直肠癌弥散峰度成像与D2-40、CD31、S-100及肿瘤细胞增殖指数的相关性研究
王莉莉,和健伟,黄刚,杜斌斌,赵雪梅,张文文,周星,马娅琼
直肠癌弥散峰度成像与D2-40、CD31、S-100及肿瘤细胞增殖指数的相关性研究
王莉莉,和健伟,黄刚*,杜斌斌,赵雪梅,张文文,周星,马娅琼
作者单位:甘肃省人民医院放射科,兰州 730000
目的 探讨弥散峰度DKI参数(diffusion-kurtosis image,DKI)参数与直肠癌免疫组化D2-40、CD31及Ki-67之间的关系。能够在术前评估其表达程度,从而能够间接反映肿瘤细胞的增殖指数等,为临床提供直肠癌术前恶性程度评估的依据。材料与方法 搜集2016年1月至9月经病理证实的直肠癌患者69例,所有病例经常规扫描加DKI,得到DKI与扩散张量成像(diffusion tensor imaging,DTI)参数:各向异性指数(fractional anisotropy,FA),垂直扩散张量(radial diffusivity,D⊥)、平均扩散系数(mean diffusivity,MD)、轴向扩散张量(axial diffusivity,D//),平均峰度(mean kurtosis,MK)、径向峰度(radial kurtosis,K⊥)、轴向峰度(axial kurtosis,K//)。免疫组化D2-40、CD31、S-100及Ki-67由UltraView-DAB染色进行处理得到。D2-40、CD31根据表达分为阴性与阳性组,采用独立样本t检验进行各参数间的对比。用ROC曲线评判各参数对两组的诊断效度,分析各MK等值与Ki-67的关系采用Pearson相关分析法。结果 MK、K⊥在D2-40、CD31阳性组参数值高于阴性对照组,MD在阳性参数组低于阴性参数组,差异有统计学意义(P<0.05)。Pearson相关分析法得出Ki-67与D⊥、D//、MD之间呈负相关,而与MK、K⊥、K//之间呈正相关性。应用ROC曲线分析各参数对直肠癌D2-40、CD31表达阴性与阳性的诊断鉴别能力。结论 DKI成像可反映直肠癌组织的复杂程度,可在术前无创地评估免疫组化D2-40、CD31、S-100及Ki-67的表达水平,从而可间接反映肿瘤细胞增殖程度。
弥散峰度成像;平均峰度;免疫组织化学;Ki-67抗原
近年来,随着生活水平的提高,直肠癌已是严重威胁人类健康的消化系统恶性肿瘤之一。磁共振成像作为评价直肠癌常用的影像方法,其功能成像的临床应用亦非常广泛,DKI是以非高斯模型为基础的一种新功能成像技术,较传统DTI更加敏感反映组织微结构的复杂程度[1]。直肠癌的肿瘤增殖指数、CD31、D2-40及S-100与预后密切相关,但最后确诊需要术后病理免疫组化,是有创的检查。本研究是研究DKI参数值与CD31、D2-40及S-100的关系,能够在术前无创地评估其表达程度,从而间接反映脉管侵犯及肿瘤增殖指数、为术前恶性程度的评估提供依据。
1 材料与方法
1.1 一般资料
搜集甘肃省人民医院直肠癌患者69例,所有患者已签署知情同意书,共纳入69例,男41例,女28例,年龄35~70岁,平均(50.1±16.2)岁。 纳入标准:(1)术前1周进行磁共振DKI检查;(2)术前未经放疗或化疗;(3)术后经病理证实是直肠癌;(4)术后免疫组化CD31、D2-40及S-100资料完整。排除标准:(1)图像伪影大;(2)后处理软件图像上无明显肿瘤信号;(3)术后病理证实间质瘤以及炎症的患者;(4)乙状结肠与直肠交界区癌。
1.2 DKI扫描
选用Siemens Skyra 3.0 T超导磁共振成像设备,表面线圈为18通道相控矩阵。所有患者在增强之前,先进行DKI扫描,采用自旋回波-平面回波(SE-EPI) 序列,30个扩散敏感梯度场,b值分别为0、1000 s/mm2、2000 s/mm2。
1.3 DKI分析
所需MR图像以DICOM格式存储,后处理过程是基于扩散峰度成像模型,通过DKI软件进行处理得到D//、D⊥、MD、K//、K⊥、MK及FA图;ROI实质区选择强化最明显区域,为减少误差,所有ROI选取由同一医师操作,每例各放置三次不同位置ROI,同一患者ROI范围选取尽量保持一致。
1.4 免疫组化染色
D2-40、CD31及Ki-67检测均严格按照试剂说明书进行操作。机器采用BenCHMARK-XT machine and Mul-timer系统,Ultra View-DAB染色。
1.5 统计分析
2 结果
2.1 DKI图像
以伪彩图的形式显示直肠癌组织的部位、范围、复杂程度等情况。
2.2 统计结果
MK、K⊥在D2-40,CD31阳性组参数值高于阴性对照组,差异有统计学意义(P<0.05),以MK在两组间的差异更显著(P<0.05),MD在D2-40,CD31表达阳性组参数值低于阴性对照组,差异有统计学意义(P<0.05);而FA、K//、D⊥在两组间无统计学意义;各参数在S-100表达阴性与阳性两组间的差异无统计学意义(表1)。
2.3 采 用ROC曲线分析法
各参数对直肠癌CD31、D2-40表达阴性与阳性的诊断鉴别能力,用曲线下面积显示。
2.4 Pearson相关分析法
统计所有病例的免疫组化Ki-67值为71.86±8.31,相对应的DKI参数值分别为:MK,(0.75±0.13) mm2/s;K⊥,(0.77±0.14) mm2/s;K//,(0.75±0.13) mm2/s;MD,(1.43±0.44) mm2/s;D//,(1.62±0.42) mm2/s;D⊥,(1.91±0.37) mm2/s。Ki-67值分别与各DKI参数值进行相关性分析,与MK、K⊥及K//有明显正相关性,r值分别为0.66、0.58、0.60,P值分别为0.00、0.00、0.06;与MD、D⊥及D//之间呈负相关性,r值分别为-0.21、-0.22、-0.52, P值分别为0.06、0.00、0.02 (图1~3)。
3 讨论
纽约大学Jenson等[2]于2005年提出KI技术,香港大学的学奎团队进一步对参数进行完善[3]。已知,在b值小于1000 s/mm2时,水分子扩散信号分布呈正态分布;当b值大于1000 s/mm2时,在活体组织中,水分子的扩散由于受到生物组织的细胞膜、细胞器、细胞间隙等限制,更加符合高b值下组织内水分子实际扩散分布,即非正态分布情况[4]。DKI作为探测非高斯分布的水分子扩散特征及运用四阶三维模式描述水分子的扩散方式,较传统的DTI更敏感把握组织微观结构的改变,因此能更加真实地反映人体内水分子扩散。DKI在帕金森病、脑肿瘤及胆管癌分级的研究中已取得成效[5-7],有学者对DKI成像对直肠癌分化程度的应用已有研究[8],而在直肠癌与免疫组化的相关研究中未见报道。

表1 对照各DKI参数在D2-40、CD31、S-100表达阴性与阳性组的差异Tab. 1 Contrast the difference of DKI parameters in D2-40,CD31 and S-100 express in the negative and positive group

图1 患者,男,65岁,A:直肠腺癌MK功能图,Ki-67表达65%的DKI图像,红染区为直肠癌组织区;B:Ki-67表达85%的DKI图像,红染区更为明显;C:直肠癌蓝色伪彩色为MD 图2 A:各参数对诊断CD31表达的曲线下面积;B:各参数对诊断D2-40表达的曲线下面积;C:各参数对诊断S-100表达的曲线下面积Fig. 1 Patient, male, 65 years old. A: MK of recta adenocarcinoma,Ki-67 express 65% of DKI image, red dye area on behalf of the rectal cancer tissue. B: Ki-67 express 85% of DKI image, red dye area is more apparent. C: A blue color represents the MD of rectal cancer. Fig. 2 A:Receiver operating characteristic curve for parameters on the diagnosis of CD31 expression. B: Receiver operating characteristic curve for parameters on the diagnosis of D2-40 expression. C: Receiver operating characteristic curve for parameters on the diagnosis of S-100 expression.
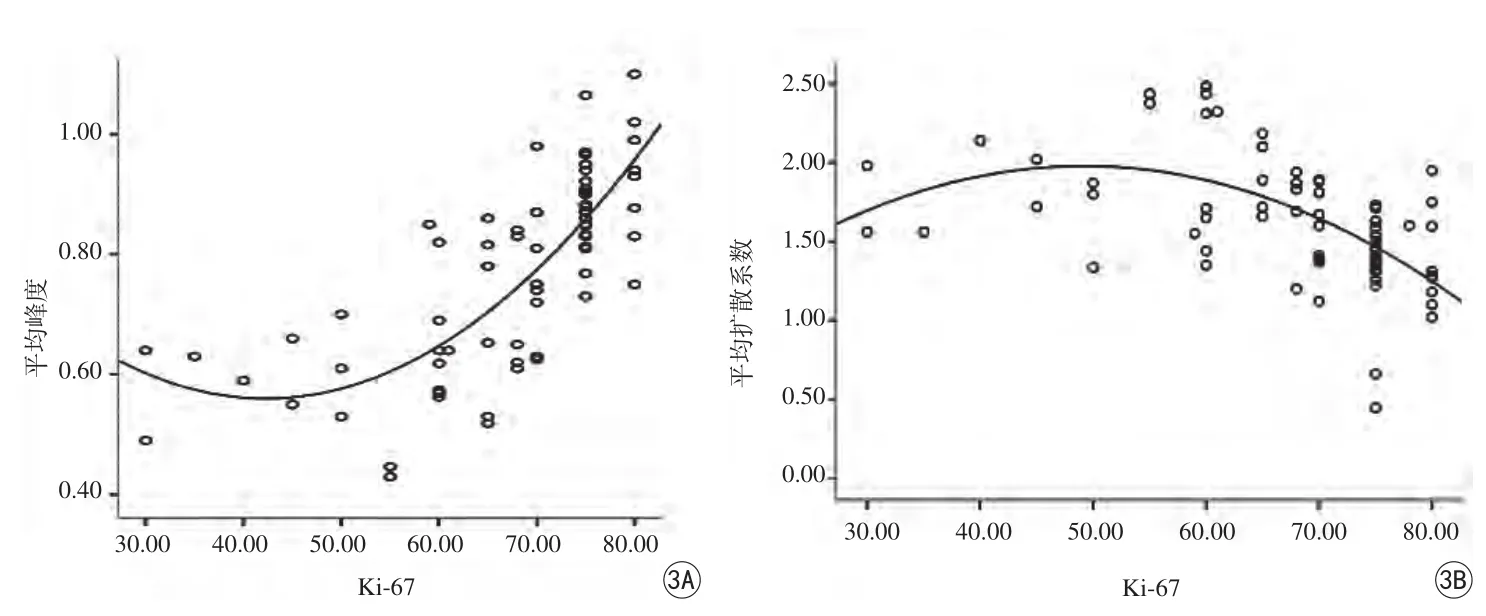
图3 A:MK与Ki-67的相关性;B:MD与Ki-67的相关性Fig. 3 A: The correlation between MK and Ki-67. B: The correlation between MD and Ki-67.
本研究所得到的参数MK值,代表空间各方向扩散峰度值的平均值,是一个反映扩散受限程度的无量纲参数,感兴趣区数值的大小取决于组织内结构的复杂程度。肿瘤细胞的增殖、分化,细胞的坏死和新生血管的生成等病理改变,正反映了肿瘤微观结构的变化。Raab等[9]对胶质瘤的研究认为低级别胶质瘤细胞结构较简单,细胞体排列相对疏松,渗透性较好,受限程度较小,而高级胶质瘤具有多形性及不典型性细胞核,含有更多新生血管及坏死组织,故结构越复杂。若结构复杂程度越高,水分子扩散受限越明显,MK值则越大,反之,则表明扩散受限越弱,MK值便越小。当肿瘤组织内细胞异型性越显著时,增生的血管越丰富,MK值就越大[10]。
CD31又称为血小板-内皮细胞粘附分子,可能参与血管生成、白细胞的迁移等。可用于证明内皮细胞组织的存在和评估肿瘤血管生成,可代表快速增长肿瘤的增殖程度。D2-40为淋巴管内皮标记物,D2-40阳性的脉管内有癌巢,提示淋巴管内有侵犯,但不能提示脉管内侵犯。CD31阳性的脉管内有癌巢,提示脉管侵犯。MK、K⊥在D2-40、CD31阳性组参数值高于阴性对照组,差异有明显统计学意义,以MK对CD31表达阴阳性的曲线下面积最大,分析认为CD31参与肿瘤血管的生成,而肿瘤的生长离不开血管生成,故随着肿瘤的生长,内部结构的复杂程度越高,血管成分即越丰富,CD31的表达也就随之增高。故CD31表达阳性组的MK值明显大于阴性组,且有统计学差异。在本次研究中,各灌注参数如S-100表达阴性与阳性之间的差异无统计学意义。两组间无明显统计学差异,可能与样本量较少有关系,将进一步研究。
Ki-67蛋白代表细胞的增殖状态,是测定细胞增殖活性的一种准确可靠的指标,也是评估肿瘤侵袭和生长的指标,在鉴别肿瘤的良恶性、确定恶性程度、评估预后和指导肿瘤术后化疗等方面有重要的意义[10-11]。笔者认为MK值与Ki-67呈正相关性,即Ki-67水平越高,增殖活跃的肿瘤细胞数增多,密度随之亦增大;相应增殖旺盛的肿瘤细胞合成的大分子蛋白增多,细胞的密度增大,从而影响扩散运动,使其受限[12]。推测原因可能是较高增生性肿瘤细胞的增加和肿瘤血管增生、坏死,因此高度增殖的肿瘤结构复杂性较低增生性肿瘤高。高Ki-67表达反映肿瘤的高增殖活性,MK值相应增加,也可预示高风险复发率和低生存率[13-15]。因此,DKI模型还可以用于未来评价新辅助化疗的效果[16]。
综上所述,DKI成像能反映组织微观结构的变化,可以反映直肠癌组织的增殖活性和复杂程度,可在术前无创评估D2-40、CD31 及Ki-67表达水平,能够较准确地反映肿瘤细胞的增殖指数、淋巴及血管等浸润,能够为临床提供直肠癌术前恶性程度评估的依据。
[References]
[1] Dai YF, Lu J, Li KC. Advances in diffusion kurtosis imaging, J Med Imaging, 2015, 5(25): 913-915.戴艳芳, 卢洁, 李坤成. 磁共振扩散峰度成像技术研究进展, 医学影像学杂志, 2015, 5(25): 913-915.
[2] Jensen JH, Helpern JA, Ramani A, et al. Diffusional kurtosis imaging: the quanti fication of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med, 2005, 53(6):1432-1440.
[3] Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue-characte rization. NMR Biomed, 2010, 23(7): 836-848.
[4] Jensen JH, Helpern JA. MRI quanti ficcation of non-guassian water diffusion by kurtosis analysis. NMR Biomed, 2010, 23(7): 698-710.
[5] Van Cauter S, Veraart J, Sijbers J, et a1. Gliomas: Diffusion kurtosis MR imaging in grading. Radiology, 2012, 263(2): 492-501.
[6] Xu ML, Xin CH, Chen HW. Application value of DKI in grading of extrahepatic cholan-giocarcinoma. Chin J Magn Reson Imaging,2016, 7(1): 34-39.徐蒙莱, 刑春华, 陈宏伟, 等. DKI技术在肝外胆管癌分级中的应用价值, 磁共振成像杂志, 2016, 7(1): 34-39.
[7] Kamagata K, Tomiyama H, Hatano T, et al. A preliminary diffusional kurtosis imaging study of Parkinson disease: comparison with conventional diffusion tensor imaging. Neuroradiology, 2014, 56(3):251-258.
[8] Raab P, Hattingen E, Franz K, et al. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology,2010, 254(6): 876-881.
[9] Raab P, Hattingen E, Franz K, et al. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology,2010, 254(3): 876-881.
[10] Veraart J, Van Hecke W, Sijbers J. Constrained maximu likelihood estimation of the diffusion kurtosis tensor using Rician noise model.Magn Reson Med, 2011, 66(3): 678-686.
[11] Sun K, Chen XS, Chai WM, et al. Breast cancer: diffusion kurtosis MR imaging. Diagnostic accuracy and correlation with clinical pathologic factors. Radiology, 2015, 277(1): 46-55.
[12] Kobayashi S, Koga F, Kajino K, et al. Apparent diffusion coef ficient value re flects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging, 2014, 39(1): 172-178.
[13] Sueta A, Yamamoto Y, Hayashi M, et al. Clinical significance of pretherapeutic Ki67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer: is it equally useful across tumor subtypes? Surgery, 2014, 155(5): 927-935.
[14] Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer:prognostic and predictive potential. Lancet Oncol, 2010, 11(2):174-183.
[15] Niikura N, Masuda S, Kumaki N, et al.Prognostic significance of the Ki67 scoringcategories in breast cancer subgroups. Clin Breast Cancer, 2014, 14 (5): 323, e3.
[16] Sueta A, Yamamoto Y, Hayashi M, et al. Clinical significance of pretherapeutic Ki-67 as a predictive parameter for response to neoadjuvant chemotherapy in breast cancer: is it equally useful across tumor subtypes? Surgery, 2014, 155(5): 927-935.
Rectal cancer on MRI diffusion-kurtosis imaging and correlation between DKI parameters and D2-40, CD31, S-100 and Ki-67 in rectal tumors
WANG Li-li, HE Jian-wei, HUANG Gang*, DU Bin-bin, ZHAO Xue-mei, ZHANG Wen-wen, ZHOU Xing, MA Ya-qiong
Department of Radiology, Gansu Provincial Hospital, Lanzhou 730000, China
*Huang G, E-mail: keen0999@163.com
Objective: To evaluate the potential association between DKI parameters and D2-40, CD31, S-100 and Ki-67 with immunohistochemical analysis of rectal cancer. Materials and Methods: Data from 69 patients who were confirmed by surgical pathology from January to September in 2016. All cases were performed by regular sequence and DKI and DTI (diffusion tensor imaging) examination. The following parameters were acquired from the entire tumors. Respectively as Dax,Dmean, Drad, Fa, Kax, Kmean, Krad. D2-40, CD31, S-100 and Ki-67 were detected by Ben CHMARK-XT machine and Mul-timer system. D2-40 and CD31 were divided into two groups according to the expression of positive or negative and independentsample t test was used for statistical analysis. Receiver operating characteristic curves and Pearson correlation were used for statistical analysis. Results: The levels of MK, K⊥ value in D2-40, CD31 positive group were signi ficant higher than negative group. The differences were statistically significant (P<0.05). Whereas MD in D2-40, CD31, positive group were signi ficantly lower than negative group, the differences were statistically signi ficant (P<0.05). Pearson correlation analysis showed Ki-67 was signi ficant positive correlation with MK, K⊥, K// parameters, whereas Ki-67 showed negative correlation with D⊥, D//, MD. ROC curve was applied to analysis of DKI parameters in D2-40, CD31 positive group and negative group. Conclusions: MK can reflect the tissue microenvironment including its component organelles, cell membranes, and water compartments, maybe preoperative noninvasive assessment of D2-40, CD31, S-100 and Ki-67 expression level, indirectly re flect the degree of tumor cell proliferation, provide a reference for colorectal malignant degree and prognosis of preoperative evaluation basis.
Diffusion-kurtosis image; Mean kurtosis; Immunohistochemistry; Ki-67 antigen
Received 6 Nov 2016, Accepted 19 Apr 2017
黄刚,E-mail:keen0999@163.com
2016-11-06
接受日期:2017-04-19
R445.2;R735.37
A
10.12015/issn.1674-8034.2017.05.006
王莉莉, 和健伟, 黄刚, 等. 直肠癌弥散峰度成像与D2-40、CD31、S-100及肿瘤细胞增殖指数的相关性研究. 磁共振成像, 2017, 8(5): 349-353.

