结晶性的多孔高分子-金属配合物的最新研究进展
——合成、表征和性质
喻 琪 陈 瑶 张振杰*,,2 程 鹏*,
结晶性的多孔高分子-金属配合物的最新研究进展
——合成、表征和性质
喻 琪1陈 瑶2,3张振杰*,1,2程 鹏*,1
(1南开大学化学学院,天津 300071)
(2药物化学生物学国家重点实验室,南开大学,天津 300071)
(3南开大学药学院,天津 300071)
由于高分子具有尺寸长短不一、柔性等特点,很难通过普通方法合成具有结晶性和多孔性的高分子材料。而高分子金属配合物材料不仅继承了高分子材料的传统性质,同时也可以获得配合物的优点,比如高结晶性和多孔性。本文系统总结了合成结晶性的多孔高分子配合物材料的方法,包括光诱导聚合法、配位自主装法、分步合成法以及后修饰法。同时介绍了它们的表征方法以及潜在应用领域,并讨论了该领域研究中存在的各种机遇与挑战。
结晶性;多孔性;高分子金属配合物;后修饰;自组装
0 Introduction
Polymer-metal coordination complexes (PMCCs)demonstrate as an emerging functional material in which polymer ligands attach to metal ions usually via coordination bonds.PMCCs are attracting increasing attention since the mid of 1990s because of the development of new synthesis strategies and theirpotentialapplicationsasfunctionalmaterialsfor conductive[1],sensing[2],self-healing[3],luminescent[4-5],stimuli responsive[6],nanoscience[7],etc[8-9].However,it is always a great challenge to prepare crystalline and porous PMCCs due to the high flexibility,random conformation of polymer ligands and lack of appropriate synthesis methods.Coordination complexes including coordination polymers (CPs)and metalorganic frameworks (MOFs)consist of central metal ions or metal clusters connected by organic ligands via coordination bonds.Due to the regular coordination geometry of metal centers (e.g.Fe favors to form 6-coordinated octahedral geometry)and the reversibility of coordination bonds,coordination complexes can form highly crystalline materials with defined structures of various topologies (e.g.pcu,nbo,rho,sql)[10-13].Coordination complexes are feasible to form large crystals which are suitable to single-crystal X-ray diffraction(SCXRD).Moreover,coordination complexes especially MOFs possess the word-record highest surface areas[14](Langmuir surface area around 10 000 m2·g-1)among all known porous materials including zeolite,carbon,mesoporous silica and so on.Coordination complexes have attracted great attentions because they demonstrate plenty of promising applications such as gas storage and separation[15],drug delivery[16],conductivity[17]and so on.PMCCs possess the potential to harness not only the advantage of polymers such as facile fabrication of films,good processability and high chemical stability,but also the advantage of coordination complexes such as structure robustness,high crystallinity,well-determined structures and permanent porosity.In this review,we summarize the reported PMCCs which possess high crystallinity and porosity,and discuss how to design and synthesize this kind of functional PMCCs.Moreover,we will discuss how to characterize PMCCs and list the challenges and chances in this area.
1 Photo-induced polymerization method
The topochemical solid-sate reaction have attracted scientists′great attention since this reaction is solvent-free,atom economic and environmental friendly.Ascribed to the mild reaction condition and gentle treatment,materials can possess their crystallinity via a single-crystal to single-crystal transformation manner under photo irradiation.Both singlecrystal,powder X-ray diffraction spectra (PXRD)and Infrared Spectroscopy (IR)spectra can help to understand the structural changes in anatomic-level[18-19].Therefore,photo-induced polymerization reaction is a feasible method to transform a crystalline coordination complex which contains photo reactive monomer into a crystalline PMCC.A wide variety of photo-active groups have been employed for photo-polymerization reactions such as olefins[20-21],anthracenes[22-24]and acetylenes[25-27]or their derivatives.For olefin groups,in order to undergo [2+2]photo-cycloaddition reaction,the C=C bonds should be aligned parallel and the distance should be lower than 0.42 nm proposed by Schmidt et al.[28].For the anthracenes,the carbon atoms in 9,10 positions of the anthracenes also follow the same rules to perform the [4+4]cycloaddition reactions[29].However,the acetylenes obey a new rule to perform 1,4-cycloaddition reaction.The distance of acetylene groups is approximately 0.35 nm,which represents the van der Waals contact between the adjacent molecules.And when the aligned angle is about 45°[30-31],acetylene groups can be polymerized via topochemical1,4-cycloaddition.In order to perform a photocycloaddition reaction,a lot of effort has been made to align the photo-active groups in a favorable stacking arrangement which is the key factor in photocycloaddition reactions with the help of selfassembly techniques such as hydrogen bonds,donor acceptor,coordination bonds and π-π stacking interactions[32-34]etc.If the photo-active groups can be stacked head to tail,when the photo-active groups link together under light source,polymer-metal coordination complexes will be obtained.This method will make it possible to synthesis large crystal of polymers.However,among these interactions,coordination bond is rarely studied and employed in the preparation of PMCCs.
Vittal et al.reported to use 1,4-bis[2-(4′-pyridyl)ethenyl]benzene(bpeb)as a photo-active ligand to form a non-porous MOF,[Zn2(bpeb)-(bdc)(fa)2][35].This MOF can undergo [2+2]cycloaddition reaction in a singlecrystal to single-crystal manner to generate a noninterpenetrated 3D structure in which bpeb ligands polymerized to form 1D chain polymers (Fig.1).The solid-state photoluminescence spectra are also recorded to tract the reaction process.The MOF before irradiation shows a strong green emission,while the MOF after irradiation has a weaker emission which is blue shifted to stronger blue emission.This new PMCC compound is unlikely prepared by a direct synthesis strategy from polymer ligands.
Vittal et al.[36]reported the crystal structure of a 3D Zn-based PMCC fused with an 1D organic polymer ligand which is made in situ by a[2+2]cycloaddition reaction of a six-fold interpenetrated 3D MOFs.The crystallinity of starting 3D MOF after irradiation under 365 nm UV lamp is retained and the structure of the 1D organic polymer is determined by single-crystal X-ray diffraction.This organic polymer ligand can be depolymerized in a SCSC fashion by heating at 250℃for 3 h,the powder X-ray diffraction (PXRD)date(Fig.2b)shows that the structure reverts back to the original MOF structure.Gas sorption study reveals that this PMCC can adsorb a small amount of CO2at 195 K and 100 kPa.

Fig.2 Reversible polymerization by[2+2]photo-cycloaddition under UV lamp and depolymerization on heating (a),PXRD patterns for as synthesized,after UV-irradiation and after heating (b)[36]
2 Coordination induced self-assembly method
According to the knowledge of traditional coordination chemistry,organic chain polymer cannot directly react with metals to form crystalline PMCCs because organic chain polymers are mostly amorphous,flexible and non-porous.Moreover,almost all coordination complexesare prepared from small organicligands.Therefore,itisalwaysa great challenge to directly prepare PMCCs from organic polymers.Until recently,Cohen et al.,for the first time, demonstrate that amorphous, linear, and nonporous polymer ligands are possible to coordinate with metalions to constructhighly crystalline coordination polymers (polyMOFs)via coordination bond induced self-assembly processes.This method makes use of the polymer ligands to react with metal ions directly to synthesize PMCCs which possess not only high crystalline and high porosity inheriting from MOFs,but also the chemical stability,flexibility,easy film formation and good proccessability from the organic polymer.
In 2015,Zhang et al.[37]reported a straightforward strategy to prepare highly crystalline MCPs from polyether ligands (pbdc-xa)which contain repeated 1,4-benzenedicarboxylic acid (H2bdc)unites.A series of polycrystalline hybrid materials with IRMOF networks were prepared upon hydrothermal reactions of pbdc-xa ligands with ZnⅡcations.PXRD and scanning electron microscope (SEM)confirm these materials exhibit the same structures as IRMOF-1.Gas-sorption studies confirm these materials are highly porous.As shown in Fig.3,Zn-pbdc-7a and Zn-pbdc-8a all exhibit typical type Ⅰ isotherms,indicating a uniform microporous structure (Fig.3b)compared to pbdc-xa polymers.Moreover,these materials can sorb more CO2than the parent MOF-5.

Fig.3 IRMOF derivatives construct from an H2bdc ligand derivative,a cross-linked H2bdc ligand,and a polymeric H2bdc polymer ligand with Zn2+ (a),N2sorption isotherms for polyMOFs (top)and CO2adsorption isotherms at 298 K (bottom)(b)[37]
Zhang et al.also demonstrate that this synthesis strategy can not only access the IRMOF structure,but also generates more MOF structures via a mixed ligands strategy (Fig.4)[38].Reaction of pbdc-xa and bridging linkers including dabco (1,4-diazabicyclo octane),bpy(4,4′-bipyridine)with Zn2+or Cu2+cations afford a series of new PMCCs.Gas sorption studies reveal that these materials exhibit relatively high CO2sorption but low N2sorption,making them promising materials for CO2/N2separations.Furthermore,these new PMCCs exhibit much higher water stability compared to their parent MOFs without polymers inside.It can be ascribed to the hydrophobicity of polymers ligands.
Very recently,Cohen et al.reported the first PMCCs with a UiO-66 architecture,prepared from polymer with various alkyl spacers,molecular weights,and dispersities[39].Indeed,PXRD confirm that polyUiO-66 form only with polymers of a certain linker spacing(pbdc-xa,x=3~8).The morphology and particle size of polyUiO-66 are investigated using SEM.The pbdc-6au is obtained composed of very small,crystalline nanostructures (Fig.5a).Pbdc-8a-u exhibit thin and brittle crystalline films (Fig.5b).The pbdc-10a does not form crystalline polyUiO-66 and only amorphous materialis produced.The sorption behaviorof polyUiO-66 is provided by nitrogen gas adsorption.From Fig.5c and Fig.5d,the surface area of polyUiO-66 materials were about 200~400 m2·g-1,which were lower than the parent UiO-66 (1 000~1 500 m2·g-1).This result is ascribed to the pore filling of methylene in the polymer ligands, however, indicate the polyMOF is micro-and meso-porous material.
Johnson et al.[40]synthesized a series of uniform oligomeric polyMOF ligands with alkyne end groups via an iterative exponential growth (IEG)strategy.When these ligands are coupled with Zn ions,a novel“block co-polyMOF” (BCPMOF)is yielded.Revealed by PXRD,BCPMOFs possessed the MOF-5 (IRMOF-1)structure same as polyMOFs reported by Cohen et al.and represent a higher stability than the parent MOF-5 and polyMOF.SEM and transmission electron microscope (TEM) (Fig.6)images reveal BCPMOFs can form a thin polymer film.
3 Two-step synthesis method
Some coordination complexes such as metalorganic polyhedral (MOPs)are inherently porous.Therefore,a two-step synthesis starting from coordination complexes with polymerization groups can afford crystalline and porous PMCCs. This approach includes two steps:first preparation of coordination complex with polymerization groups and second cross-linking of coordination complexes through homopolymerization or copolymerization reactions.
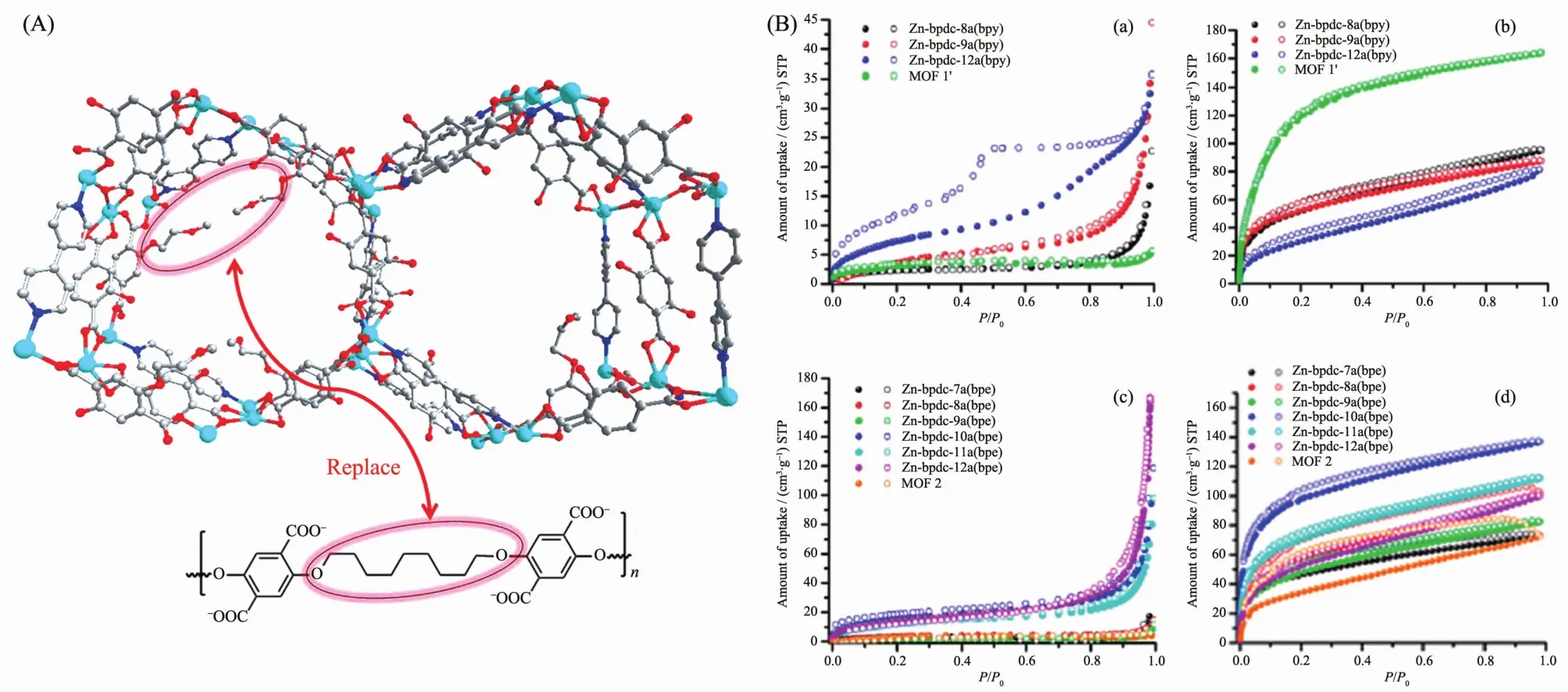
Fig.4 Design concept for creating a polyMOF analogue of MOF via replacing dangling groups by polymer chains (a),N2sorption and CO2sorption isotherms for polyMOFs (b)[38]
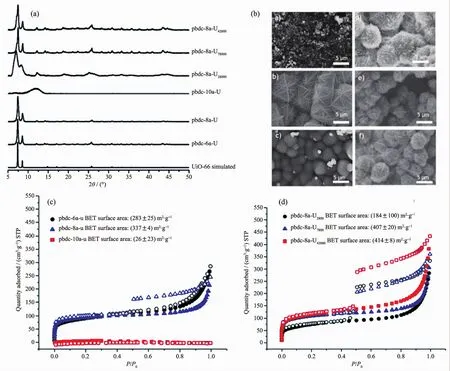
Fig.5 PXRD patterns,SEM images and N2sorption isotherms of polyMOF prepared from different polymer ligands (a~d)[39]
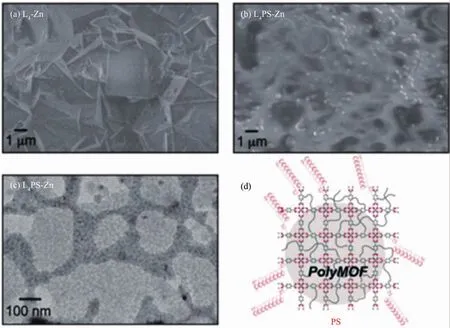
Fig.6 SEM images of L4-Zn (a),L4PS-Zn dried at RT (b)and TEM image of L4PS-Zn dried at RT (c),Schematic of L4PS-Zn depicting a crystalline polyMOF domain embedded within a PS matrix (d)[40]
Kitagawa et al.[41]in Japan reported the divergent and convergent synthesis of coordination star polymers(CSPs)by using MOPs as a multifunctional core.The great rhombicuboctahedral MOPs as a multifunctional core consists of a total of 24 isophthalic acid ligands interconnected via 12 dicopper paddle wheel clusters.Reversible addition-fragmentation chain transfer polymerization mediated with the MOP led to MOP-star polymers.Atomic force microscope (AFM)images show CSPs exhibit the nanoscale particles.
Thibonnet et al.[42]reported titanium-doped porous polymers obtained from specific Ti-containing monomerswith polymerization groups.Thisfree radicalco-polymerization reaction affords several titanium-containing polymers,which were dried under supercriticalconditions to afford porous organic aerogels.As shown in Fig.8,the IR spectra reveal that the strong signal at 3 400 cm-1for the Ti aerogel may be corresponded to the presence of hydrogen bonded OH groups.Moreover,two carbonylsignalsare observed at 1 708 and 1 200 cm-1which confirm the existence of Ti carboxylate complexes.The porosity is investigated using N2adsorption-desorption isotherms.The BET value of PMCCs was about 454 m2·g-1.There are mesopores and microporosity characterized through pore size distribution.
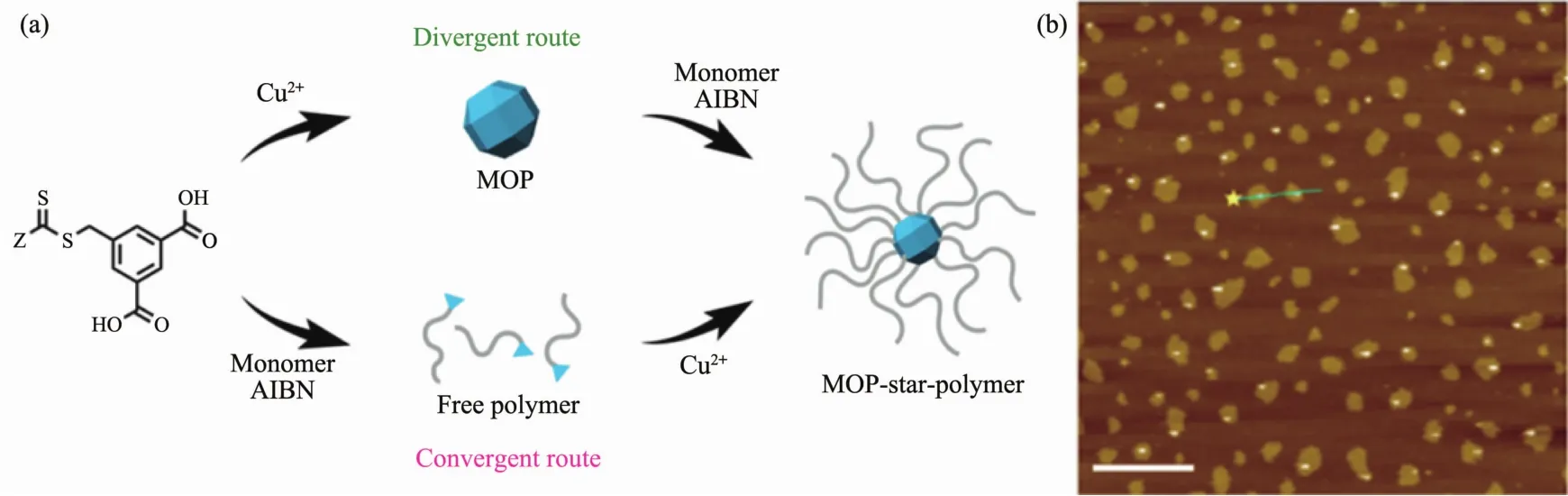
Fig.7 Schematic diagram of the divergent route for MOP-core CSPs (a)and AFM height image of individual particles of MOP with scale bar=1 μm (b)[41]
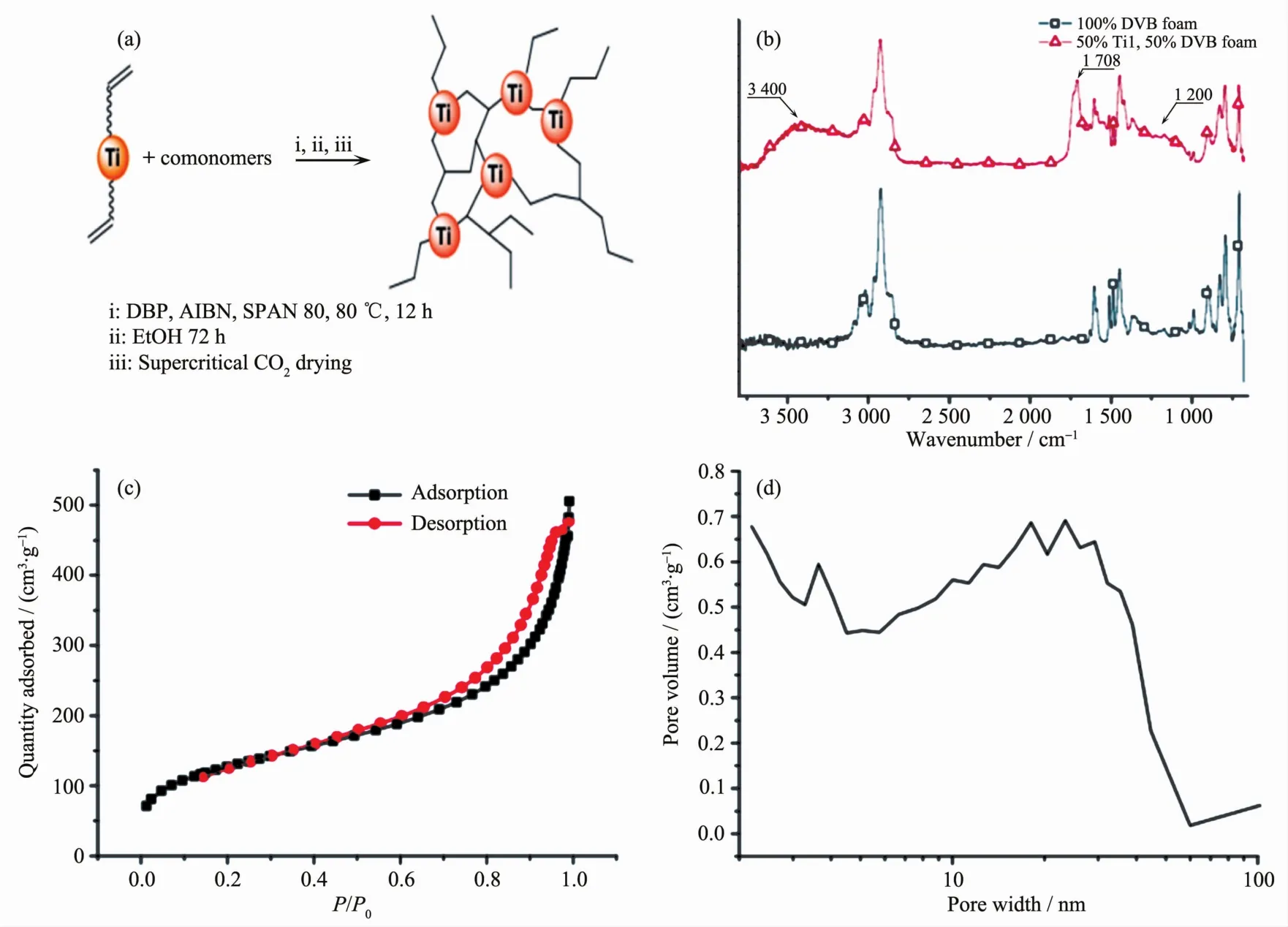
Fig.8 Different porous co-polymers obtained with Ti-complex (a)and IR spectra of a 50/50 Ti1/DVB co-polymer foam and a DVB homopolymer foam (b),N2adsorption-desorption isotherms of polymer (c,d)[42]
A facile and scalable route to prepare PMCCs were employed by Dai et al.[43]The bifunctional 1-vinylimidazole (VIm)with a coordinating site and a polymerizable organic group is introduced as the ligand.Subsequently,the radical polymerization of[Zn(VIm)4][NO3]2coordination complex is carried out under solvothermal conditions to gain a higher degree ofcross-linking.Thismaterialexhibitsexcellent stability in boiling water and can be stable up to 390℃in air.Fig.9 displays the abundant pores within a large domain of CIN-1.It is observed that mesopores exist side by side among CIN-1 particles.This strategy will inspire a number of stable metal-supported porous polymers by careful selection of ligands,thus opening a new pathway to porous PMCCs.
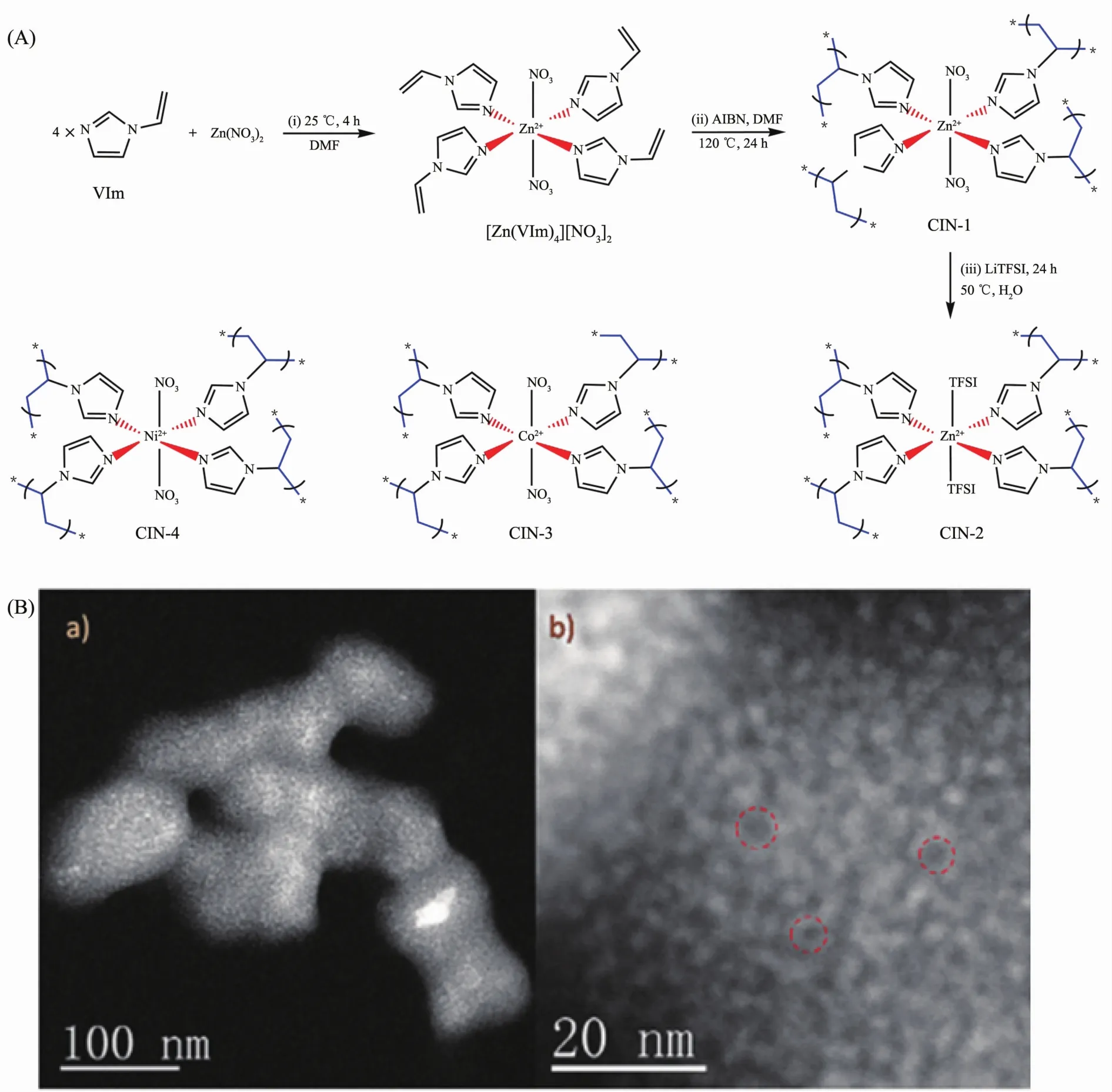
Fig.9 Strategy to prepare coordination-supported imidazolate networks (A)and STEM-HAADF images of CIN-1 at 60 kV (B)[43]

Fig.10 Depiction of polymer imprinting with metal-oxo-hydroxo carboxylate clusters,the adsorption of Fe3+on unimprinted polymer and imprinted polymer[44]
By using the same synthesis strategy,Walton′s group[44]synthesized three new ion-oxo-hydroxo cluster coordinated by vinyl-derivatized carboxylates,[Fe6O2(OH)2(O2CC(Cl)=CH2)12(H2O)2] (1),[{Fe(O2CC(Cl)=CH2)(OMe)2}10] (2)and [Fe6O2(OH)2(O2C-Ph-(CH)=CH2)12(H2O)2](3).Polymerization these Fe-based coordination complexes afford a series of PMCCs.For E-L(nonimprinted copolymer of chloroacrylic acid and egdma)the maximum iron uptake is 1.1 mg·g-1of polymer,for E-1 (egdma polymer imprinted with 1)this figure is 1.8 mg·g-1of polymer.Hence,the imprinted polymer shows a greater than 60%increase in the amount of iron removed from solution compared to the nonimprinted polymer.
4 Post-synthetic modification method
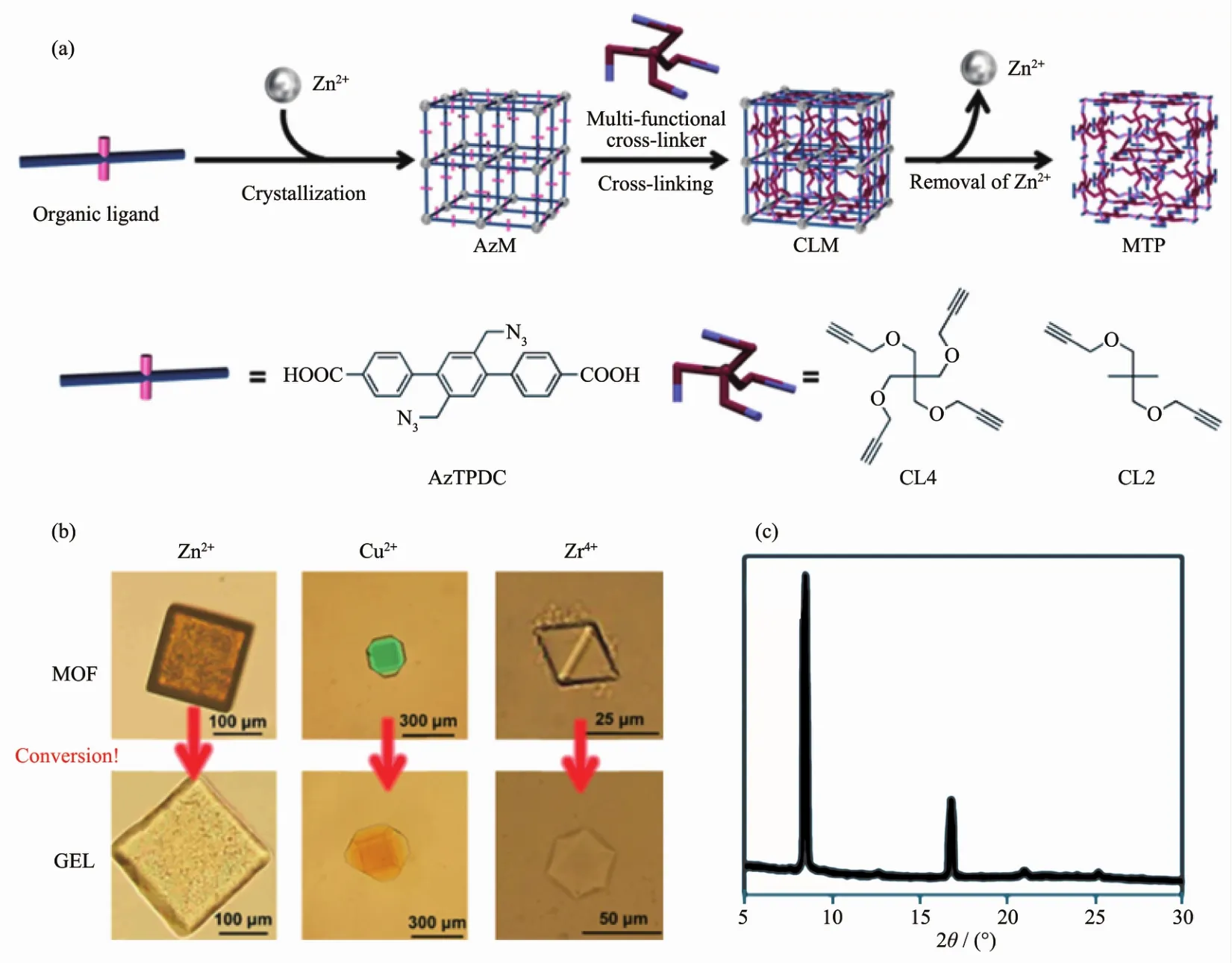
Fig.11 Schematic illustration of cross-linking of the organic linkers in MOF and subsequent decomposition to obtain polymer gel(a),Photographs of resulting MOF-templated polymers from various combinations of organic ligands and metal ions (b),Powder X-ray diffraction pattern of a single piece of whole chimera-type hybrid (c)[45]
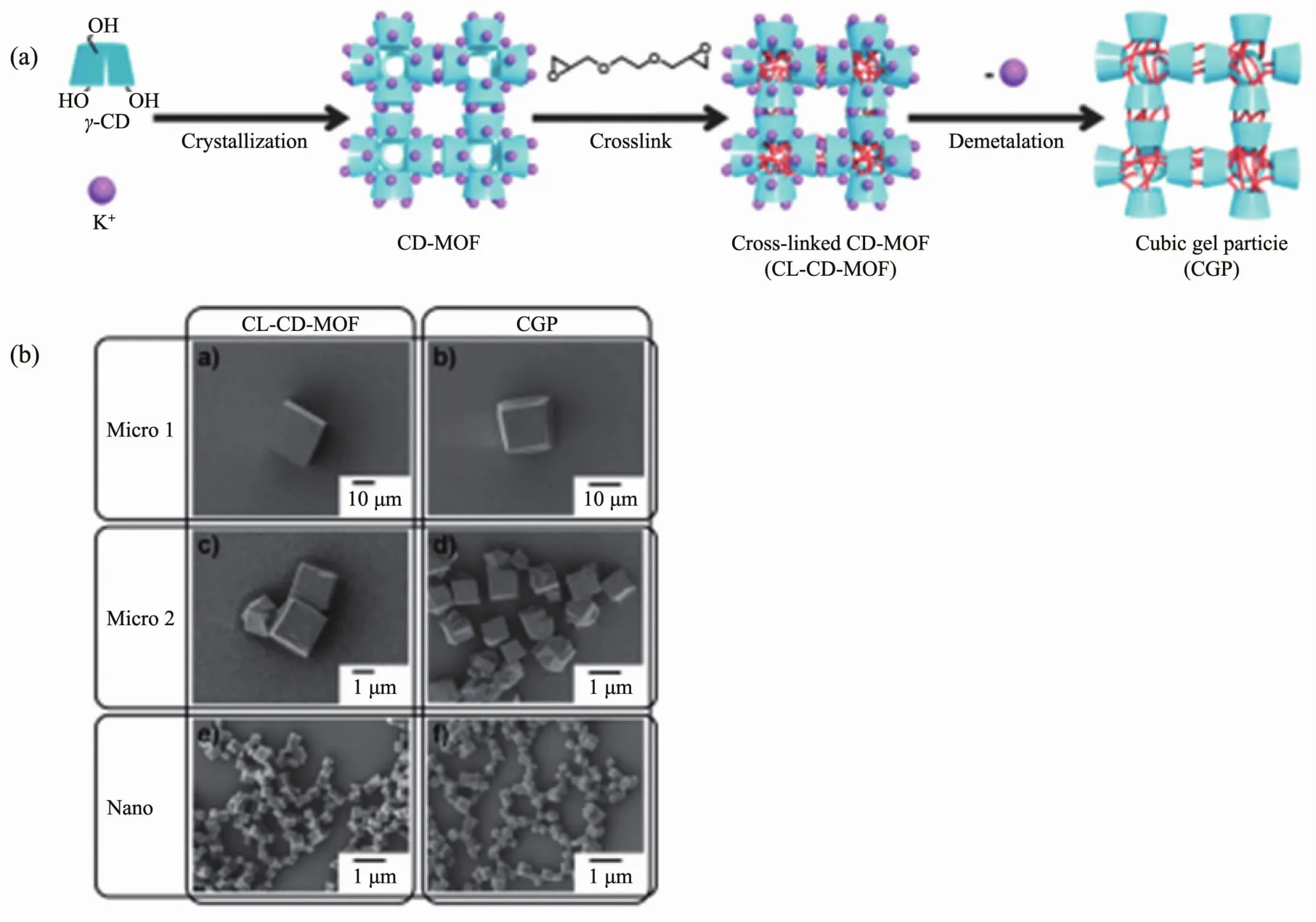
Fig.12 Schematic images for the CC method using CD-MOF to obtain a cubic gel particle (a),SEM images of CL-CD-MOF and CGP with different sizes (b)[46]
Compared with other porous materials,a great advantage of MOFs is the ability to perform postsynthetic modifications (PSMs).The clear crystal structures and defined pores of MOFs make them as suitable platforms to perform post-synthetic PSMs in MOF crystals without destroying MOFs′crystallinity.Therefore,if MOFs are functionalized with polymerization groups in their structures,it is possible to employ PSMs method to form crystalline and porous PMCCs.
An azide-functionalized terphenyl dicarboxylic acid derivative was selected as the organic ligand for the PSM strategies[45].Starting from dicarboxylic acid ligands,treatment of Zn2+,Cu2+and Zr4+provid colorless cubic and green truncated octahedral MOF crystals under the solvothermal reactions.The polymer gels(PG)are produced from the transformation of various MOFs via inner cross-linking of the organic linkers in the void space of MOFs,followed by decomposition of the metal coordination.As expected,all crystals are successfully transformed into polymer gels with the same shape as the corresponding MOF,indicating that the PSM method should be applicable to many MOFs systems.
The uniform cubic gel particles with well-defined edges and square faces using internal cross-linking of the CD-MOF crystals followed by loss of coordinating metal ions[46].The cubic gel particles retain the shape and size of the original CD-MOF crystals,indicating that by controlling the recrystallization conditions a wide range of sizes of CGPs,from millimeters to nanometers,can be produced.Moreover,a variety of polyhedral gel particles from the MOF crystals with controlled polyhedral shapes can be produced.
5 Characterization and application of coordination polymers
Similarto the characterization methods for traditional coordination polymers,single crystal and powder X-ray diffraction data can be used to study the crystallinity of PMCCs.For example,the structure of PMCCs in Fig.2a are firstly determined via the single crystal X-ray diffraction.Furthermore,PXRD is used to confirm that bulky samples possesse the same structure as single crystal.In addition,IR,photolu-minescence spectra (PL)and solid nuclear magnetic resonance (NMR)data can be employed to study whether there are polymer groups formed.As shown in Fig.13a,the complete polymerization ofthe[Zn(VIm)4][NO3]2monomer is indicated by the disappearance of the characteristic peak for vinyl group(1 650 cm-1)in IR spectra[43].Solid state photoluminescence spectra in Fig.14 are recorded for 1 (MOF)and 2 (MOPF)after photopolymerization.Compound 1 shows a strong green emission while 2 has a weaker emission which is blue shifted to more strong blue emission which may be due to the loss of extended conjugation upon polymerization[35].The presence of a broad signal at δ 46.5 indicates the formation of the cyclobutane ring in the Solid NMR spectra[36]in Fig.15.SEM,TEM and AFM are widely applied to check the PMCCs particles′surface morphology,fracture characteristics of cross section and the nanoscale.The SEM images[39]in Fig.5 show that the obtained PMCCs are composed of tiny crystalline nanostructures.TEM images[43]in Fig.9 display the abundant pores within a large domain of CIN-1,wormhole-like mesopores side by side are observed in a high-resolution image,and the apparent pore sizes are in the range of 4~7 nm.AFM spectra[41]in Fig.7 are used to visualize the shape of individual particles of MOP.All particles are clearly shown to possess an individual core that is covered by a polymeric corona.
PMCCs have been reported to possess some promising applications such as catalysis,gas separation and film fabrications.For example,Dai et al.reported thatCINsare interesting catalystsforselective oxidations[43].CINs were used in phenol oxidation with water as solvent and H2O2as oxidant.No products are observed in the blank run.However,the CIN-3 with Co2+centers coordinated by imidazole ligand shows a good activity in the oxidation of phenol,and a high turnover frequency (TOF)of 779 h-1was achieved.As a solid catalyst,CIN-3 could be easily recovered by centrifugation and reused for at least three cycles with slight loss in efficiency.Hence,CIN-3 could be considered as a promising catalyst in the catalytic oxidation of phenol.
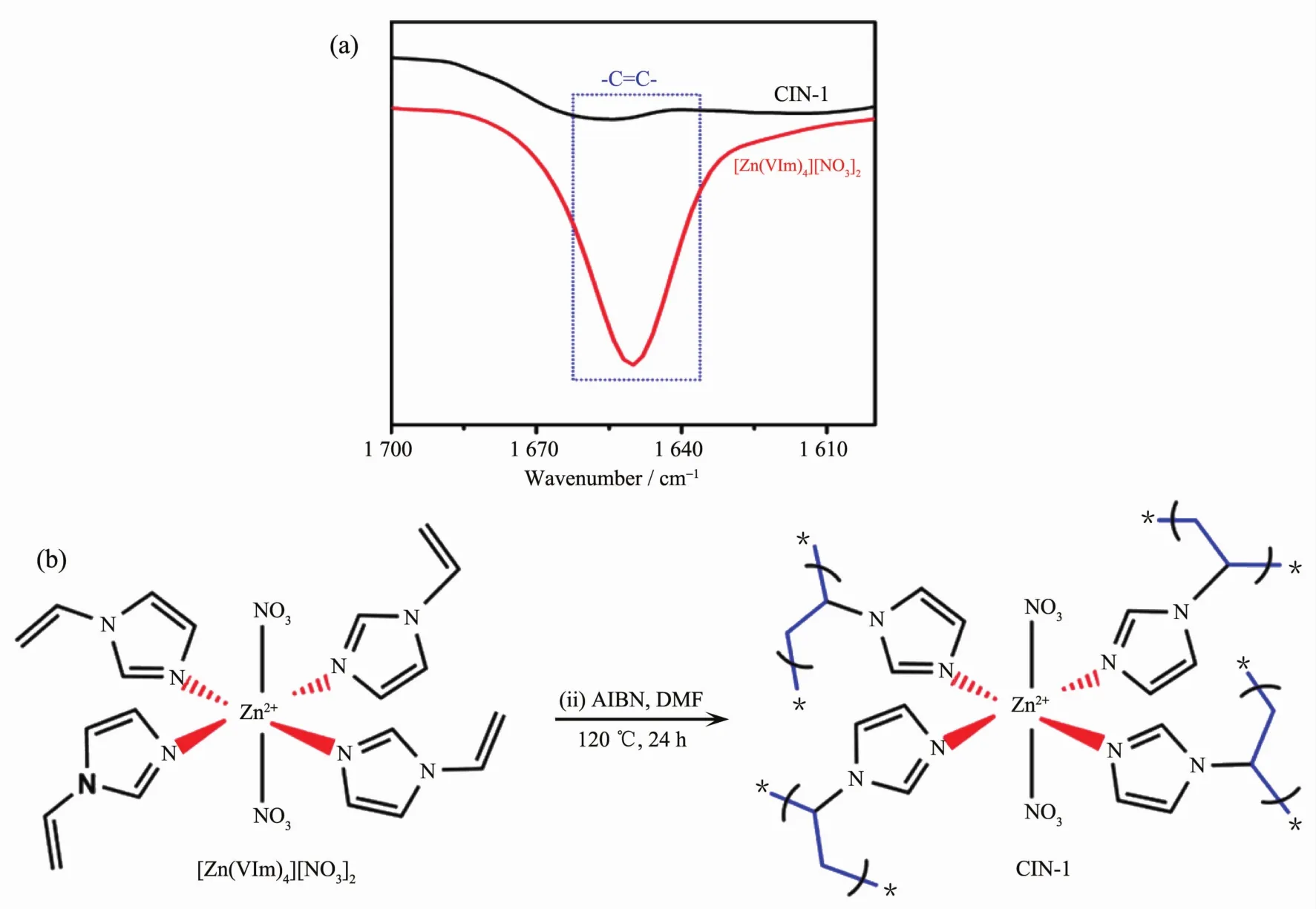
Fig.13 IR spectra of[Zn(VIm)4][NO3]2and CIN-1 (a),formation process of[Zn(VIm)4][NO3]2to CIN-1 (b)[43]
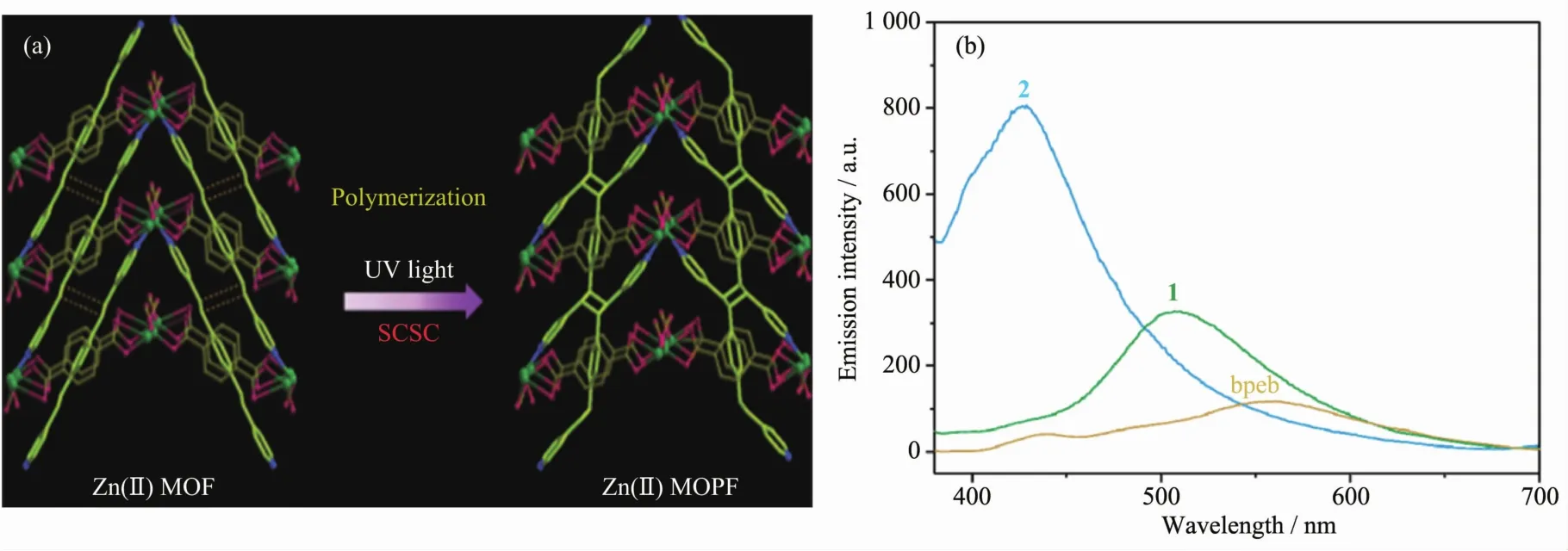
Fig.14 SCSC transformation from MOF (1)to MOPF (2)by polymerization (a)and their UV spectra (b)[35]
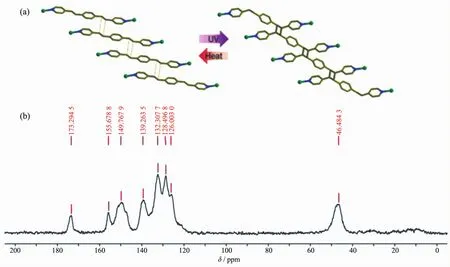
Fig.15 SCSC transformation (a)and its13C CPMAS solid-state NMR spectra (b)[36]
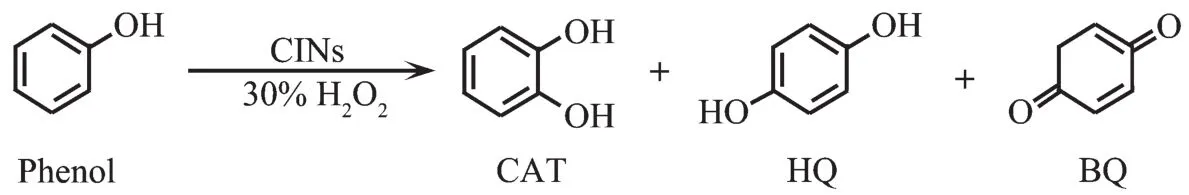
Fig.16 Catalytic oxidation of phenol by CINs[43]
Cohen et al.reported that PMCCs could be applied in gas separation[38].The observed selective adsorption of PMCCs can be attributed to a kinetic sieving effect,where the small windows limit the diffusion of larger N2molecules into pores resulting in reduced adsorption.Attempts to quantitatively evaluate the CO2/N2and CO2/CH4separation performance at 273 and 298 K for these PMCCs are performed in Fig.17.The PMCCs can absorb significant amounts of CO2at 100 kPa and 298 or 273 K,however low N2and methane uptake.The relative high CO2sorption but very low N2sorption makes these PMCCs as promising materials for CO2/N2separation.
Except the above application,PMCCs also are easy to fabricate films as shown in Fig.18.Cohen et al.reported that at a low temperature,rather than forming spheroidal structures,Zn-pbdc-7a and Znpbdc-8a produce crystalline films,showing an intergrown network of crystallites.The films are about 20 μm thick.Such films may prove useful for small molecule and gas separations[37].

Fig.17 CO2,CH4and N2sorption isotherms at 273 K (a)and CO2,CH4and N2sorption isotherms at 298 K for polyMOFs (b)
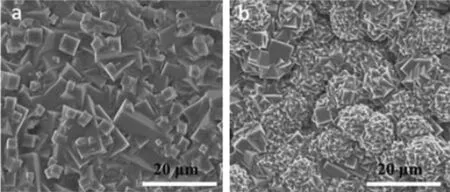
Fig.18 Film morphology of Zn-pbdc-7a (a)and Zn-pbdc-8a (b)
6 Summary and outlook
In this review article,we have discussed four approaches how to construct polymer-metal coordination complexes (PMCCs)with good crystalline and high porosity.We also display the characterization methods of PMCCs.Although PMCCs are in the process of rapid development,there are still some challenges and potentialchances.Forthe first approach,the limited numbers of coordination complexes for realizing the single crystal to single crystal manner is a big challenge in the research.Therefore,design coordination complexes with novel topologies suitable for photo-polymerization is of great significance in the future research.During the transformation process,crystals are easy to lose their crystallinity.So how to control the completeness of polymerization and retain the crystallinity of PMCCs is deserved to explore.For the second approach,it is difficult to synthesize crystalline PMCCs because the organic chain polymers are mostly amorphous,flexible and non-porous.Finding appropriate polymer ligands react directly with metal ions is extremely urgent.For the third approach,finding right coordination complexes with polymerization groups is very important and theconditionsforhomopolymerization orcopolymerization of the complex is also needed to be explored to find the appropriate condition reactions.For the fourth approach,how to control the reaction condition to retain the crystallinity of coordination polymers is the key in research.These techniques will enable the as-yet unexplored precise structural control of PMCCs on the molecular,which are advanced materials for novel properties and applications discovered in the future.
Acknowledgements:We acknowledge the China Young 1000 Talents program and NSFC (Grant No.21601093).
[1]Holliday B J,Swager T M.Chem.Commun.,2005,1:23-26
[2]Holliday B J,Stanford,T B,Swager T M.Chem.Mater.,2006,18:5649-5651
[3]Burnworth M,Tang L,Kumpfer J R,et al.Nature,2011,472:334-337
[4]Dobrawa R,Lysetska M,Ballester P,et al.Macromolecules,2005,38:1315-1325
[5]Dobrawa R,Wurthner F.Chem.Commun.,2002,17:1878-1879
[6]Beck J B,Rowan S J.J.Am.Chem.Soc.,2003,125:13922-13923
[7]Zou S,Hempenius M A,Schnherr H,et al.Macromol.Rapid Commun.,2006,27:103-108
[8]Whittell G R,Hager M D,Schubert U S,et al.Nat.Mater.,2011,10:176-188
[9]Whittell G R,Manners I.Adv.Mater.,2007,19:3439-3468
[10]Guillerm V,Kim D,Eubank J F,et al.Chem.Soc.Rev.,2014,43:6141-6172
[11]Navarro J A R,Barea E,Galindo M A,et al.J.Solid State Chem.,2005,178:2436-2451
[12]Zhang Y B,Furukawa H,Ko N,et al.J.Am.Chem.Soc.,2015,137:2641-2650
[13]Wang T C,Vermeulen N A,Kim I S,et al.Nat.Protoc.,2016,11:149-162
[14]Farha O K,Eryazici I,Jeong N C,et al.J.Am.Chem.Soc.,2012,134:15016-15021
[15]Li J R,Kuppler R J,Zhou H C.Chem.Soc.Rev.,2009,38:1477-1504
[16]Rojas S,Wheatley P S,Quartapelle-Procopio E,et al.CrystEngComm,2013,15:9364-9367
[17]Nagarkar S S,Anothumakkool B,Desai A V,et al.Chem.Commun.,2016,52:8459-8462
[18]Liu D,Wang H F,Abrahams B F,et al.Chem.Commun.,2014,4:1-3
[19]Liu D,Ren Z G,Li H X,et al.Angew.Chem.Int.Ed.,2010,49:4767-4770
[20]Hasegawa M.Adv.Phys.Org.Chem.,1995,30:117-171
[21]Garai M,Santra R,Biradha K.Angew.Chem.Int.Ed.,2013,52:5548-5551
[22]Champness N R.Nat.Chem.,2014,6:757-759
[23]Kissel P,Murray D J,Wulftange W J,et al.Nat.Chem.,2014,6:774-778
[24]Kory M J,Wrle M,Weber T,et al.Nat.Chem.,2014,6:779-784
[25]Itoh T,Shichi T,Yui T,et al.Langmuir,2005,21:3217-3220
[26]Sun A,Lauher J W,Goroff N S.Science,2006,312:1030-1034
[27]Arai M,Okada S.Chem.Lett.,2006,35:1012-1013
[28]Schmidt G M.Pure Appl.Chem.,1971,27:647-678
[29]Bhola R,Payamyar P,Murray D J,et al.J.Am.Chem.Soc.,2013,135:14134-14141
[30]Sarkar A,Okada S,Komatsu K,et al.Macromolecules,1998,31:5624-5630
[31]Fahsi K,Deschamps J,Chougrani K,et al.CrystEngComm,2013,15:4261-4279
[32]Bhattacharya S,Stojakovi J,Saha B K,et al.Org.Lett.,2013,15:744-747
[33]Yang S Y,Deng X L,Jin R F,et al.J.Am.Chem.Soc.,2014,136:558-561
[34]Papaefstathiou G S,Zhong Z,Geng L,et al.J.Am.Chem.Soc.,2004,126:9158-9159
[35]Park I H,Chanthapally A,Lee H H,et al.Chem.Commun.,2014,50:3665-3667
[36]Park I H,Chanthapally A,Zhang Z J,et al.Angew.Chem.Int.Ed.,2014,53:414-419
[37]Zhang Z J,Nguyen H T H,Miller S A,et al.Angew.Chem.Int.Ed.,2015,54:6152-6157
[38]Zhang Z J,Nguyen H T H,Miller S A,et al.J.Am.Chem.Soc.,2016,138:920-925
[39]Ayala S,Zhang Z,Cohen S M.Chem.Commun.,2017,53:3058-3061
[40]MacLeod M J,Johnson J A.Polym.Chem.,2017,8:4488-4493
[41]Hosono N,Gochomori M,Matsuda R,et al.J.Am.Chem.Soc.,2016,138:6525-6531
[42]Cadra S,Velasquez E,Moreau L,et al.Tetrahedron Lett.,2011,52:3982-3986
[43]Zhang P,Yang S,Chisholm M F,et al.Chem.Eur.J.,2017,23:10038-10042
[44]Harben S M,Mosselmans J F W,Ryan A T,et al.Dalton Trans.,2012,41:208-218
[45]Ishiwata T,Furukawa Y,Sugikawa K,et al.J.Am.Chem.Soc.,2013,135:5427-5432
[46]Furukawa Y,Ishiwata T,Sugikawa K,et al.Angew.Chem.Int.Ed.,2012,51:10566-10569
Recent Progress of Crystalline and Porous Polymer-Metal Coordination Complexes:Synthesis,Characterization and Properties
YU Qi1CHEN Yao2,3ZHANG Zhen-Jie*,1,2CHENG Peng*,1
(1College of Chemistry,Nankai University,Tianjin 300071,China)
(2State Key Laboratory of Medicinal Chemical Biology,Nankai University,Tianjin 300071,China)
(3College of Pharmacy,Nankai University,Tianjin 300071,China)
It is always a great challenge to synthesize crystalline and porous polymer materials due to the random configuration and flexibility of polymers.However,polymer-metal coordination complexes (PMCCs)can exhibit high porosity and crystallinity because PMCCs possess not only the properties of polymers such as flexibility and good processability but also the properties of coordination complexes such as high crystallinity and porosity.Herein,this review article summarizes the methods how to construct porous and crystalline PMCCs including photo-induced polymerization method,coordination bond induced self-assembly method,two-step synthesis method and post-synthetic modification method.Finally,we discusse the characterization methods and the potential applications of PMCCs,and list the challenges and chances in the field.
crystallinity;porosity;polymer-metal coordination complexes;post-synthetic modification;self-assembly
O641.4
A
1001-4861(2017)11-1991-14
10.11862/CJIC.2017.243
2017-08-07。收修改稿日期:2017-08-22。
国家自然科学基金(No.21601093)和中国青年千人计划资助项目。
*通信联系人。E-mail:zhangzhenjie@nankai.edu.cn,pcheng@nankai.edu.cn

