Genetic polymorphism and natural fertility in women
Fulvia Gloria-Bottini, Neri A, Pietropolli A, Magrini A, Bottini E
Department of Biomedicine and Prevention, University of Rome Tor Vergata, School of Medicine, Rome, Italy
Genetic polymorphism and natural fertility in women
Fulvia Gloria-Bottini✉, Neri A, Pietropolli A, Magrini A, Bottini E
Department of Biomedicine and Prevention, University of Rome Tor Vergata, School of Medicine, Rome, Italy
Women fertility Genetic polymorphism Phosphoglucomutase locus 1 Adenosine deaminase locus 1 Acid phosphatase locus 1 Adenylate kinase locus 1 Haptoglobin
Objective:To investigate the cooperative interaction among five genetic systems(phosphoglucomutase locus 1, adenosine deaminase locus 1, acid phosphatase locus 1,adenylate kinase locus 1, and haptoglobin) concerning their effects on natural fertility in humans. Natural fertility has been evaluated by a model of age related differences between the distributions of types among pregnant women.Methods:A total of 137 nonsmoking consecutive puerperaes from the white population who had delivered their first born baby in the Maternity Department of S. Massimo Hospital of Penne were studied. The phenotypes of the five systems studied were determined by starch gel electrophoresis. Statistical analysis was performed using the statistical package for the social science.Results:There was a highly significant negative correlation between maternal age and the number of genetic factors showing a lower maternal age at the birth of the first child, which suggested a positive cooperative interaction among these factors concerning their effects on fertility.Conclusions:In the relationship of mother-fetus, besides nutritional factors, genetic factors involved in immunological interaction of the embryo with the mother are of paramount importance.Haptoglobin and adenosine deaminase locus 1 polymorphisms are involved in immune reactions and our data indicate that genetic variability within these systems gives a more important contribution to variation of human fertility as compared to acid phosphatase locus 1, phosphoglucomutase locus 1 and adenylate kinase locus 1 that are mainly involved in metabolic functions.
1. Introduction
Previous studies have shown an association of natural fertility with haptoglobin (Hp), phosphoglucomutase locus 1 (PGM1) and adenosine deaminase locus 1 (ADA1) phenotypes[1-3]. We have investigated the cooperative interaction among these systems concerning their effects on fertility. Acid phosphatase locus 1(ACP1) and adenylate kinase locus 1 (Ak1) genetic polymorphismshave been also considered.
Natural fertility has been evaluated by the model of age related differences between the distribution of types among pregnant women proposed by Gimerfalb and Bottini[3]. According to this model, types that have a higher natural fertility should be over represented among pregnant women of younger age as compared to types with a lower natural fertility. In the present analysis, smokingwomen have been excluded and only women who have delivered their first live born infant have been considered. Nonsmoking women only have been included. Hp*1/*1 and ADA1*1/*1 show a consistent reduction of maternal age as compared to carriers of Hp*2 and ADA1*2 alleles respectively. Intermediate reduction of maternal age is observed for ACP1*B/*B and ACP1*A/*C phenotypes as compared to other ACP1phenotypes. The lowest difference is observed for Ak1*1/*1 compared to carriers of Ak1*2 allele and for carriers for PGM1*1 allele as compared to PGM1*2/*2 genotype.
1.1. Hp genetic polymorphism
Hp is a genetic polymorphism showing three phenotypes (Hp*1/*1,Hp*1/*2, Hp*2/*2). In consideration of its anti-inflammatory and immunomodulatory properties[4,5], it may be involved in human reproduction[6]. Hp*1/*1 is composed of small polymers that may diffuse more readily[7] explaining the greater natural fertility of Hp*1/*1 women as compared to women carrying the Hp*2 allele[2].
1.2. ADA1 genetic polymorphism
The genetic polymorphism of ADA1is due to two codominant alleles (ADA1*1 and ADA1*2[8]. ADA1deaminates irreversibly adenosine to inosine. Adenosine regulates many physiological function including immune interactions. In the liver, adenosine counteracts insulin action[9] while in adipocyte it facilitates this action. Since ADA1*1 is more active than ADA1*2, this may influence adenosine concentration and in turn maternal-fetal immunological interactions.
1.3. ACP1 genetic polymorphism
The genetic polymorphism of ACP1is due to the presence of three alleles at an autosomal locus[10]. The three alleles have an activity decreasing in the order ACP1*C>ACP1*B>ACP1*A[10]. The enzyme regulates flavo-enzyme activity, energy metabolism, glycolytic rate and cellular growth[11,12]. There are strong differences in enzymatic activity among genotypes pointing to an important role in many cellular functions. *A/*A and *A/*B genotypes show a low enzymatic activity while *B/*C genotype shows a high activity,*B/*B and *A/*C show an intermediate activity.
1.4. PGM1 genetic polymorphism
The enzyme catalyzes the reversible reaction glucose 1 phosphate glucose 6 phosphate. PGM1shows a polymorphism due to the presence of two codominant alleles at an autosomal locus with activity increasing in the order PGM1*1 < PGM1*2[13,14]. The central role in glycide metabolism, the organ specificity and the presence of the polymorphism in all human populations suggest an important role of the enzyme in tissue functions and that may have an important role in intrauterine development.
1.5. Ak1 genetic polymorphism
The enzyme catalyzes the reversible reaction ATP+AMP←→ADP with an important role in the synthesis of DNA and RNA[15]. AK1shows a polymorphism due to the presence of two common alleles at an autosomal locus with enzymatic activity decreasing in the order AK1*1>Ak1*2 [16].
2. Materials and methods
A total of 137 nonsmoking consecutive puerperaes from the White population who had delivered their first born baby in the Maternity Department of S. Massimo Hospital of Penne were studied. Verbal informed consent was obtained from these women to participate to the study that was approved by the Sanitary Direction of the Hospital. The data were collected a few years ago before the institution of an Ethical Committee. Nonsmoking women only have been included. The phenotypes of the five genetic systems were determined by starch gel electrophoresis[2,8,10,13,16].
Correlation analysis and eta (η) statistics were performed using the Statistical Package for the Social Science (SPSS). Eta statistics is a measure of the strength of association: η2measures the proportion of variance of dependent variable explained by the independent variable.
3. Results
Table 1 showed maternal age at delivery of the first child in relation to the phenotype of Hp, ADA1, Ak1, PGM1and ACP1genetic polymorphisms. Hp*1/*1 and ADA1*1/*1 showed a consistent reduction of maternal age as compared to carriers of Hp*2 and ADA1*2 alleles respectively. Intermediate reduction of maternal age was observed for ACP1*B/*B and ACP1*A/*C genotypes as compared to other ACP1phenotypes. The lowest difference was observed for Ak1*1/*1 compared to carriers of Ak1*2 allele and for carriers for PGM1*1 allele as compared to PGM1*2/*2 genotype.
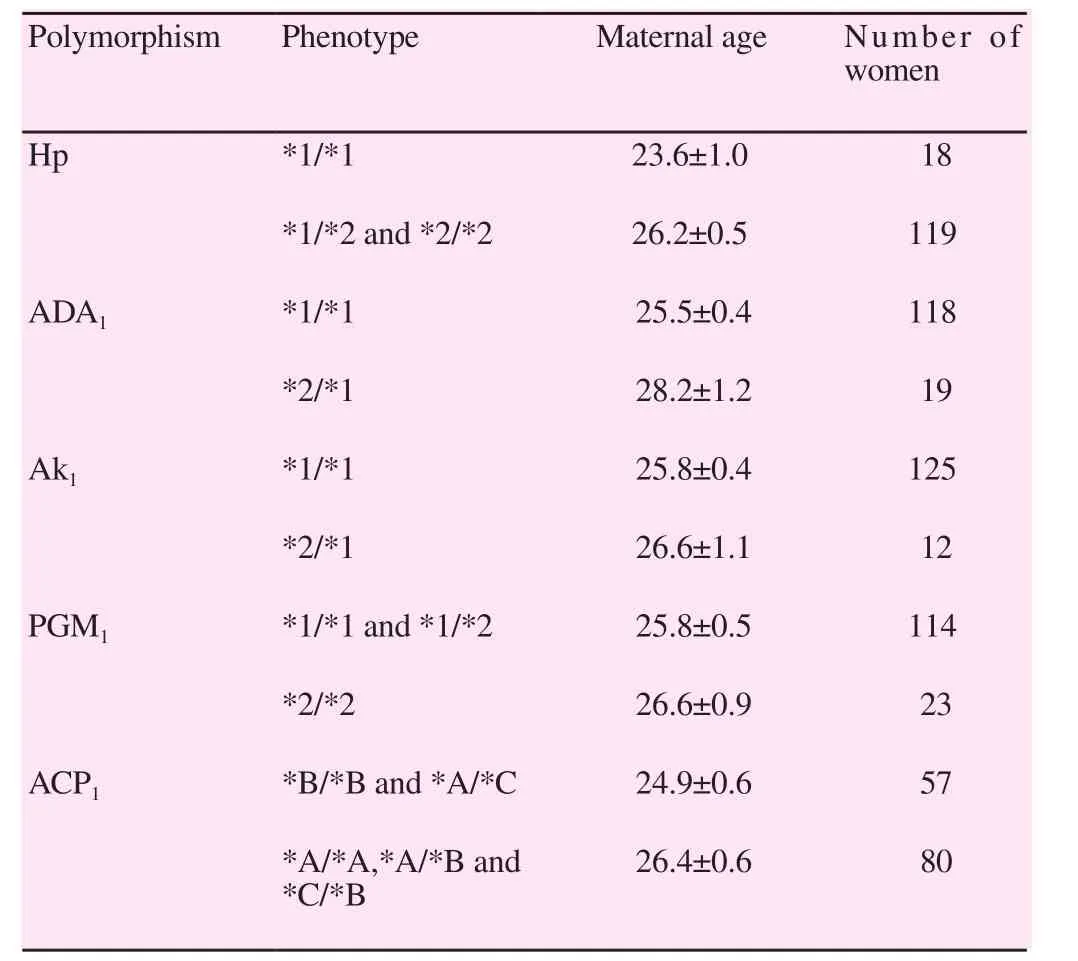
Table 1 Maternal age at delivery of the first child in relation to phenotype of Hp,ADA1, Ak1, PGM1, ACP1 genetic polymorphisms.
We have examined the relationship between the number of genetic factors associated with early reproduction and maternal age at first delivery considering only Hp and ADA1. There was a highly significant negative correlation between the number of genetic factors associated with early reproduction and maternal age (correlation coefficient=-0.23, P=0.007; η2=0.05) suggesting a positive cooperative interaction between the two genetic factors concerning their effects on fertility.
Considering all the five genetic factors studied, there was also a high significant negative correlation between the number of factors and maternal age at delivery of the first child. Compared to the analysis with two factors, the correlation coefficient was higher(-0.28 vs. -0.23) and the proportion of the variance of age explained by the number of factors was higher (0.08 vs. 0.05).
4. Discussion
As stated by Gimelfarb and Bottini[3] considering for example women with type A and women with type B, in absence of limiting factors except natural selection if type A is more fertile than type B,type A will produce more children and faster than type B. Assuming that there is a limit to this number and both types have the same limit, type A will reach such limit faster than type B. Therefore, the difference in the distribution of types among puerperae of different ages could represent an index of maternal selection at reproductive level.
Our analysis suggests an additive action of the genetic factors examined in female natural fertility suggesting that natural fertility depends on many genetic factors, each one with a relatively small effect. It is likely that environmental factors also play an important role
In the mother-fetus relationship, besides nutritional factors, genetic factors involved in immunological interaction of the embryo with the mother are of paramount importance since the first steps of zygote implantation. Hp and ADA1polymorphisms are involved in immune reaction and our data indicate that genetic variability within these systems gives the most important contribution to variation of human fertility. ACP1, PGM1and Ak1are mainly involved in metabolic functions and our data suggest a less important contribution to variation of human fertility.
The relatively low number of women examined represents a limitation of this study. If confirmed in other clinical settings, the results could have practical importance in the prediction of success in assisted reproduction.
Conflict of interest statement
The authors declare that there was no conflict of interest.
[1] Nicotra M, Bottini N, Grasso M, Gimelfarb A, Lucarini N, Cosmi E, et al.Adenosine deaminase and human reproduction: a comparative study of fertile women and women with recurrent spontaneous abortion. J Reprod Immunol 1998; 39: 266-270.
[2] Bottini N, Gimelfarb A, Gloria-Bottini F, La Torre M, Lucarelli P,Lucarini N. Haptoglobin genotype and natural fertility in humans. Fertil Steril 1999; 72: 293-296.
[3] Gimelfarb A, Bottini E. Age differences between distributions of genotypes among pregnant women: Evidence of fertility selection. Genet Res 1989; 53: 207-214.
[4] Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996; 42: 1589-1600.
[5] Smithies O. Zone electrophoresis in starch gels: grouped variations in the serum proteins of normal human adults. Biochem J 1955; 61: 629-641
[6] Berkova N, Lemay A, De Grandpré P, Goupil S, Maheux R. Immunoblot detection of decreased antibodies to haptoglobin-like protein in the serum of infertile women with or without endometriosis. Biol Reprod 1997; 57:178-185.
[7] Javid J. The effect of haptoglobin polymer size on hemoglobin binding capacity. Vox Sang 1965; 10: 320-325.
[8] Spencer N, Hopkinson D, Harris H. Adenosine deaminase polymorphism in man. Ann Hum Genet 1968; 32: 9-14.
[9] Yasuda N, Inoue T, Horizoe T, Nagata K, Minami H, Kawata T, et al.Functional characterization of the adenosine receptor contributing to glycogenolysis and gluconeogenesis in rat hepatocytes. Eur J Pharmacol 2003; 459: 159-166.
[10] Spencer N, Hopkinson DA, Harris H. Quantitative differences and gene dosage in the human red cell acid phosphatase polymorphism. Nature 1964; 201: 299-300.
[11] Bottini E, Gloria-Bottini F, Borgiani P. ACP1and human adaptability 1.Association with common diseases: A case-control study. Hum Genet 1995; 96: 629-637.
[12] Bottini N, Bottini E, Gloria-Bottini F, Mustelin T. Low-molecular weight protein tyrosine phosphatase and human disease: Insearch of biochemical mechanism. Arch Immunol Ther Exp (Warsz) 2002; 50: 95-104.
[13] Spencer N, Hopkinson DA, Harris H. Phosphoglucomutase polymorphism in man. Nature 1964; 204: 742-745.
[14] McAlpine PJ, Hopkinson DA, Harris H. The relative activities attributable in three phosphoglucomutase loci (PGM1, PGM2, PGM3) in human tissues. Ann Hum Genet 1970; 34:169-175.
[15] Van Rompay AR, Johansson M, Karlsson A. Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases.Pharmacol Ther 2000; 87: 189-198.
[16] Fildes RA, Harris H. Genetically determined variation of adenylate kinase in man. Nature 1966; 209: 261-263.
29 October 2017 Revision 10 November 2017 Accepted 30 November 2017 Available online 1 January 2018
10.4103/2305-0500.220980
✉First and corresponding author: Fulvia Gloria-Bottini, MD, Department of Biomedicine and Prevention, University of Tor Vergata, Via Montpellier, 100133 Rome,Italy.
Tel: +39 06 30889514
E-mail: gloria@med.uniroma2.it
This is an open access article distributed under the terms of the Creative Commons Attribution-Non Commercial-Share Alike 3.0 License, which allows others to remix,tweak and buid upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
For reprints contact: reprints@medknow.com
©2018 Asian Pacific Journal of Reproduction Produced by Wolters Kluwer- Medknow
How to cite this article: Fulvia Gloria-Bottini, Neri A, Pietropolli A, Magrini A,Bottini E. Genetic polymorphism and natural fertility in women. Asian Pac J Reprod 2018; 7(1): 19-21.
 Asian Pacific Journal of Reproduction2018年1期
Asian Pacific Journal of Reproduction2018年1期
- Asian Pacific Journal of Reproduction的其它文章
- Non-ischemic priapism in dog: Case report
- Sperm dosage and site of insemination in relation to fertility in bovines
- Heritability and variance components estimates for growth traits in Saudi Ardi goat and Damascus goat and their crosses
- Effects of reduced glutathione on Boer goat semen freezability
- Biofertilizing efficiency of Sargassum polycystum extract on growth and biochemical composition of Vigna radiata and Vigna mungo
- Effect of water extract of dates palm (Phoenix dactylifera) on semen characteristics and oxidative status in serum of male New Zealand rabbits under heat stress
