磁性固相萃取技术在真菌毒素检测中的应用研究进展
赵仁勇 ,安 娟 ,崔文航 ,何丽君 *
(河南工业大学 1.粮油食品学院;2.化学化工与环境学院,河南 郑州 450001)
磁性固相萃取技术在真菌毒素检测中的应用研究进展
赵仁勇1,安 娟1,崔文航2,何丽君2*
(河南工业大学 1.粮油食品学院;2.化学化工与环境学院,河南 郑州 450001)
真菌毒素具有强烈的毒性,在食品和农作物中广泛存在,对人类和动物的健康造成了极大的安全隐患。快速高效对真菌毒素进行定量检测十分必要,样品前处理是整个分析环节的关键步骤。磁性固相萃取(magnetic solid-phase extraction,MSPE)是一种以磁性材料为吸附剂的新型样品前处理技术,因操作简单、萃取时间短、萃取效率高,以及吸附剂易从样品溶液中分离等优点在食品样品前处理中引起广泛关注。介绍了MSPE的萃取过程、磁性吸附剂的种类及其制备方法,综述了MSPE近年在真菌毒素检测分析中的应用,并对该技术的发展趋势进行了展望。
真菌毒素;磁性固相萃取;磁性吸附剂;样品前处理
0 引言
真菌毒素是一类由丝状真菌产生的有毒次级代谢产物,广泛存在于农产品及其制品中,具有强烈的毒性、致癌性、致畸性和致突变性[1]。目前已发现的真菌毒素有400多种,其中污染广泛、对人体健康危害较大的真菌毒素有十几种,主要包括黄曲霉毒素(aflatoxin,AF)、脱氧雪腐镰刀菌烯醇(deoxynivalenol,DON)、 赭曲霉毒素 A(ochratoxin A,OTA)、玉米赤霉烯酮(zearalenone,ZEN)、伏马毒素(fumonisin,FB)等[2-3]。考虑真菌毒素在食品及其供应链中普遍存在以及它们对人类和动物健康的危害,许多地区和国家都建立了一系列真菌毒素限量标准和检测方法,以严格控制真菌毒素在食品中的残留。
目前真菌毒素的测定方法主要有酶联免疫吸附法(enzyme-linked immunosorbent assay,ELISA)[4]、色谱法[5-11]及荧光光谱法[12-13]。ELISA法虽然检测效率高,但重复性较差、干扰因素多,适用于大量样品的筛查。近年来色谱法及色谱-质谱联用技术等现代仪器分析方法得到了迅速发展及推广,这些方法具有灵敏度高、检测限低、重现性好等优点,在真菌毒素检测方面得到了广泛应用。
由于食品样品基质十分复杂,且真菌毒素在样品基质中的含量极低,在色谱分析前如何准确、快速、高效地对样品基质中的真菌毒素进行前处理至关重要。样品前处理是整个分析过程中的重要环节,而前处理效果往往是决定仪器分析成功与否的关键。目前应用于真菌毒素的样品前处理方法主要有免疫亲和层析净化[14]、分散液液微萃取[15-16]、固相萃取[17-18]、固相微萃取[19-20]、基质固相分散萃取[21]和QuEChERS[22-23]技术等,这些方法通常用于单一或同类真菌毒素的样品前处理过程,存在操作复杂、耗时、成本高等缺点。磁性固相萃取技术是一种新型样品前处理技术,该技术以其快速、简单、高效、绿色等优点在有机污染物[24-25]、金属离子[26]和生物活性物质[27]测定中得到广泛应用。作者综述了MSPE在测定真菌毒素中的应用,包括MSPE的萃取过程、磁性吸附剂的种类及其制备方法,也对MSPE在真菌毒素检测中的发展趋势进行了展望。
1 MSPE技术简介
1.1 MSPE过程
MSPE技术是一种以磁性材料作为吸附剂的固相萃取技术。在MSPE过程(图1)中,磁性吸附剂与样品基质混合,在振荡或超声等辅助条件下,样品中的目标分析物被吸附到磁性吸附剂表面,通过外部磁场作用将含目标分析物的磁性吸附剂与样品基质分离,目标分析物经洗脱剂从磁性吸附剂上洗脱下来后用于仪器检测[24-25,28]。MSPE技术克服了传统固相萃取技术在过柱或过滤操作中耗时的缺点,且萃取过程中磁性吸附剂与目标分析物接触面积足够大,能够使目标分析物的相转移快速完成,萃取效率较高。另外,MSPE的磁性吸附剂可重复利用,降低了分析检测的成本。
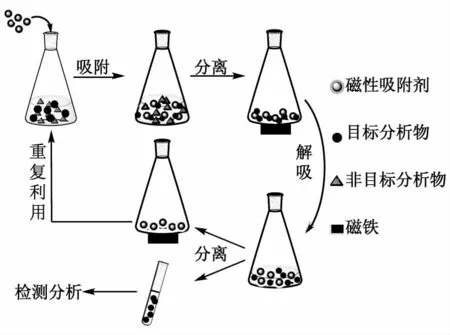
图1 磁性固相萃取过程示意图Fig.1 The schematic diagram of MSPE procedure
1.2 磁性吸附剂
磁性吸附剂在MSPE中起着至关重要的作用。磁性吸附剂由磁性颗粒及负载于磁性颗粒表面的功能分子组成,磁性颗粒的磁性使得吸附剂与样品基质在外界磁场下快速分离,而功能分子的种类决定了吸附剂对目标分析物的萃取性能。常用的磁性颗粒有四氧化三铁(Fe3O4)、三氧化二铁(γ-Fe2O3)、铁氧体和金属合金等,在这些磁性材料中,Fe3O4以其制备方法简单、性能稳定、成本低等优点在MSPE中得到广泛应用[24]。但纯的Fe3O4对目标分析物没有萃取性能,且其尺寸小、比表面积大,裸露在空气中易被氧化从而造成磁性的损失,因此人们通过适当的物理或化学方法对Fe3O4颗粒表面进行改性,将某些具有特殊结构的物质引入颗粒表面,改变磁性颗粒的表面结构和性能,以实现对某些目标分析物的有效萃取[25]。
在真菌毒素检测中,磁性吸附剂的改性分子有石墨烯、聚多巴胺、碳纳米管以及单克隆抗体等。图2是几种磁性吸附剂的结构示意图。Es'Haghi等[29]利用化学共沉淀法制备了石墨烯-Fe3O4,在Fe3O4合成的同时直接负载在石墨烯表面,并将其作为磁性吸附剂应用于谷物中黄曲霉毒素的萃取。Taherimaslak等[30]通过化学键合法,将二巯基乙酸乙二酯固载于3-甲氧基硅烷基-1-丙硫醇修饰的Fe3O4磁性颗粒表面,制备出磁性吸附剂EGBMAMSPT-Fe3O4,结构如图 2a 所示。Socas-Rodríguez等[31]利用多巴胺在弱碱性溶液中的自聚合能力,将聚多巴胺修饰在Fe3O4表面(如图2b),由于磁性吸附剂表面带有大量的活性基团,使得吸附剂在样品基质中具有更好的生物相容性和分散性;同时,吸附剂带有的氨基和邻苯二酚基团可以与目标分析物间形成氢键,产生π-π相互作用,实现对目标分析物的萃取。Wu等[32]通过一系列化学反应,依次将酰胺基团、γ-Fe2O3磁性纳米颗粒、琥珀酰酐修饰于苯乙烯-丙烯酰胺纳米微球表面,减小苯乙烯-丙烯酰胺纳米微球的空间位阻并使其带有磁性后,再引入OTA适体,制备出OTA适体-γ-Fe2O3磁性吸附剂,其结构如图2c所示。修饰磁性材料的功能物质与方法多种多样,这也为磁性材料及MSPE技术在真菌毒素检测中的应用提供了很大的可能性,具有广阔的研究前景。
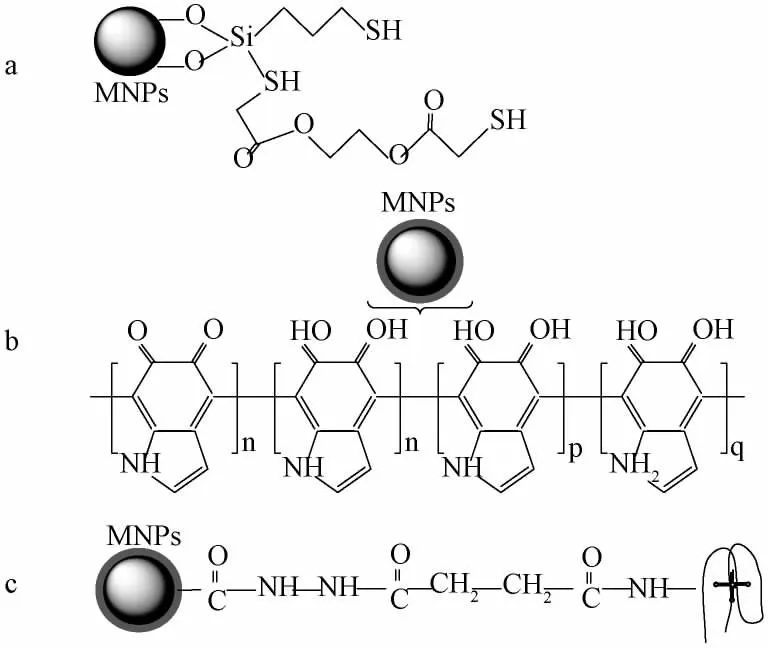
图2 磁性吸附剂结构示意图Fig.2 The structure diagram of representative magnetic adsorbents
2 MSPE技术在真菌毒素检测中的应用
目前,MSPE与不同分离检测技术联用主要可检测的真菌毒素包括黄曲霉毒素、脱氧雪腐镰刀菌烯醇、赭曲霉毒素A、玉米赤霉烯酮、伏马毒素等(表 1)。
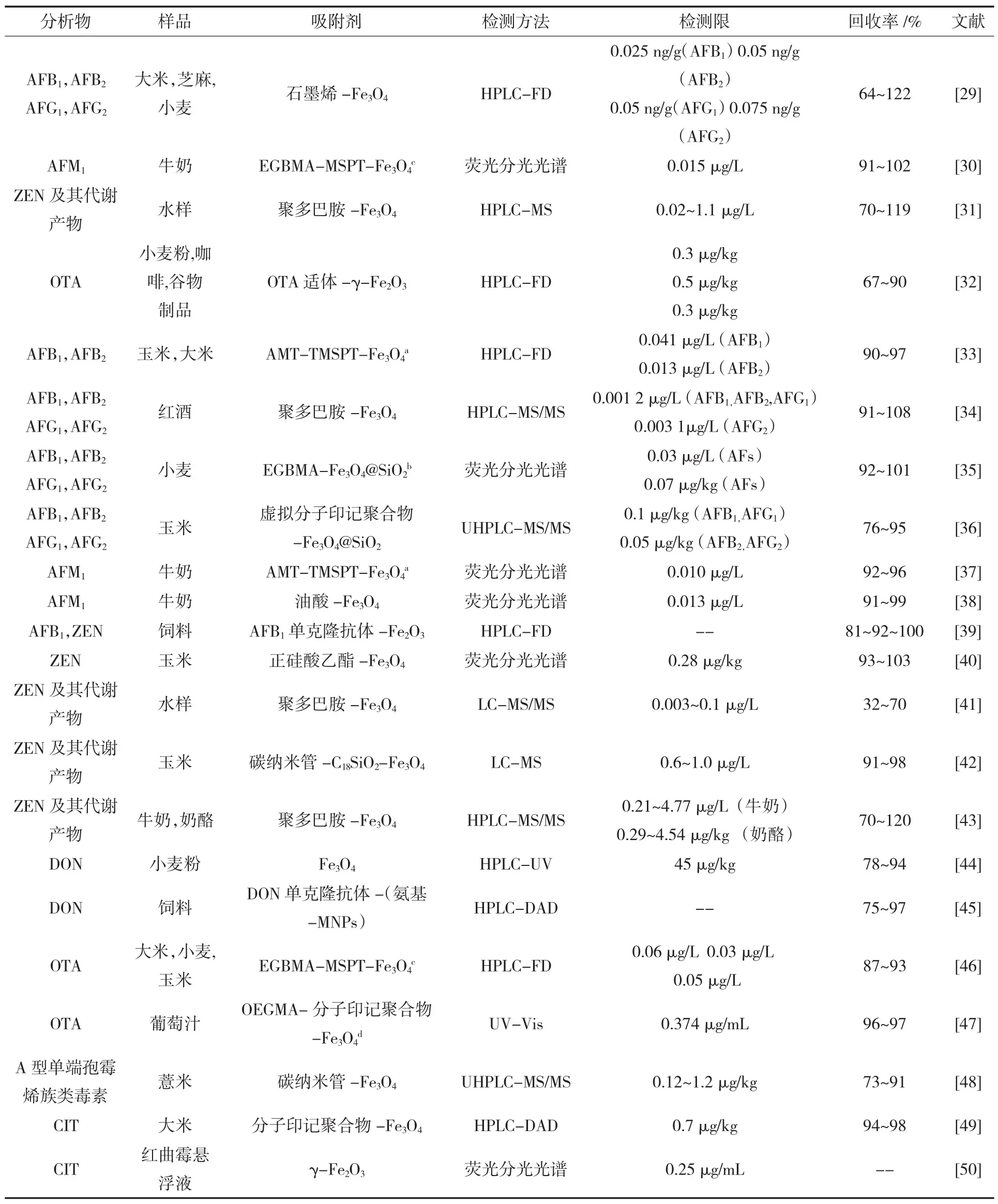
表1 MSPE在真菌毒素检测中的应用Table 1 Application of MSPE for determination of mycotoxin
2.1 黄曲霉毒素(AF)
AF是真菌次级代谢产物的一种,其毒性较强、污染较广泛的是 AFB1、AFB2、AFG1和 AFG2[51-52]。此外,AFM1、AFM2在牛奶中的污染也十分广泛。
Hashemi等[33]将 2-氨基-5-巯基-1,3,4-噻二唑类席夫碱固载在3-(三甲氧基硅烷基)-1-丙硫醇修饰的Fe3O4磁性纳米颗粒表面,将其作为MSPE吸附剂,结合高效液相色谱-荧光检测器(HPLC-FD),研究了该磁性吸附剂对谷物中AFB1和AFB2的萃取性能。该吸附剂具有的氨基和巯基基团可以和AFB1和AFB2内酯环上的羰基基团相互作用,从而达到对AFB1和AFB2的有效萃取。该方法集样品提取、净化和富集过程于一体,分析时间(9 min)比免疫亲和层析净化法(35 min)更短,吸附剂制备简单,稳定性好,可重复利用。Es'Haghi研究小组[29]制备了石墨烯-Fe3O4磁性吸附剂,采用MSPE技术,结合HPLC-FD法研究了该吸附剂对大米、 小麦和芝麻中 AFB1、AFB2、AFG1和 AFG2的萃取性能,该方法克服了免疫亲和层析净化法的诸多缺点,如操作繁琐、耗时耗溶剂、成本高等。Mccullum课题组[34]制备了聚多巴胺-Fe3O4磁性纳米颗粒,采用MSPE技术,结合HPLC-MS/MS研究了该吸附剂对红酒中 AFB1、AFB2、AFG1和 AFG2的萃取性能。Manafi等[35]制备了二巯基乙酸乙二酯修饰的Fe3O4@SiO2磁性纳米颗粒,结合MSPE-荧光光谱法研究了吸附剂对 AFB1、AFB2、AFG1和 AFG2的萃取性能,并应用于小麦样品中AF的分析检测,富集倍数为97倍。Tan等[36]采用MSPE-UHPLCMS/MS研究了虚拟分子标记聚合物修饰的磁性纳米材料对玉米中 AFB1、AFB2、AFG1和 AFG2的萃取性能。
Taherimaslak 等[30]、Hashemi等[37]、Amolidiva 等[38]分别制备了不同种类的磁性吸附剂,采用MSPE-荧光光谱法考察了吸附剂对AFM1的萃取性能,并应用于牛奶中AFM1的检测。Hyunjung等[39]采用MSPE技术研究了修饰有AFB1单克隆抗体的磁性纳米材料对饲料中AFB1的萃取性能。Taherimaslak研究小组[30]制备的吸附剂(EGBMA-MSPT-Fe3O4)具有碳氢链骨架和巯基基团(在萃取体系中以SH2+形式存在),而AFM1由双呋喃环和香豆素组成,具有共轭体系和疏水性质,吸附剂对AFM1的吸附主要取决于其碳氢链骨架和AFM1之间的疏水作用以及吸附剂的巯基基团和AFM1的内酯环之间的范德华力。
磁性吸附剂及MSPE技术在各类样品基质中AF的萃取发挥了极大的作用,简化了繁琐的样品前处理操作,降低了分析成本。但目前研究AF与磁性吸附剂之间作用机理的报道还较少,磁性吸附剂种类也有待进一步深入研究。
2.2 玉米赤霉烯酮(ZEN)
ZEN及其代谢产物玉米赤霉醇(ZAL)和玉米赤霉烯醇(ZOL)是一类具有类雌激素作用的真菌毒素,其危害次于AF,在小麦、玉米、大麦等农作物中广泛存在[53-54]。
Hashemi等[40]制备了正硅酸乙酯修饰的Fe3O4磁性纳米颗粒作为MSPE的吸附剂,并将此样品前处理技术与分散液液微萃取联用,研究了其对玉米中ZEN的萃取性能,联用技术大大提高了富集倍数。该方法对ZEN的检测限为0.28 μg/kg,低于食品安全国家标准中的限量(60 μg/kg),具有回收率高(93.4%~103.1%)、萃取过程有机溶剂用量少、重复性好、方法准确度高等诸多优点。Socas-Rodríguez 课题组[31]和 Capriotti课题组[41]制备 了多孔型聚多巴胺修饰的磁性纳米吸附剂,并采用MSPE-HPLC-MS/MS研究了吸附剂对水样中ZEN及 α-ZAL、β-ZAL、α-ZOL 和 β-ZOL 的萃取性能。结果表明,方法线性良好,检测限低至0.003 μg/L、回收率高。Hyunjung等[39]制备了ZEN单克隆抗体修饰的磁性纳米材料,并将其作为MSPE过程中的吸附剂应用于饲料中ZEN的检测。Moreno研究小组[42]采用MSPE-HPLC-MS,以多层碳纳米管-C18SiO2纳米复合物修饰的磁性纳米材料为吸附剂,研究了其对玉米样品中ZEN及ZEL、ZOL的萃取性能。González-Sálamo 等[43]采用 MSPE 技术将聚多巴胺修饰的多孔型磁性纳米材料应用于牛奶和酸乳中雌激素类真菌毒素 (ZEN、ZEA、α-ZAL、β-ZAL、α-ZOL和 β-ZOL) 的提取富集, 并结合HPLC-MS分析检测。
2.3 脱氧雪腐镰刀菌烯醇(DON)
DON又称呕吐毒素,属于B型单端孢霉烯族毒素,在谷物及其制品和动物饲料中广泛存在[55]。
Karamiosboo等[44]以Fe3O4为磁性吸附剂,采用MSPE技术研究了其对小麦粉中DON的萃取性能。该方法将磁性吸附剂、提取剂和样品混合,使得DON的提取和净化过程在一步操作中完成,磁性吸附剂主要起净化作用,并影响目标分析物的提取效率。与免疫亲和层析净化方法相比,具有操作简单、萃取时间短、有机溶剂用量少、成本低等优点。Lee等[45]将DON单克隆抗体修饰在磁性纳米颗粒上,应用于动物饲料中DON的萃取。该方法整个过程仅需5 min左右,为动物饲料及食品中真菌毒素的检测提供了一种新途径。
2.4 赭曲霉毒素A(OTA)
OTA是赭曲霉毒素中对人类和动植物影响最大、毒性最强,在自然界中分布最广泛的一种,在谷物、咖啡、坚果及其制品、干果、豆类、葡萄及葡萄酒中均有发现[56-57]。
Mashhadizadeh等[46]将二巯基乙酸乙二酯固载于3-甲氧基硅烷基-1-丙硫醇修饰的Fe3O4磁性纳米颗粒表面,并将其作为MSPE技术的磁性吸附剂,与HPLC-FD结合应用于大米、小麦和玉米中OTA的分析,建立了一种新型OTA样品前处理方法,方法检测限为0.03 μg/L,富集倍数为24倍。该研究在考察其他真菌毒素对OTA回收率的影响时发现,磁性吸附剂对AFs、ZEN和DON也有吸附效果,回收率在71%~94%之间。Turan等[47]将OTA的分子印记聚合物修饰于Fe3O4磁性纳米颗粒表面,采用MSPE技术结合紫外-可见分光光度法应用于葡萄汁中OTA的检测,该方法具有选择性好、回收率高、吸附效果好等优点,检测限为0.374 μg/mL,且吸附剂可重复使用12次。Wu课题组[32]将 OTA的适体固载于磁性纳米微球表面,考察了其对OTA的萃取效果,并结合HPLC-FD,用于谷物制品、小麦粉和咖啡中OTA的检测,方法灵敏度高、选择性好。
2.5 其他真菌毒素
MSPE技术在真菌毒素检测中的应用除上述真菌毒素外,其他真菌毒素也有报道,如T-2毒素、HT-2毒素、二乙酰镳草镰刀菌烯醇(diacetoxyscirpenol,DAS)、 新 茄 病 镰 刀 菌 烯 醇(neosolaniol,NEO)、桔霉素(citrinin,CIT)等。T-2 毒素、HT-2毒素、DAS、NEO等属A型单端孢霉烯族毒素,在小麦、玉米、大米等谷物中广泛存在[58]。CIT常与OTA和AFB1在谷物中同时出现[59]。
Dong等[48]将Fe3O4磁性纳米颗粒与多壁碳纳米管结合,制备了磁性多壁碳纳米管材料,并采用MSPE-UHPLC-MS/MS研究了其对T-2毒素、HT-2毒素、DAS和NEO的萃取性能,成功应用于薏米种子中4种A型单端孢霉烯族毒素的检测,定量限为 0.3~1.5 μg/kg,回收率为 73.6%~90.6%,该方法具有检测限低、回收率高、萃取时间短等优点。Urraca等[49]合成了磁性分子印记聚合物,采用MSPEHPLC-UV,将其应用于大米中CIT的检测,检测限为 0.7 μg/kg,回收率为 94%~97%,与 SPE 相比,省去了过滤操作,操作简单、快速高效。Magro等[50]合成了磁赤铁矿纳米颗粒,研究了其对红曲霉悬浮液中CIT的脱除效果,脱除率达70%。
综上所述,MSPE技术在真菌毒素分析检测中的应用体现出诸多优势,使得真菌毒素的样品前处理过程更为简单,萃取时间缩短,萃取效率和回收率提高,且无明显样品基质的杂质干扰,对液态样品基质和固态样品基质均适用,该技术在真菌毒素检测中具有十分广阔的应用前景。
3 展望
本文介绍了MSPE的萃取过程、磁性吸附剂的种类及其制备方法,综述了其在真菌毒素检测分析中的应用。MSPE技术以其快速、简单、绿色及低成本等优点,在真菌毒素检测中得以广泛应用,但目前磁性吸附剂种类较少,应用受到一定的限制,不能达到对多种真菌毒素同时萃取的目的。此外,在MSPE过程中,多种真菌毒素的存在也影响着对其他种类真菌毒素的萃取效率,萃取选择性不高。因此,在保证萃取效率的前提下,研发更多种类的新型磁性吸附剂,实现对样品中多种真菌毒素的萃取分离,又能针对某一种类真菌毒素进行选择性萃取,以及简化磁性吸附剂制备过程并对磁性吸附剂与真菌毒素间的作用机理进行深入研究是MSPE技术应用于真菌毒素检测中需要关注的问题。
[1]GEARY P A,CHEN G,KIMANYA M E,et al.Determination of multi-mycotoxin occurrence in maize based porridges from selected regions of Tanzania by liquid chromatography tandem mass spectrometry (LC-MS/MS),a longitudinal study[J].Food Control,2016,68:337-343.
[2]AIKO V,MEHTA A.Occurrence,detection and detoxification of mycotoxins [J].Journal of Biosciences,2015,40(5):1-12.
[3]FERE F S.Worldwide occurrence of mycotoxins in rice[J].Food Control,2016,62:291-298.
[4]BAKIRDERE S,YAROGLU T,TIRIK N,et al.Determination of trace aflatoxin levels in agricultural products and foodstuffs using ELISA system after immunoaffinity clean-up procedure[J].Minerva Biotecnologica,2014,26(4):235-240.
[5]GHALI R,BELOIAER I,HDIRI S,et al.Simultaneous HPLC determination of aflatoxins B1,B2,G1and G2in Tunisian sorghum and pistachios[J].Journal of Food Composition&Analysis,2009,22(7):751-755.
[6]FAN C,CAO X,LIU M,et al.Determination of Alternaria mycotoxins in wine and juice using ionic liquid modified countercurrent chromatography as a pretreatment method followed by high -performance liquid chromatography[J].Journal of Chromatography A,2016,1436(7):133-140.
[7]RODRÍGUEZ-CARRASCO Y,BERRADA H,FONT G,et al.Multi-mycotoxin analysis in wheatsemolina using an acetonitrile-based extraction procedure and gas chromatography-tandem mass spectrometry [J].Journalof Chromatography A,2012,1270(24):28-40.
[8]JUAN C,COVARELLI L,BECCARI G,et al.Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy [J].Food Control,2015,62:322-329.
[9]BACALONI A,CAVALIERE C,CUCCI F,et al.Determination of aflatoxins in hazelnuts by various sample preparation methods and liquid chromatography-tandem mass spectrometry[J].Journal of Chromatography A,2008,1179(2):182-189.
[10] VACLAVIK L,VACLAVIKOVA M,BEGLEY T H,et al.Determination of multiple mycotoxins in dietary supplements containing green coffee bean extracts using ultrahigh-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS)[J].Journalof Agricultural and Food Chemistry,2013,61(20):4822-4830.
[11]SARTORI A V,MATTOS J S D,MORAES M H P D,et al.Determination of aflatoxins M1,M2,B1,B2,G1,and G2and ochratoxin A in UHT and powdered milk by modified QuEChERS method and ultra high -performance liquid chromatography tandem mass spectrometry [J]. Food Analytical Methods,2015,8(9):2321-2330.
[12] HASHEMIJ,KRAM G A,ALIZADEH N.Enhanced spectrofluorimetric determination of aflatoxin B1in wheat by second-order standard addition method [J].Talanta,2008,75(4):1075-1081.
[13]NASR M R J,ABEDINZADEGAN M.Enhanced synchronous spectrofluorimetric determination of aflatoxin B1in pistachio samples using multivariate analysis [J].Analytica Chimica Acta,2007,582(2):288.
[14]李军,于一茫,田苗,等.免疫亲和柱净化-柱后光化学衍生-高效液相色谱法同时检测粮谷中的黄曲霉毒素、玉米赤霉烯酮和赭曲霉毒素 A[J].色谱,2006,24(6):581-584.
[15] AFZALI D,GHANBARIAN M,MOSTAFAVI A,et al. A novel method for high preconcentration of ultra trace amounts of B1,B2,G1and G2aflatoxins in edible oils by dispersive liquid-liquid microextraction after immunoaffinity column clean-up [J].Journal of Chromatography A,2012,1247(1247):35-41.
[16]RUAN C,DIAO X,LI N,et al.Determination of ochratoxin A and citrinin in fruits using ultrasound-assisted solvent extraction followed by dispersive liquid-liquid microextraction with HPLC with fluorescence detection[J].Analytical Methods,2016,8(7):1586-1594.
[17]ZHU W,REN C,NIE Y,et al.Quantification of ochratoxin A in Chinese liquors by a new solid-phase extraction clean-up combined with HPLC-FLD method [J].Food Control,2015,64:37-44.
[18]WANG M, JIANG N, XIAN H, et al.A single-step solid phase extraction forthe simultaneous determination of 8 mycotoxins in fruits by ultra-high performance liquid chromatography tandem massspectrometry[J].Journal of Chromatography A,2016,1429:22-29.
[19]KHAYOON W S,SAAD B,SALLEH B,et al.Micro-solid phase extraction with liquid chromatography-tandem mass spectrometry for the determination of aflatoxins in coffee and malt beverage[J].Food Chemistry,2014,147:287-294.
[20]ES'HAGHI Z,SORAYAEI H,SAMADI F,et al.Fabrication of a novel nanocomposite based on sol-gel process forhollow fiber-solid phase microextraction of aflatoxins:B1and B2,in cerealscombined with high performane liquid chromatography-diode array detection[J].Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences,2011,879(28):3034-3040.
[21]RUBERT J,SOLER C,MAES J.Opti mization of matrix solid-phase dispersion method for simultaneous extraction of aflatoxins and OTA in cereals and its application to commercial samples[J].Talanta,2010,82(2):567-574.
[22]ZHOU Q,LI F,CHEN L,et al.Quantitative analysis of 10 mycotoxins in wheat flour by ultrahigh performance liquid chromatography-tandem massspectrometrywith amodified QuEChERS strategy [J].JournalofFood Science,2016,81(11):T2886-T2890.
[23] KOESUKWIWATU, SANGUANKAEW K,LEEPIPATPIBOON N. Evaluation of a modified QuEChERS method for analysis of mycotoxins in rice [J].Food Chemistry,2014,153(9):44-51.
[24]JIANG Q,LIU Q,CHEN Q,et al.Dicationic polymeric ionic-liquid-based magnetic material as an adsorbent for the magnetic solid-phase extraction of organophosphate pesticides and polycyclic aromatic hydrocarbons[J].Journal of Separation Science,2016,39 (16):3221-3229.
[25]ZHENG X,HE L,DUAN Y,et al.Poly(ionic liquid)immobilized magnetic nanoparticles as new adsorbent for extraction and enrichment of organophosphorus pesticides from tea drinks[J]. Journal of Chromatography A,2014,1358:39-45.
[26] XIANG G,LIL,JIANG X,etal.Thiolmodified magnetic silica sorbent for the determination of trace mercury in environmental water samples coupled with cold vapor atomic absorption spectrometry [J].Analytical Letters,2013,46(4):706-716.
[27]XU K,WANG Y,LI Y,et al.A novel poly(deep eutectic solvent)-based magnetic silica composite for solid-phase extraction of trypsin[J].Analytica Chimica Acta,2016,946:64-72.
[28]刘勤,何丽君,杨君,等.离子液体基磁性固相萃取技术的研究进展[J].分析测试学报,2015,34(7):860-866.
[29]ES'HAGHIZ,BEHESHTIH R,FEIZY J.Extraction ofaflatoxinsfrom food samples using graphene-based magnetic nanosorbents followed by high-performance liquid chromatography:a simple solution to overcome the problems of immunoaffinity columns [J].Journal of Separation Science,2014,37(18):2566-2573.
[30]TAHERIMASLAK Z,AMOLI-DIVA M,ALLAHYARY M,et al.Magnetically assisted solid phase extraction using Fe3O4nanoparticles combined with enhanced spectrofluorimetric detection foraflatoxin M1determination in milk samples [J].Analytica Chimica Acta,2014,842:63-69.
[31]SOCAS-RODRÍGUEZ B,HERNÁNDEZBORGES J,SALAZAR P,et al.Core-shell polydopamine magnetic nanoparticles as sorbent in micro-dispersive solid phase extraction for the determination ofestrogenic compounds in water samples prior to highperformance liquid chromatography-mass spectrometry analysis [J].Journal of Chromatography A,2015,1397:1-10.
[32]WU X,HU J,ZHU B,et al.Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of ochratoxin A in food samples [J]. Journal of ChromatographyA,2011,1218 (41):7341-7346.
[33] HASHEMIM,TAHERIMASLAKZ,RASHIDI S. Application of magnetic solid phase extraction for separation and determination of aflatoxins B1and B2in cereal products by high performance liquid chromatographyfluorescence detection [J]. Journal of Chromatography B,2014,960:200-208.
[34] MCCULLUM C,TCHOUNWOU P,DING L S,etal.Extraction ofaflatoxinsfrom liquid foodstuff samples with polydopamine-coated superparamagneticnanoparticlesforHPLCMS/MS analysis[J].Journal of Agricultural&Food Chemistry,2014,62(19):4261-4267.
[35] MANAFI M H,ALLAHYARI M,POURGHAZI K,et al.Surfactant-enhanced spectrofluorimetric determination of total aflatoxins from wheat samples after magnetic solid-phase extraction using modified Fe3O4,nanoparticles[J].Spectrochimica Acta PartA Molecular&Biomolecular Spectroscopy,2015,146:43-49.
[36] TAN L,HE R,CHEN K,et al.Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic nanoparticles [J]. Microchimica Acta,2016,183(4):1469-1477.
[37] HASHEMIM,TAHERIMASLAKZ,RASHIDI S.Enhanced spectrofluorimetric determination of aflatoxin M1in liquid milk after magnetic solid phase extraction[J].Spectrochimica Acta Part A Molecular& Biomolecular Spectroscopy,2014,128C(14):583-590.
[38]AMOLIDIVA M,TAHERIMASLAK Z,ALLAHYARIM,etal.Application ofdispersive liquid-liquid microextraction coupled with vortex-assisted hydrophobic magnetic nanoparticles based solid-phase extraction for determination of aflatoxin M1in milk samples by sensitive micelle enhanced spectrofluori-metry[J].Talanta,2015,134(5):98-104.
[39]HYUNJUNG K,SUNGHEE K,JINKYU L,et al.A novelmycotoxin purification system using magnetic nanoparticles for the recovery of aflatoxin B1and zearalenone from feed[J].Journal of Veterinary Science,2012,13(4):363-9.
[40] HASHEMIM,TAHERIMASLAKZ,PARVIZI S,et al.Spectrofluorimetric determination of zearalenone using dispersive liquid-liquid microextraction coupled to micro-solid phase extraction onto magnetic nanoparticles[J].Rsc Advances,2014,4(85):45065-45073.
[41]CAPRIOTTI A L,CAVALIERE C,BARBERA G L,etal.Polydopamine-coated magnetic nanoparticles for isolation and enrichment of estrogenic compounds from surface water samples followed by liquid chromatographytandem mass spectrometry determination[J].Analytical and Bioanalytical Chemistry,2016,408(15):1-10.
[42] MORENOV,ZOUGAGH M,ÁNGELRÍOS.Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nanoC18SiO2,composites for solid phase extraction of mycotoxins prior to their determination by LCMS [J].Microchimica Acta,2016,183(2):871-880.
[43]GONZÁLEZ-SÁLAMO J,SOCAS-RODRÍGUEZB,HERNÁNDEZ-BORGESJ,etal.Core -shell poly (dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LCMS analysis[J].Food Chemistry,2017,215:362-368.
[44] KARAMIOSBOOR,MAHAMM,MIRABOLFATHY M. Magnetic nanoparticle solid phase extraction-HPLC-UV for determination of deoxynivalenol in wheat flour[J].Analytical Methods,2015,7(24):10266-10271.
[45]LEE H M,SONG SO,CHA S H,et al.Development of a monoclonal antibody against deoxynivalenol for magnetic nanoparticlebased extraction and an enzyme-linked immunosorbent assay[J].Journal of Veterinary Science,2013,14(2):143-150.
[46]MASHHADIZADEH M H,AMOLI-DIVA M,POURGHAZI K.Magnetic nanoparticles solid phase extraction for determination of ochratoxin A in cereals using high -performance liquid chromatography with fluorescence detection [J]. Journal of Chromatography A,2013,1320(20):17-26.
[47]TURAN E,SAHIN F.Molecularly imprinted biocompatible magnetic nanoparticles for specific recognition of ochratoxin A [J].Sensors&Actuators B Chemical,2016,227:668-676.
[48] DONG M,SIW,WANG W,etal.Determination of type A trichothecenes in coix seed by magnetic solid-phase extraction based on magnetic multi-walled carbon nanotubes coupled with ultra-high performance liquid chromatography-tandem mass spectrometry[J].Analytical & Bioanalytical Chemistry,2016,408(24):1-9.
[49]URRACA JL,HUERTAS-PÉREZ JF,CAZORLA G A,et al.Development of magnetic molecularly imprinted polymers for selective extraction:determination of citrinin in rice samples by liquid chromatography with UV diode array detection [J].Analytical and Bioanalytical Chemistry,2016,408(11):3033-3042.
[50]MAGRO M,MORITZ D E,BONAIUTO E,et al.Citrinin mycotoxin recognition and removal by naked magnetic nanoparticles [J].Food Chemistry,2016,203:505-512.
[51]LEONTOPOULOS D,SIAFAKA A,MARKAKI P.Black olives as substrate for Aspergillus parasiticus,growth and aflatoxin B1,production[J].Food Microbiology,2003,20 (1):119-126.
[52]ZUCKERMAN A J.IARC monographs on the evaluation of carcinogenic risks to humans[J].JournalofClinicalPathology,1995,48(7):161-179.
[53] 章英.建立IAC-HPLC检测谷物中的玉米赤霉烯酮和赭曲霉毒素A[D].南昌:南昌大学,2007.
[54]ZINEDINE A,SORIANO J J,MANES J.Review on the toxicity,occurrence,metabolism,detoxification,regulations and intake of zearalenone: an oestrogenic mycotoxin[J].Food and Chemical Toxicology,2007,45(1):1-18.
[55]杨俊.脱氧雪腐镰刀菌烯醇降解菌的筛选、降解酶的纯化及其降解效果的初步研究[D].合肥:合肥工业大学,2014.
[56]SAITO K,IKEUCHI R,KATAOKA H.Determination ofochratoxinsin nutsand grain samples by in-tube solid-phase microextraction coupled with liquid chro-matographymass spectrometry [J]. Journal of Chromatography A,2011,1220(1):1-6.
[57]ZUCKERMAN J A.IARC monographs on the evaluation of carcinogenic risks to humans[J].JournalofClinicalPathology,1995,48(1):229-230.
[58]MAOFENG D,WENSHUAI S,KEQIU J,et al.Multi-walled carbon nanotubes as solid-phase extraction sorbents for simultaneous determination oftype A trichothecenes in maize,w heat and rice by ultra-high performance liquid chromatography-tandem mass spectrometry [J].Journal of Chromatography A,2015,1423:177-182.
[59]FLAJS D,PERAICA M.Toxicological properties of citrinin [J].Archives of Industrial Hygiene&Toxicology,2009,60(4):457-464.
ADVANCES IN APPLICATION OF MAGNETIC SOLID-PHASE EXTRACTION FOR MYCOTOXIN ANALYSIS
ZHAO Renyong1,AN Juan1,CUI Wenhang2,HE Lijun2
(1.School of Food Science and Technology;2.School of Chemistry Chemical and Environmental Engineering,Henan University of Technology,Zhengzhou450001,China)
Mycotoxins are of security risks to human and animals because of their strong toxicity and widespread contamination in food and crops,therefore the rapid and effective analysis of mycotoxins are required. Sample pretreatment plays a key role in the whole analysis process. Magnetic solid-phase extraction (MSPE) is a novel sample pretreatment technique,which uses magnetic materials as adsorbents. Due to its advantages including simple operation,fast extraction,high extraction efficiency,as well as easy separation of adsorbent from sample solution,MSPE has attracted much attention in sample pretreatment of mycotoxins. In this paper,the extraction procedure of MSPE,the type and preparation of magnetic adsorbents and the applications of MSPE in mycotoxin analysis were reviewed,and the future trends of MSPE technique for mycotoxin analysis were also discussed.
mycotoxins;magnetic solid-phase extraction;magnetic adsorbent;sample pretreatment
TS 201.2 文献标志码:A
1673-2383(2017)05-0118-09
http://kns.cnki.net/kcms/detail/41.1378.N.20171030.0936.044.html
网络出版时间:2017-10-30 9:36:44
2017-03-15
国家自然科学基金河南省联合基金重点项目(U1604234);国家自然科学基金项目(21577031);河南省现代农业玉米产业技术体系资助项目(S2010-02-G06)
赵仁勇(1969—),男,湖南澧县人,教授,研究方向为粮食资源转化与利用。
*通信作者

