 mM glutathione to semen diluent improve freezability of Boer buck spermatozoa through DNA protection from deterioration and oxidative stress reduction. Moreover, 10 mM of glutathione exerts cytotoxic effects on Boer buck semen.
1. Introduction
Goats are considered as one of important backbone blocks in the economy of rural countries like Egypt, as they are chief source for meat, milk and skin. So, all goats, producing countries shall intendto increase this animal population by all possible means. It is clear that artificial insemination and its related assisted reproductive technologies are considered the most important of these applications.Goat’s artificial insemination can achieve widespread progression of buck semen, limiting the spread of sexually transmitted diseases andfacilitating genetic improvement programs in goat’s industry.
Semen manipulation during cryopreservation is associated with a plethora of changes in spermatozoa such as capacitation like effects[1], reduction in integrity of the plasma and acrosomal membranes[2], diminution in motility and ability to penetrate oocyte in vitro[3]. Moreover, cryopreservation leads to a significant reduction in the level of spermatozoa antioxidants[4], predisposing the sperm to the generated reactive oxygen species (ROS) such as superoxide radical, hydroperoxyl radical and hydroxyl radical[5], which cause sperm cell damage[6]. Generally, there is a state of equilibrium between ROS production and their scavenging systems which are delicate. This balance can be distrusted by excessive centrifugation and freezing/thawing processes during cryopreservation resulting in excessive ROS production in sperm-processing media[7]. ROS excess during cryopreservation has been coupled with reduced post-thaw motility[8], mainly due to changes in membrane transportation[9].Additionally, excessive ROS results in reduction in the sperm viability, membrane integrity and other sperm functions[8]; which in turn affects the sperm fertilizing potentials. Therefore, the amount of ROS should be restricted to the minimum requirements to preserve the cell functions[6]. The sperm cell membrane contains high levels of polyunsaturated fatty acids contents that are easily damaged by ROS through the process of peroxidation[10-12]; the latter is considered as bio-index or a key to evaluate the degree of sperm membranes damage by the excessive ROS[13], which in turn affects the future fertility characteristics of semen.
Sperm motility, viability and fertilizing ability can be improved or preserved by addition of various motility enhancing agents or antioxidants in semen diluent. The antioxidants can neutralize or reduce the risk of damage to spermatozoa during cryopreservation process by combating harmful effects of ROS or enhancing its antioxidant enzymes[14,15]. In between, glutathione which is considered as a thiol tripeptide (γ-glutamyl cysteinyl glycine)has antioxidative potentials and can maintenance the intracellular redox conditions[16,17]. Glutathione is considered as a natural reservoir of redox force depending upon its sulphydryl group in reduced glutathione (GSH) and oxidized glutathione forms, which quickly protects different cell types against oxidative stress[18].Furthermore, glutathione has roles in protein synthesis regulation,cellular detoxification, and leukotriene synthesis depending on its components from glutamate, cysteine, and glycine amino acids[17].In semen, glutathione plays the same roles against the excessive generated ROS during centrifugation process that removes its seminal plasma containing antioxidant, also during the process of freezing/thawing[8,16,19]. Furthermore, glutathione plays a cofactor role for glutathione peroxidase which uses glutathione to reduce hydrogen peroxide to H2O and lipoperoxides to alkyl alcohols[15],even though it has been found that cryopreservation process reduced the content of GSH of spermatozoa and seminal plasma[8,16].Therefore, depending on the scarcity of information regarding the effects of GSH on Boer buck spermatozoa, the current study is planned to investigate the effects of GSH on Boer buck semen freezability, then to maintain the qual ity of buck semen and enhance its fertilizing potentials.
2. Materials and methods
2.1. Animals and semen collection
Semen samples from 5 mature fertile Boer bucks (2 years old) were collected by artificial vagina twice weekly. Only semen samples of at least 80% initial motility and 3 ×109sperm/mL were used.The spermatozoa were separated from seminal plasma through twice centrifugation (10 min/2 000 rpm). Pelleted sperms were resuspended with semen extender and pooled, split into 5 portions and diluted at 30 ℃ with Tris-based extender (6% glycerol and 20% egg yolk), and supplemented with different concentrations of glutathione(2, 5, 7 and 10 mM) and those without glutathione served as a control. The extended semen was cooled to 5 ℃ throughout 60 min in a cold cabinet and equilibrated at 5 ℃ for a period of 2.5 h. After equilibration, the cooled semen was loaded in straws (IMV, L’Aigle,France/0.25 mL), then exposed to liquid nitrogen vapor (6 cm above liquid nitrogen) / 15 min. Finally, the straws were stored in the liquid nitrogen till analysis. Frozen straws were thawed in a water bath at 37 ℃/30 s. Post-thawing viability, acrosomal integrity and motility were assessed according to Salamon and Maxwell[2].
2.2. Biochemical analysis
Aspartate-aminotransferase, alanine-aminotransferase and alkaline phosphatase enzyme concentrations were measured according to Burtis et al[20] to estimate membrane stabilizing roles of GSH.Additionally, lipid peroxidation was estimated by malondialdehyde(MDA) generation that was determined by thiobarbituric acid test as described by Cortossa et al[21].
2.3. Assessment of sperm DNA integrity
DNA integrity and the incidence of DNA fragmentation were detected using neutral single cell gel electrophoresis assay (comet assay) according to Boe-Hansen et al[22]. Frozen-thawed spermatozoa were diluted in phosphate buffer saline, embedded in agarose, and then subjected to lysis, DNA decondensation, electrophoresis, and finally DNA was stained with 50 µL of 20 µg/mL ethidium bromide(Sigma). Stained sperms were visualized by fluorescent microscope.Compact and bright fluorescent appearance of the sperm heads in the comet assay denotes its intact nuclei, while its DNA fragmentation was denoted by a tail behind its head due to DNA migration during the electrophoresis giving comet appearance[23]. To assess the comet assay results, software computer program was used to judge the sperm nuclei.
2.4. Statistical analysis
Costat Cottort software program (1986), version 3.03, was used to analyze the current data. Results were expressed as means ± SEM.Least significant difference at 1% (P<0.01) and 5% probability(P<0.05) was used to compare values.
3. Results
Table 1 illustrated that addition of GSH to Boer buck semen extender improved its freezability compared to control group,in a dose-dependent pattern. Where, 5 mM of glutathione in Boer buck semen extender appeared to be the best concentration that significantly increased (P<0.05) its post-thawing sperm motility, viability index and acrosomal integrity [(66.67±5.50)%,168.30±18.59 and (12.75±2.45)%, respectively] compared with control semen samples [(40.00 ±2.88)%, 95.00±8.90 and(25.75±3.46)%, respectively]. Moreover, 10 mM of glutathione seemed to be cytotoxic to Boer buck semen; where the viability index was lowered 89.00±15.99 compared with the control 95.00±8.90.
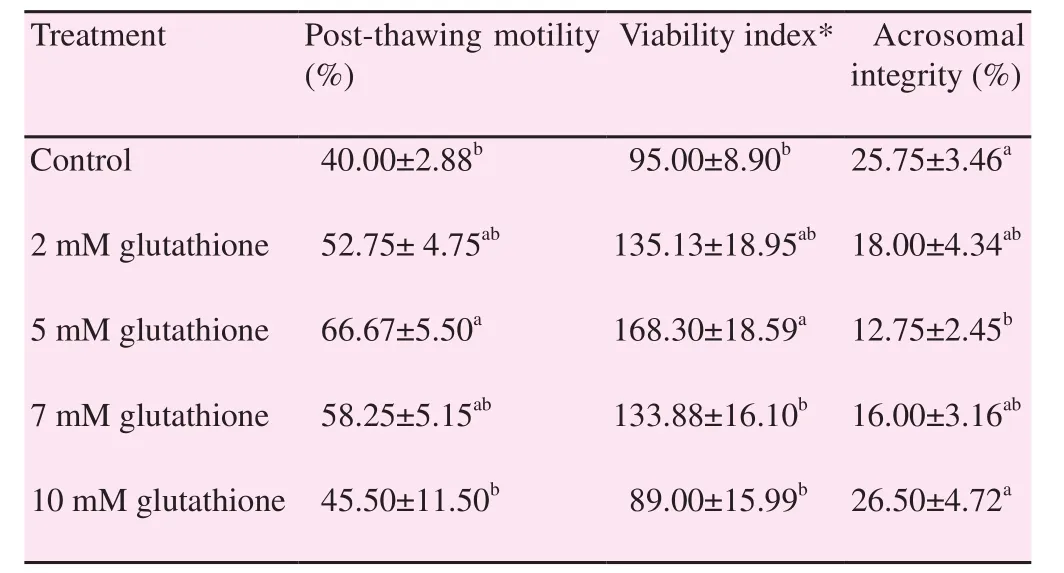
Table 1 Effect of GSH at different concentrations on Boer buck semen freezability(mean ± SEM).
Table 2 clarified that 5 mM glutathione addition in Boer buck semen extender resulted in significant reduction of aspartateaminotransferase, alanine-aminotransferase and alkaline phosphatase enzyme concentrations [(55.50±1.72) U/L, (19.75±2.46) U/L,(20.66±3.40) U/L, respectively] compared with the control extender[(100.50±7.75) U/L, (26.75±2.10) U/L and (26.45±2.88) U/L, respectively].Moreover, 5 mM of GSH in Boer buck semen extender significantly(P<0.05) increased the total antioxidant capacity and reduced lipid peroxidation of frozen-thawed buck semen [(0.51±0.07) mµ/mL and (8.68±2.72) nmol/mL, respectively] compared with the control semen [(0.18±0.02) mµ/mL, and (24.92±5.80) nmol/mL,respectively].
Table 3 and Figure 1 show the influence of glutathione addition to the semen extender on the DNA integrity of the frozen-thawed Boer buck spermatozoa. The present data pointed that 5 mM glutathione in Boer buck semen extender significantly (P<0.05) decreased its DNA fragmentation percent, tail length and tail moment after freezethawing processes [(2.32±0.27)%, (1.64±0.49) µm and 3.55±0.36,respectively] compared with GSH free extender [(6.66±0.84)%,(4.09±0.47) µm and 26.47±0.51, respectively].
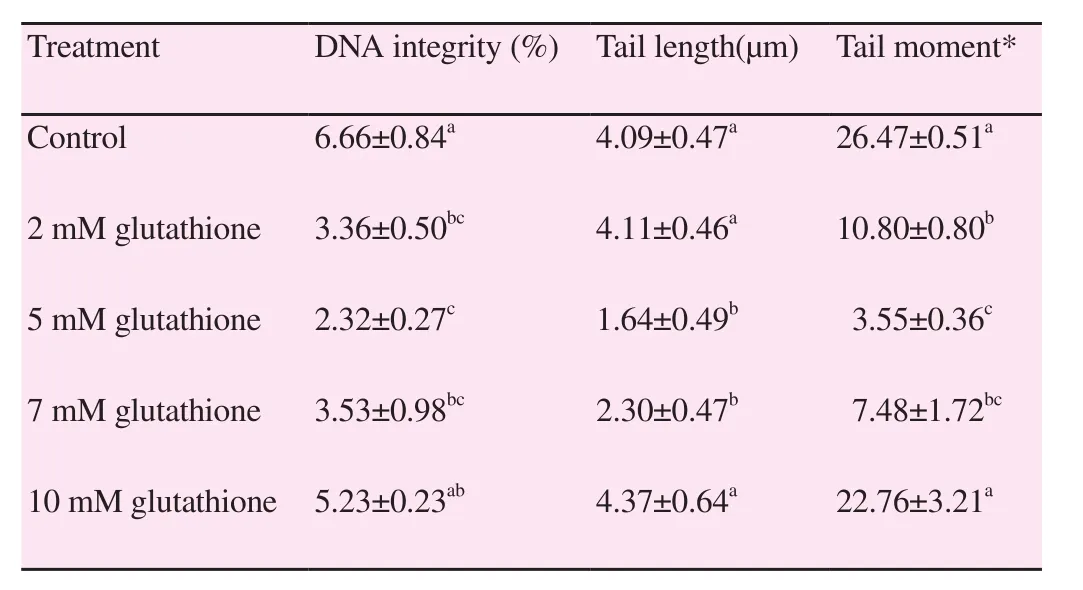
Table 3 Effect of glutathione at different concentrations on DNA integrity of Boer buck spermatozoa (mean ± SEM).
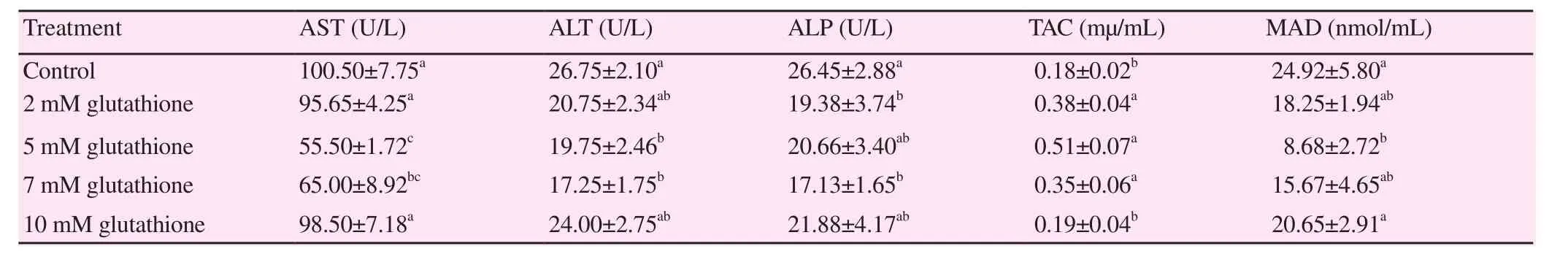
Table 2 Effect of GSH at different concentrations on Boer buck semen biochemical activity.
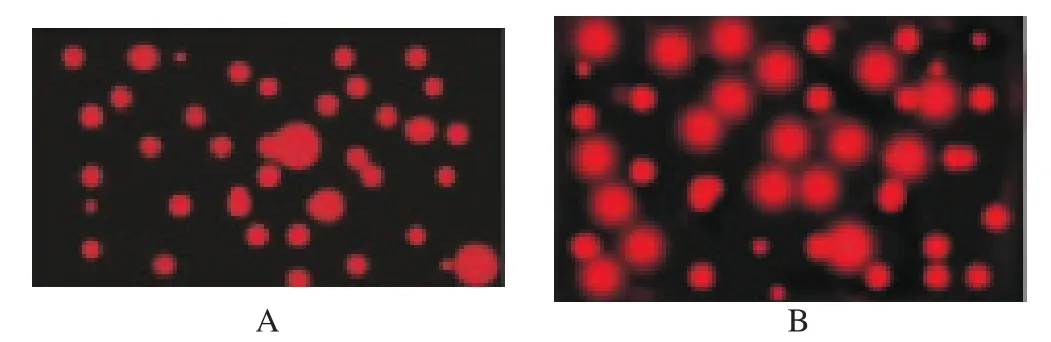
Figure 1. Single cell gel electrophoresis.
4. Discussion
Assisted reproductive techniques such as artificial insemination are used in goat’s industry with the objectives of increasing its genetic gain and producing livestock with improved reproductive efficiency.Despite these advantages, artificial insemination in goats is poorly applied due to mediocre outcomes when frozen/thawed spermatozoa are used. Moreover, few artificial insemination records are conducted worldwide using frozen-thawed Boer buck semen. Therefore, there is an interest in the use of this technology to enhance Boer buck semen industry.
When spermatozoa were frozen / thawed, they were subjected to various stressors (physical, physiological, osmotic and chemical) that resulted in disruption of the transbilayer phospholipids asymmetry of mammalian sperm, thus, damaging the plasma membrane, increasing its susceptibility to lipid peroxidation[7,24-27], predisposing the mammalian sperm to gross morphologic damage and decreasing motility and fertilizing capability. The current results explain that GSH supplementation to Boer buck semen extender improved its freezability. These results are in harmony with those of Sinha et al[14], Foote et al[28], Badr et al[29] and Badr et al[30], who confirmed obvious increase in post-thaw motility and acrosomal integrity of buck and bovine spermatozoa when GSH was added to the semen extender. GSH protective effects on sperm fertility characteristics were dose-dependent. The current study declared that addition of 5 mM glutathione into the semen diluent was satisfactorily effective to improve the freezability of Boer buck spermatozoa and these results comes in harmony with the study of Sinha et al[14]. On the other hand, the current results run in disagreement with Noei Razliqi et al[15] and Sarangi et al[31] who reported 1mM; while Badr et al[30] reported 2 mM and Foote et al[28] reported 0.05-1.00 mM of glutathione had beneficial effects on total motility, plasma membrane integrity, functionality and viability with lowering number of apoptotic spermatozoa. The difference in concentrations might be due to species, breed, extender differences or due to all.
It is well-known that cryopreservation prompts ROS production which affects the sperm functions[32]. Low scavenging and antioxidant power in sperm extender gave the chance for the generated ROS to drastically affect the sperm fertility characteristics like motility, viability, and penetration capability by interacting with its lipids, proteins and DNA[33]. The current study results were presented that addition of glutathione to Boer buck semen extender reduced aspartate-aminotransferase, alanine-aminotransferase and alkaline phosphatase enzyme concentrations, enhanced its total antioxidant capability, and reduced lipid peroxidation of the frozenthawed semen; and this comes in harmony with Noei Razliqi et al[15]and Sarangi et al[31] who reported that glutathione had enhancing effects on semen characteristics. The improved post thawing motility in glutathione treated group (5 mM) might be elucidated by the intimate link between ROS, glutathione and motility. Glutathione efficiently combated ROS, so it not only guarded the sperm cells against the decrease in phosphorylation process of its axonemal proteins but also prevented membrane fluidity reduction[34],thus preventing the sperm immobilization. Plus, the reduction in glutathione concentration due to cryopreservation process resulted in changes in membrane transportation potential[9], which in turn affected its motility, penetration and fertilizing potential.
It is well established that the lipid peroxides generated by peroxidation during cryopreservation process can damage the sperm structure and eventually lower its metabolism and motility[35].Lipid peroxides are unstable compounds; they decompose reactive carbonyl compounds, mainly MDA[36]. So MDA could be used as biochemical indicator for lipid peroxidation in spermatozoa[13]. The current study results reported that the presence of GSH in Boer buck semen extender reduced MDA level in frozen-thawed spermatozoa.These results came in agreement with Sikka[13] and Sarangi et al[31] who reported that glutathione addition to the semen extender reduced lipid peroxidation level in the sperm cell membrane. The end products of lipid peroxidation not only affected the sperm membrane and motility but also damaged its DNA integrity either through DNA bases oxidation or by binding with MDA[13,37],resulting in DNA deterioration, damage and an apoptotic-like changes[38-40], which appeared in the form of strand breaks and cross linking[13]. In harmony with these previous reports, the current study results showed that there were positive effects of GSH on Boer buck sperm freezability depending upon its roles in combating the oxidative hazardous of ROS on DNA, thus reducing its fragmentation[27,41]. Moreover, the current study results shed more light on the ideal concentration of GSH that should be incorporated in Boer buck semen. The present results confirmed that increased levels of glutathione inclusion in Boer buck semen extender had cytotoxic effects on the sperm functions. These results run in accordance with Aitken et al[42] who stated that high antioxidants levels in semen diluents accompanied with impaired sperm functions might be due to high sperm susceptibility to the cytotoxic effect of hydroperoxyl radical. Therefore, it is vital to select the ideal antioxidant concentrations to maintain the natural balance between ROS production and scavenging activities[29].
In conclusion, this study confirms that the presence of GSH in Boer buck semen extender at a concentration of 5 mM improves its freezability. The protective glutathione effects against the cryoinjury are directly related to lipid peroxidation reduction and sperm DNA protection from deterioration by increasing total antioxidant capacity. Additionally, glutathione effect in Boer buck semen is dose-dependent, where 10 mM of glutathione is considered cytotoxic to Boer buck semen. Furthermore, the current study results open new windows to explore the practical application of antioxidants to improve the quality of post-thaw goat semen.
Conflict of interest statement
The authors declare that there was no conflict of interest.
We appreciate Dr. H. M. Hassen (ARRI) for the fruitful efforts in single cell gel electrophoresis (comet) assay.
[1] Gillan L, Evans G, Maxwell WM. Capacitation status and fertility of fresh and frozen-thawed ram spermatozoa. Reprod Fertil Dev 1997; 9(5): 481-487.
[2] Salamon S, Maxwell WMC. Frozen storage of ram semen. II. Causes of low fertility after cervical insemination and methods of improvement.Anim Reprod Sci 1995; 38(1-2): 1-36.
[3] Gillan L, Maxwell WM. The functional integrity and fate of cryopreserved ram spermatozoa in the female tract. J Reprod Fertil 1999; S54: 271-283.
[4] Bilodeau JF, Blanchette S, Gagnon C, Sirard MA. Thiols prevent H2O2- mediated loss of sperm motility in cryopreserved bull semen.Theriogenology 2001; 56(2): 275-286.
[5] Silva PFN. Physiology of peroxidation process in mammalian sperm[dissertation]. Utrecht: Utrecht University; 2006.
[6] Agarwal A, Saleh RA, Bedaiwy MA. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil Steril 2003; 79(4): 829-843.
[7] Anghel A, Zamfirescu S, Dragomir C, Nadolu D, Elena S, Florica B. The effects of antioxidants on the cytological parameters of cryopreserved buck semen. Romanian Biotechnol Lett 2010; 15(3): 26-32.
[8] Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev 2000; 55(3): 282-288.
[9] Alvarez JG, Storey BT. Spontaneous lipid peroxidation in rabbit epididymal spermatozoa: its effects on sperm motility. Biol Reprod 1982;27: 1102-1108.
[10] Baumber J, Ball BA, Gravance CG, Medina V, Davies-Morel MC. The effect of reactive oxygen species on equine sperm motility, viability,acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl 2000; 21: 895-902.
[11] Neild DM, Brouwers JP, Colenbrander B, Aguero A, Gadella BM. Lipid peroxide formation in relation to membrane stability of fresh and frozenthawed stallion spermatozoa. Mol Reprod Dev 2005; 72: 230-238.
[12] García BM, Fernández LG, Ferrusola CO, Rodríguez AM, Bolaños JMG,Martinez HR, et al. Fatty acids and plasmalogens of the phospholipids of the sperm membranes and their relation with the post-thaw quality of stallion spermatozoa. Theriogenology 2011; 75(5): 811-818.
[13] Sikka SC. Oxidative stress and role of antioxidant in normal and abnormal sperm function. Front Biosci 1996; 1: 78-86.
[14] Sinha MP, Sinha AK, Singh BK, Prasad PL. The effect of glutathione on the motility, enzyme leakage and fertility of frozen goat semen. Anim Reprod Sci 1996; 41(3-4): 237-243.
[15] Noei Razliqi R, Zhandi M, Shakeri M, Towhidi A, Sharafi M, Emamverdi M, et al. Protective role of glutathione in buck semen cryopreservation.Iran J Vet Res 2015; 16(3): 298-300.
[16] Gadea J, Gumbao D, Cánovas S, García-Vázquez FA, Grullón LA,Gardón JC. Supplementation of the dilution medium after thawing with reduced glutathione improves function and the in vitro fertilizing ability of frozen-thawed bull spermatozoa. Int J Androl 2008; 31(1): 40-49.
[17] De Oliveira RA, Wolf CA, Viu MA, Gambarini ML. Addition of glutathione to an extender for frozen equine semen. J Eq Vet Sci 2013;33: 1148-1152.
[18] Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol 2005; 5: 5-17.
[19] Triwulanningsih E, Situmorang P, Sugiarti T, Sianturi RG,Kusumaningrum D. The effect of glutathione in sperm diluents on the quality of bovine chilled semen. Indonesian J Anim Vet Sci 2003; 8(2):64-69.
[20] Burtis CA, Ashwood ER, Bruns DE, Sawyer BG. Tietz fundamentals of clinical chemistry. 6th ed. Missouri: Saunders, Elsevier Publishing Company; 2008.
[21] Cortossa S, Aon MA, Winslow RL, O′Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J 2004; 87(3):2060-2073.
[22] Boe-Hansen GB, Morris ID, Ersbøll AK, Greve T, Christensen P. DNA integrity in sexed bull sperm assessed by neutral Comet assay and sperm chromatin structure assay. Theriogenology 2005; 63(6): 1789-1802.
[23] Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. A comparison of baseline and induced DNA damage in human spermatozoa from fertile and infertile men, using a modified comet assay. Mol Hum Reprod 1996; 2(8): 613-619.
[24] Alvarez JG, Storey BT. Evidence for increased lipid peroxidative damage and loss of superoxide dismutase activity as a mode of sublethal cryodamage to human sperm during cryopreservation. J Androl 1992;13(3): 232-241.
[25] Bucak MN, Atessahin A, Varı lı Ö, Yüce A, Tekin N, Akçay A. The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen: Microscopic and oxidative stress parameters after freeze-thawing process. Theriogenology 2007; 67(5): 1060-1067.
[26] Sarıözkan S, Bucak MN, Tuncer PB, Uluta PA, Bilgen A. The influence of cysteine and taurine on microscopic-oxidative stress parameters and fertilizing ability of bull semen following cryopreservation. Cryobiology 2009; 58(2): 134-138.
[27] Funahashi H, Sano T. Select antioxidants improve the function of extended boar semen stored at 10 ℃.Theriogenology 2005; 63(6): 1605-1616.
[28] Foote RH, Brockett CC, Caproth MT. Motility and fertility of bull in whole milk extender containing antioxidant. Anim Reprod Sci 2002;71(1-2): 13-23.
[29] Badr MR, Abd el Hafez SM, Abd El Fatah EM. Influence of antioxidants on DNA integrity, mitochondrial function and fertilizing potentials of cryopreserved buffalo spermatozoa. Assiut Vet Med J 2009; 55(120): 296-317.
[30] Badr MR, Abd El- Malak MG, Ghattas TA. Effect of glutathione on the freezability and in vitro fertilizing potentials of bovine spermatozoa.Assiut Vet Med J 2012; 58(134): 374-381.
[31] Sarangi A, Singh P, Virmani M, Yadav AS, Sahu S, Ajithakumar HM, et al. Effect of antioxidants supplementation on the quality of Beetal buck semen stored at 4 ℃.Vet World 2017; 10(10): 1184-1188.
[32] Watson PF. The cause of reduced fertility with cryopreserved semen.Anim Reprod Sci 2000; 60-61: 481-492.
[33] Hellstrom WJ, Bell M, Wang R, Sikka SC. Effect of sodium nitroprusside on sperm motility, viability and lipid peroxidation. Fertil Steril 1994;61(6): 1117-1122.
[34] De Lamirande E, Gagnon C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects.Hum Reprod 1995; 10(suppl 1): 15-21.
[35] Chatterjee S, Gagnon C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing and thawing. Mol Reprod Dev 2001; 59(4): 451-458.
[36] Mennella MRF, Jones R. Properties of spermatozoal superoxide dismutase and lack of involvement of superoxides in metal-ion-catalyzed lipid peroxidation reactions in semen. J Biochem 1980; 191(2): 289-297.
[37] Fraser L, Strzezek J. Effects of freezing-thawing on DNA integrity of boar spermatozoa assessed by the neutral comet assay. Reprod Domest Anim 2005; 40(6): 530-536.
[38] Kotamraju S, Hogg N, Joseph J, Keefer LK. Inhibition of oxidized low density lipoprotein-induced apoptosis in endothelial cells by nitric oxide.J Biol Chem 2001; 276: 17316-17323.
[39] Slowi ska M, Karol H, Ciereszko A. Comet assay of fresh and cryopreserved bull spermatozoa. Cryobiology 2008; 56(1): 100-102.
[40] Badr MR, Abd El- Malak MG, Hassan HM. Effect of trehalose on cryopreservation, oxidative stress and DNA integrity of buffalo spermatozoa.J Reprod Infert 2010; 1(2): 50-57.
[41] Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update 2003; 9(4): 331- 345.
[42] Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, et al.Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod 1998; 59(5): 1037-1046.
7 November 2017 Revision 18 November 2017 Accepted 1 December 2017 Available online 1 January 2018
10.4103/2305-0500.220983
✉Corresponding author: El-Raey M, Department of Theriogenology, Faculty of Veterinary Medicine, Benha University, P.O: 13736, Tokh, Kaliobia, Egypt.
E-mail: elraay_310@yahoo.com
Tel: +201003828279
Rawash ZM, Artificial Insemination and Embryo Transfer Department,Animal Reproduction Research Institute, Al Haram.
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix,tweak and buid upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
For reprints contact: reprints@medknow.com
©2018 Asian Pacific Journal of Reproduction Produced by Wolters Kluwer- Medknow
How to cite this article: Rawash ZM, Ebtihal A Ibrahim, El-Raey M.Effects of reduced glutathione on Boer goat semen freezability. Asian Pac J Reprod 2018;7(1): 33-38.
 Asian Pacific Journal of Reproduction2018年1期
Asian Pacific Journal of Reproduction2018年1期
- Asian Pacific Journal of Reproduction的其它文章
- Non-ischemic priapism in dog: Case report
- Sperm dosage and site of insemination in relation to fertility in bovines
- Heritability and variance components estimates for growth traits in Saudi Ardi goat and Damascus goat and their crosses
- Biofertilizing efficiency of Sargassum polycystum extract on growth and biochemical composition of Vigna radiata and Vigna mungo
- Effect of water extract of dates palm (Phoenix dactylifera) on semen characteristics and oxidative status in serum of male New Zealand rabbits under heat stress
- Genetic polymorphism and natural fertility in women
