混合溶液对维生素C的紫外-可见/荧光双光谱检测
郑金慧,赵 博,王晓红,乌英嘎,王 斌,3,刘宗瑞
(1.内蒙古民族大学化学化工学院,内蒙古 通辽 028043;2.长春理工大学化学与环境工程学院,吉林 长春 130022;3.天然产物化学及功能分子合成内蒙古自治区重点实验室,内蒙古 通辽 028042)
郑金慧1,赵 博2,王晓红1,乌英嘎1,王 斌1,3,刘宗瑞1
(1.内蒙古民族大学化学化工学院,内蒙古 通辽 028043;2.长春理工大学化学与环境工程学院,吉林 长春 130022;3.天然产物化学及功能分子合成内蒙古自治区重点实验室,内蒙古 通辽 028042)
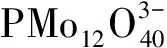
多金属氧酸盐;可逆变色性质;稀土发光;维生素C;光谱检测
维生素在动植物及人体新陈代谢过程中起着极其重要的作用,维生素含量的高低常作为疾病诊断的重要指标,因此开发简便易行、检出限低的维生素C检测方法一直受到科学家的普遍关注.[1-2]
多金属氧酸盐简称多酸(POMs)是由带正电荷的抗衡离子与带负电荷的多酸阴离子构成,具有多样化结构、可逆的氧化还原以及光、电、磁等性质,在催化、能源、材料、医学等领域有着广阔的应用前景.[3-4]多酸在光照、电场、化学还原剂等作用下呈现出无色与蓝色的可逆变化,基于多酸可逆的变色性质,人们将多酸应用于荧光开关的能量受体.[5-6]文献[7]通过层层静电组装技术(LBL)构筑了PEI/Eu-GeWM11O39杂化红光薄膜,薄膜在外加电压-0.85~0.85 V下呈现出可逆的电致变色/荧光开关性质;文献[8]基于功能互补原理,制备了红光吡啶钌与多酸的杂化功能薄膜,在外加电压-0.70~0.70 V下,薄膜呈现出可逆的电致变色/荧光开关性质;文献[9]通过LBL技术构筑了不同荧光性质的量子点/多酸杂化白光薄膜,薄膜在不同电压下呈现出多色可调控荧光开关性质;文献[10]通过溶胶-凝胶技术构筑了Eu(SiW10Mo)2自支持薄膜,薄膜在紫外-可见光照射下呈现出可逆的光致变色-荧光开关性质;文献[11]通过LBL技术构筑了多酸/量子点杂化红光薄膜,薄膜在紫外-可见光辐射下呈现出可逆的光致变色-荧光开关性质.

1 实验部分
1.1 仪器与试剂
仪器:UV-670型双光束紫外-可见分光光度计(上海美普达);Nicilet Nexus470型傅里叶红外光谱仪(美国NICOLET);CHI660e型电化学工作站(上海辰华);F-4600型荧光光谱仪(日本岛津).
试剂:抗坏血酸、六水合硝酸铽(Tb(NO3)3·6H2O)、磷钼酸(H3PMo12O40)均购买于国药集团化学试剂有限公司,均为分析纯;pH=2.5的缓冲溶液用0.5 mol/L H2SO4与0.5 mol/L Na2SO4配制.
1.2 H3PMo12O40晶体的制备
将2 g粉末状的H3PMo12O40·xH2O溶于10 mL 1 mol/L的HCl溶液中,在室温下放置3~4 d,有黄色棒状晶体析出,用减压过滤的方法收集晶体.
1.3 H3PMo12O40晶体的表征
1.3.1 H3PMo12O40的红外分析

1.3.2 H3PMo12O40的紫外-可见光谱分析
图2为0.011 mmol/L H3PMo12O40水溶液的紫外-可见光谱.H3PMo12O40在紫外区波长为210 nm附近处出现明显的吸收峰,归属于端氧、桥氧到金属的电荷转移吸收带(LMCT).[17]而该化合物在可见区没有吸收,表明对可见区的检测信号没有干扰.
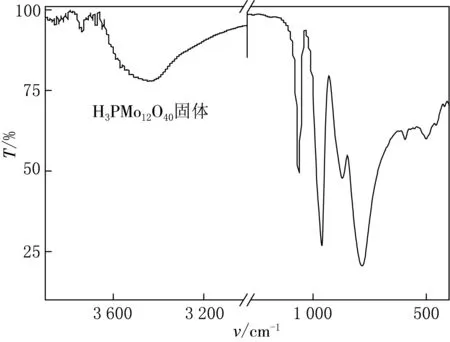
图1 H3PMo12O40的红外光谱

图2 H3PMo12O40溶液的紫外-可见光谱
1.4 溶液的循环伏安曲线
H3PMo12O40在pH=2.5的缓冲溶液中呈现出一系列可逆的氧化还原峰,对应于Mo6+/Mo5+可逆的氧化还原过程见图3[18],维生素C则呈现出一对不可逆的氧化还原峰.在相同条件下维生素C的氧化电位(0.47 V)比H3PMo12O40的还原电位(0.51 V)更负,表明二者可以发生氧化还原反应.


2 结果与讨论

图5 不同浓度的维生素C在溶液中的紫外-可见动力学光谱
2.2 紫外-可见光谱法检测维生素C


图6 不同浓度的维生素C下溶液的紫外-可见光谱(a)与864 nm处吸光度对维生素C的浓度做图的工作曲线(b)
2.3 荧光光谱法检测维生素C

图7 不同浓度的维生素C下溶液的荧光光谱(a)与546 nm处荧光强度的对数与维生素C浓度的工作曲线(b)
3 结论

[1] WANG YI,ZHANG PU,MAO XUANXIANG,et al.Seed-mediated growth of bimetallic nanoparticles as an effective strategy for sensitive detection of vitamin C[J].Sens Actuators B Chemical,2016,231: 95-101.
[2] WU WEI,SUN ZHEN-LIAN,ZHANG WEI.Simple and rapid determination of vitamin c in vegetables and fruits by a commercial electrochemical reader[J].Food Anal Method,2016,9(11):3187-3192.
[3] HARALAMPOS N M,LAIA V N,LEROY C.Polyoxometalate based open-frameworks(POM-OFs)[J].Chem Soc Rev,2014,43(16): 5679-5699.
[4] DIANA J L,ANA C G,MARTYN P,et al.Zinc-substituted polyoxotungstate@amino-MIL-101(Al) an efficient catalyst for the sustainable desulfurization of model and real diesels[J].Eur J Inorg Chem,2016,2016(32): 5114-5122.
[5] JAMES J W,ALAN M B,ROBERT J F,et al.Hybrid polyoxometalate materials for photo(electro-)chemical applications[J].Coord Chem Rev,2016,306: 217-234.
[6] WANG SHIMING,LIU LIN,HUANG ZHIYONG,et al.Vanadium substituted Keggin-type POM-based electrochromic films showing high performance in a Li+-based neutral non-aqueous electrolyte[J].RSC Adv,2016,6(45): 38782—38789.
[7] WANG BIN,YIN ZHENDONG,BI LIHUA,et al.An electroswitchable fluorescence thin-film based on a luminescent polyoxometalate cluster[J].Chem Commun,2010,46(38): 7163-7165.
[8] WANG BIN,BI LIHUA,WU LIXIN. Electroswitchable fluorescent thin film controlled by polyoxometalate[J].J Mater Chem,2011,21(1): 69-71.
[9] GU HONG-XI,BI LI-HUA,FU YU,et al.Multistate electrically controlled photoluminescence switching[J].Chem Sci,2013,4(12): 4371-4377.
[10] WANG ZHONGLIANG,MA YING,ZHANG RUILI,et al.Reversible luminescent switching in a [Eu(SiW10MoO39)2] 13-agarose composite film by photosensitive intramolecular energy transfer[J].Adv Mater,2009,21(17): 1731-1741.
[11] QIN BING,CHEN HONGYUE,LIANG HUI,et al.Reversible photoswitchable fluorescence in thin films of inorganic nanoparticle and polyoxometalate assemblies[J].J Am Chem Soc,2010,132(9): 2886-2888.
[12] WANG BIN,MENG RUIQI,BI LIHUA,et al.A novel detection of hydrogen peroxide based on a luminescent polyoxometalate[J].Dalton Trans,2011,40(19): 5298-5301.
[13] WANG BIN,MENG RUIQI,XU LINGXIAO,et al.A novel detection of nitrite,iodate and bromate based on a luminescent polyoxometalate[J].Anal Methods,2013,5(4): 885-890.
[14] ZHAI YANLING,ZHU CHENGZHOU,REN JIANGTAO,et al.Multifunctional polyoxometalates-modified upconversion nanoparticles: integration of electrochromic devices and antioxidants detection[J].Chem Commun,2013,49(24): 2400-2402.
[15] 王斌,王晓红,乌英嘎,等.多酸Eu-PMo12O40可逆变色-荧光开关性质对维生素C的光谱检测[J].无机化学学报,2016,32(6):994-1000.
[16] YOU WANSHENG,WANG ENBO,HE QINGLIN,et al.Synthesis and crystal structure of a new supermolecular compound: [C12H24O6][H3PMo12O40] center dot 22H2O(C12H24O6=18-crown-6)[J].J Mol Struct,2000,524(3): 133-139.
[17] SASCA V,STEFANESCU M,POPA A.Thermal behavior of the polyoxometalates derived from H3PMo12O40and H4PVMo11O40[J].J Therm Anal Calorim,2003,72(1): 311-322.
[18] HILA G,DEVESH K,NARAHARI G S,et al.An antimony(Ⅴ) substituted Keggin heteropolyacid,H4PSbMo11O40: why is its catalytic activity in oxidation reactions so different from that of H4PVMo11O40?[J].J Mol Catal A Chem l,2012,356: 152-157.
[19] MARK R A,JING JING,BENJAMIN P B,et al.Series behavior of lanthanoid(Ⅲ) complexes with the a-1-Wells-Dawson heteropolyoxoanion in acetonitrile: electrochemistry and Ln coordination[J].Dalton Trans,2010,39(34): 7980-7992.
[20] ZHAI YANLING,ZHU ZHIJUN,ZHU CHENGZHOU,et al.Reversible photo-chem-electrotriggered three-state luminescence switching based on core-shell nanostructures[J].Nanoscale,2013,5(10): 4344-4350.

ZHENG Jin-hui1,ZHAO Bo2,WANG Xiao-hong1,WU Ying-ga1,WANG Bin1,3,LIU Zong-rui1
(1.College of Chemistry and Chemical Engineering,Inner Mongolia University for the Nationalities,Tongliao 028043,China;2.School of Chemistry and Environmental & Engineering,Changchun University of Science & Technology,Changchun 130022,China;3.Inner Mongolia Key Laboratory for the Natural Products Chemistry and Functional Molecular Synthesis,Tongliao 028042,China)
polyoxometalates;reversible color-changing property;rare earth luminescence;vitamin C;spectroscopy
1000-1832(2017)04-0096-05
10.16163/j.cnki.22-1123/n.2017.04.019
2017-05-24
国家自然科学基金资助项目(21501102,21501014);内蒙古自治区自然科学基金资助项目(2015BS0207);内蒙古民族大学科学研究基金资助项目(NMDGP1501,NMDSS1627);内蒙古民族大学“天然产物化学及功能分子合成自治区重点实验室”开放课题(MDK2016010).
郑金慧( 1993—),女,硕士研究生;通信作者:刘宗瑞(1954—),男,教授,主要从事多酸功能材料研究.
O 611.3学科代码150·10
A
(责任编辑:石绍庆)

