Prepration of Solid Base Catalyst Added with Tung Shell Ash and Its Application in Methyl Esterification of Tung Oil
LIU Wenzhu, TANG Kehua, 2*, LI Shuo
(1.Key Laboratory of Hunan Forest Products and Chemical Industry Engineering,Jishou University,Zhangjiajie 427000, China; 2.Zhangjiajie Tung Fair Technologies Co., ltd.,Zhangjiajie 427000, China)
Prepration of Solid Base Catalyst Added with Tung Shell Ash and Its Application in Methyl Esterification of Tung Oil

LIU Wenzhu
LIU Wenzhu1, TANG Kehua1, 2*, LI Shuo1
(1.Key Laboratory of Hunan Forest Products and Chemical Industry Engineering,Jishou University,Zhangjiajie 427000, China; 2.Zhangjiajie Tung Fair Technologies Co., ltd.,Zhangjiajie 427000, China)
Solid catalyst added with tung shell ash(KOH-TSA-Al2O3) was prepared using Al2O3and KOH as raw materials. The catalyst was used in the process of methyl esterification of tung oil. Its catalysis efficiency was analyzed. Effects of KOH dosage, sintering temperature and tung shell ash dosage on the conversion efficiency of tung oil were studied with the solo conditions experiment and orthogonal design. The final results showed that the efficiency of methyl esterification catalyzed by the optimized solid catalyst was up to 76.34%, under the conditions of 6 g KOH, 4 g Al2O3, 3 g tung shell ash, and 700 ℃ for 10 h. The prepared catalyst was analyzed with alkalinity detected, scanning electron microscope (SEM) and X-ray diffraction (XRD). The results showed that the surface catalyst was like tree branch and new phase of CaO, MgO and SiO2was generated. Compared with 0#diesel standard in China, the biodiesel prepared under the catalyst was showed better quality.
solid catalyst; tung shell ash; methyl esterification; tung oil
Homogeneous catalysis is in general used in the industrial preparation of methyl ester oils. Liquid acid or alkali catalyst used in the process enables the fast speed and high conversion rate to the catalytic reaction, but at the same time, industrial waste water and environmental pollution are unavoidable because of the washing process to clear the impurities in the product. So the post-processing is complex in preparing methyl ester oils with homogeneous catalysis[1-2]. The disadvantages of homogeneous catalysis process can be avoided when using solid base catalyst to prepare methyl ester oils, which is benefit to automation control and large scale continuous in production process. There were plenty of methods to prepare solid base catalyst. Meng, et al[3]studied reaction activity of solid base catalysts KF/CaO prepared under different temperatures in catalyzing methyl esterification of soybean oil. Ebiura, et al[4]studied reaction activity of triglyceride and methyl alcohol catalyzed by the solid K2CO3/Al2O3. The studies[5-6]showed that solid alkali with high specific surface area was generally prepared by compounds including potassium. The control of quantity and the state of potassium in the raw materials were the critical step to get catalyst that had certain specific surface area and pore structure. Up to now, there was no reported research about how to add any rich potassium plant materials into solid base catalyst. The present paper studied the solid catalyst added with tung shell ash, with Al2O3as substrate and KOH as main active component. Tung tree is an important industrial oil plant in China. The mass fraction of α-tung oil acid ester in tung oil, which is extracted from the seed of tung tree, is up to 82.0%[7]. Tung oil has been used for thousand years, but its usage was restricted to antiseptic treatment to wooden ship and wooden house in the old days[8-10]. In modern times, tung oil is used as material in the products as heat-resistant insulating material, high temperature resistant friction material, marine paint and synthetic resin[11-13].These expand the use of tung oil in the field of chemistry, electronic, plastic, rubber and casting. Currently, the research of chemical products about tung oil is focused on tung oil biodiesel[8,14], tung oil dimer acid and its ester[11,15], and modified polymer materials with tung oil. In tung oil chemical industry, methyl esterification of tung oil is an important process. The process was optimized and the best prepared catalyst was used in the process of methyl esterification of tung oil to study the catalysis efficiency of the catalyst.
1 Materials and Methods
1.1 Raw materials
Tung oil was purchased from Zhangjiajie Jiajiayao Oil company, with the acid value of 6 mg/g. Tung shell ash was prepared in muffle furnace under 400 ℃ for 4 h. The reagents were all analytically pure, including menthol, butanone, KOH,γ-Al2O3, sodium periodate, ethanediol, ethyl alcohol, NaOH and phenolphthalein.
1.2 Instruments
DF-101S hot type constant temperature heating magnetic stirrer; D7401-ZH motor stirrer; JA2003 and JY5002 electronic scales; SX- 4-10 chamber electric furnace S-3400 N scanning electron microscope, Japan Hitachi company; DMAX-2400 diffractometer, Japan Shimadzu company.
1.3 Preparation of solid base catalyst
The impregnation method was used in this process. Firstly, certain percentage KOH (2-7 g) and Al2O3(8-3 g), and certain amount of tung shell ash (1-9 g) were added into distilled water. After it was placed for several hours, the mixture was calcined under certain temperature (500-900 ℃) for 10 h. The product after being calcined was the solid base catalyst.
1.4 Methyl esterification of tung oil catalyzed by solid base catalyst
1.4.1 Methyl esterification of tung oil process Firstly, dehydrated tung oil was prepared after vacuum dehydrating of tung oil. Then 100 g dehydrated tung oil, 55 g menthol and 20 g butanone were added into a 500 mL flask. When the temperature of the liquid in the flask reached 60 ℃, 2 g solid base catalyst was added into the reaction system and the temperature was kept at 60 ℃. After 2 h, the reaction system was cooled naturally. The solid base catalyst was removed out of the flask when the temperature of liquid fell to 30 ℃. Then the product was removed out of the flask and washed with distilled water, and the liquid was standed and layered after requirements were met. The material in oil layer was collected as sample for measurement.
1.4.2 Catalytic efficiency for methyl esterification Glycerol content was determinated according to the titration method in ISO 1066-1975[16-17]. Then the conversion efficiency of tung oil was calculated according to the glycerol content.
1.4.3 Optimization method for methyl esterification With the conversion efficiency of tung oil in methyl esterification process as indicator, the solo effect of KOH dosage(4,5,6 g), sintering temperature(600,700,800℃) and tung shell ash dosage(2,3,4 g) were studied. Then the experiment conditions were optimized with orthogonal design according to the results of single factor experiments.
1.5 Analysis and characterization
1.5.1 Alkalinity detection for the catalyst The alkalinity was titrated by using Hammett indicators[18], which was formulated as 0.1% benzene solution. 50 mg of catalyst was added into 5 mL cyclohexane and shocked for 30 min. And then a few drops of indicator was added into the solution, and the changes of the surface color of the indicator was observed. The Hammett indictors used were shown in Table 1.

Table 1 Hammett indicators
1.5.2 Analysis of SEM for the catalyst The samples were handled with spraying before scanning. The SEM images were filmed on Hitachi S-4800 field emission scanning electron microscope,and its acceleration voltage was 5 000 V.
1.5.3 Analysis of XRD for the catalyst The XRD spectrums were obtained fromRigaku D/MAX-2500PC powder diffractometer. The test conditions were: Tube voltage 60 kV, tube current 300 mA, power 18 kW, scanned area 1-18°, and scanning speed 3(°)/min.
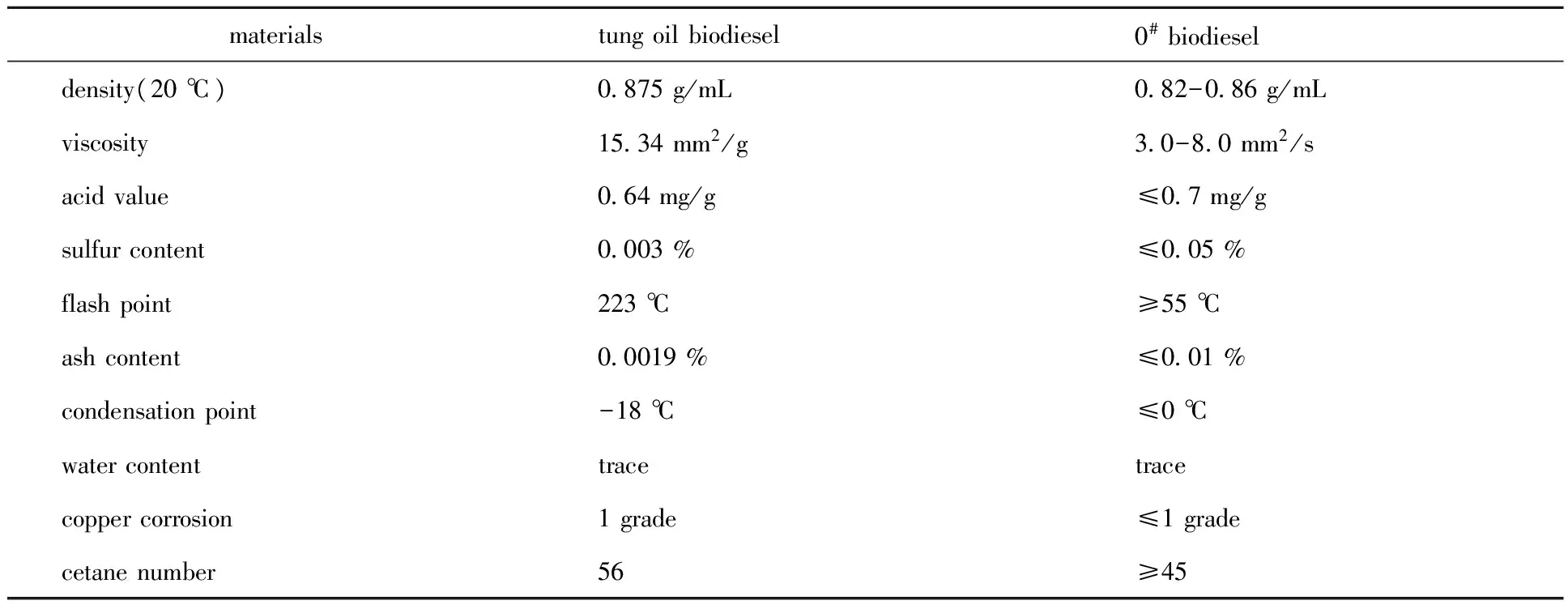
1.5.4 Determination of the quality of tung oil biodiesel In order to evaluate the basic properties of tung oil biodiesel, the density, viscosity, acid value, sulfur content, flash point, ash content, condensation point, water content, copper corrosion and cetane number of tung oil biodiesel prepared under the optimal conditions were measured[19-20]. They were compared with the standards of 0#diesel.
2 Results and Discussion
2.1 Test of mineral elements in tung shell ash
The mineral elements in tung shell ash were analyzed, the results of which were as follows: Fe 0.37%, SiO27.70%, Al2O30.91%, CaO 4.19%, MgO 1.29%, K2O 18.36% and Na2O 0.21%. According to the results, except carbon, main mineral elements in tung shell ash were potassium, calcium and silicon, and the content of potassium reached up to 18.36%. The main elements K, Si and Ca were all general substrate or active component used in the preparation of solid base, so the solid base catalyst added with tung shell ash should have preferable catalytic activity theoretically.
2.2 Effect of conditions to tung oil methyl efficiency
2.2.1 KOH dosage Under 700 ℃, tung shell ash 3 g, total KOH and Al2O310 g, calcined time 10 h, different catalysts with KOH percentage, i.e., 2, 3, 4, 5, 6, and 7 g were prepared to study the effect of KOH percentage to the efficiency of methyl esterification of tung oil. In the preparation, the calcined temperature and time were both invariable. After being calcined, the prepared catalysts were added into the methyl esterification of tung oil reaction system in a certain proportion to tung oil so that their catalytic efficiency was tested. The results were shown in Fig.1(a).
As the data shown in Fig.1(a), the methyl esterification efficiency of tung oil was increased gradually when KOH percentage was lower than 4 g. While the KOH percentage reached up to 6 g, the efficiency was decreased slightly. It may be caused by the non-uniform distribution of potassium oxide which was appeared because of the increased KOH[21].
2.2.2 Calcined temperature In the preparation, tung shell ash 3 g, the KOH dosage 5 g, total KOH and Al2O310 g, calicined time 10 h, catalysts under different calcined temperatures, i.e., 500, 600, 700, 800, and 900 ℃, were prepared. In order to test their catalytic efficiency, the prepared catalysts were added into the methyl esterification of tung oil reaction system in a certain proportion to tung oil. The results were shown in Fig.1(b).
According to Fig.1(b), calcined temperature had some impacts on catalysis efficiency. The methyl esterification efficiency of tung oil reached up to the peak when the temperature was 700 ℃. The results may be caused by the change of crystal structure or the surface alkali of solid base catalyst which was influenced by the calcined temperature.
2.2.3 Tung shell ash dosage In the preparation, the KOH dosage 5 g, total KOH and Al2O310 g, calcined time 10 h, temperature 700℃, catalysts added with different dosages of tung shell ash, i.e., 1, 2, 3, 4, 5, 7, and 9 g, were prepared. After being calcined, the prepared catalysts were added into the methyl esterification of tung oil reaction system in a certain proportion to tung oil. The results were shown in Fig.1(c).

Fig.1 Effect of KOH dosage, calcined temperature and tung shell ash dosage to
According to Fig.1(c), the catalysis efficiency was relatively high when the dosage of tung shell ash was lower than 3 g, but the efficiency was decreased obviously while the added dosage was more than 3 g. The results may be related with the alkali metal and other elements included in tung shell ash.
2.3 Orthogonal experiment
According to the results of single factor experiments above, optimization of solid base catalyst preparation conditions was finished by using orthogonal experiment(L934). The results of orthogonal experiment and the analysis of variance to the experimental were shown in Table 2 and Table 3.
According to the results shown in Table 2 and Table 3, all of the arranged factors had significant influence to the catalysis efficiency. The effect of tung shell ash dosage made the most significant influence to the efficiency, then the KOH dosage, and calcined temperature made the least influence. The optimum conditions for preparation of solid base catalyst were: 6 g KOH, 4 g Al2O3, 3 g tung shell ash, and 700 ℃.
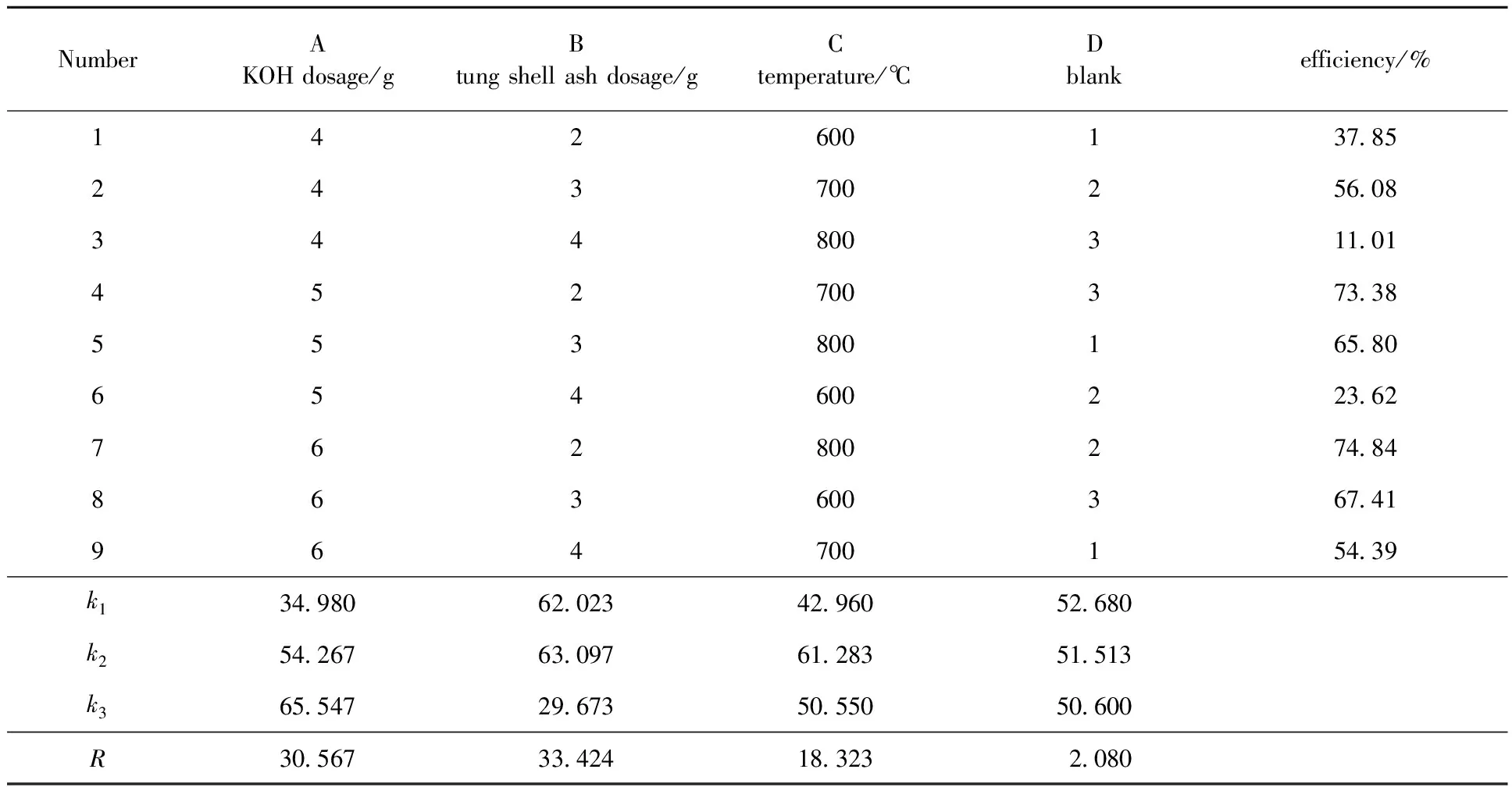
Table 2 Level of factors arrangement and results of orthogonal experiment

Table 3 Analysis of variance table for the experiment results
2.4 Compliance test
The solid base catalyst was prepared according to the optimal process conditions for preparation of solid base catalyst were, 6 g KOH, 4 g Al2O3, 3 g tung shell ash, calcined 10 h under 700 ℃. The prepared solid base catalyst was used to catalyze the reaction of methyl esterification of tung oil. In the reaction, the mass fraction of catalyst used was equal to 2% percentage of the tung oil used in the system. After 5 parallel experiments, the average catalysis efficiency was 76.34%.
2.5 Analysis and characterization
2.5.1 Alkalinity detected results The alkalinity of the solid base catalyst sintered with different KOH amounts was determined. The results were KOH 2,3 and 4 g, alkalinity 15-18.4, KOH 5,6 and 7 g, alkalinity 18.4-26.5. At the same time, the alkalinity of the solid base catalyst prepared under the optimum process was detected.
It could be seen that with increase of the amount of KOH, the alkali strength of the solid base catalyst increased. When the mass of KOH was less than 5 g, the alkali strength was 15 2.5.2 SEM analysis The SEM images of the catalyst added with tung shell ash prepared under optimal process conditions was shown in Fig.2. The surface of the catalyst was irregular, and the diaphaneity was inhomogeneity. Its fine structure was schistose, which was provided with relatively large specific surface area. According to the structure of the catalyst shown in SEM images, raw materials in the reaction system catalyzed by this solid base catalyst were easily contacted with each other on the surface of the catalyst, so the increase of catalysis efficiency was possible. Fig.2 SEM analysis of the solid base catalyst 2.5.3 XRD analysis The catalysts including different contents of KOH and without tung shell ash, which was prepared under 700° for 10 h, was analyzed by XRD. The results of XRD figure was shown in Fig.3. At the same time, the XRD figures for optimized catalysts with and without tung shell ash were compared with each other in Fig.4. As shown in Fig.3, when the KOH content was lower, the diffraction peak of KAlO2was lesser, while the diffraction peak of Al2O3was larger. The strength of diffraction peak of KAlO2was increased with the increasing of KOH content; but while the KOH content was more than 70%, the diffraction peak of KAlO2was not obvious, and replaced by the diffraction peak of K2O. Combining with the results, the efficiency of methyl esterification of tung oil rised first, then reduced later with the increasing of KOH content. KAlO2content included in the solid base catalyst played important role in the reaction of methyl esterification of tung oil. Fig.3 XRD figures for catalysts with different KOH content Fig.4 XRD figures for catalysts with(a) and without(b) tung shell ash As shown in Fig.4, the main diffraction peak in the two catalysts was both KAlO2, but its strength was a little weaker in catalyst without tung shell ash than another one. At the same time, there were diffraction peaks of CaO, MgO and SiO2appeared in the catalyst without tung shell ash. The results showed that, with the exception of KAlO2, some other active ingredients or active site in the catalyst without tung shell ash played certain positive influence to the reaction of methyl esterification of tung oil. 2.5.4 The quality of tung oil biodiesel The indexes of self-madetung oil biodiesel were analyzed. It can be seen from Table 4 that most of the indicators of tung oil biodiesel prepared were better than that of specified in the standard of 0#diesel in China. Table 4 The quality of prepared tung oil biodiesel 3.1 In conclusion, solid base catalyst by adding tung shell ash mixed with Al2O3and KOH was prepared. It was used to methyl esterification of tung oil.The optimal preparation process of catalyst added with tung shell ash were: 6 g KOH, 4 g Al2O3, 3 g tung shell ash, and calcined under 700 ℃ for 10 h. The catalysis efficiency of methyl esterification of tung oil was 76.34% in average when the catalyst prepared under the above conditions was used in the reaction system. 3.2 According to the analysis of the catalyst, the prepared KOH-TSA-Al2O3showed good structures and properties. The alkali strength of the solid base catalyst prepared under the optimum conditions was 18.4 [1]KIM H J,PARK S H,SUH H K,et al.Atomization and evaporation characteristics of biodiesel and dimethyl ether compared to diesel fuel in a high-pressure injection system[J].Energy & Fuels,2009,23(3):1734-1742. [3]孟鑫,辛忠.KF/CaO催化剂催化大豆油酯交换反应制备生物柴油[J].石油化工,2005,34(3):282-285. MENG X,XIN I.Preparation of biodiesel from soybean oil by transesterification on KF/CaO catalyst[J].Petrochemical Technology,2005,34(3):282-285. [4]EBIURA T,ECHIZEN T,ISHIKAWA A,et al.Selective transesterification of triolein with methanol to methyl oleate and glycerol using alumina loaded with alkali metal salt as a solid-base catalyst[J].Applied Catalysis A:General,2005,283(1/2):111-116. [5]TAY T,UCAR S,KARAGOZ S.Preparation and characterization of activated carbon from waste biomass[J].Journal of Hazardous Materials,2009,165(1/2/3):481-485. [6]GUO J,LUA A C.Preparation of activated carbons from oil-palm-stone chars by microwave-induced carbon dioxide activation[J].Carbon,2000,38(14):1985-1993. [7]PFISTER D P,BAKER J R,HENNA P H,et al.Preparation and properties of tung oil-based composites using spent germ as a natural filler[J].Journal of Applied Polymer Science,2008,108(6):3618-3625. [8]PARK J Y,KIM D K,WANG Z M,et al.Production and characterization of biodiesel from tung oil[J].Applied Biochemistry and Biotechnology,2008,148(1/2/3):109-117. [9]商士斌,谢晖,黄焕,等.桐油改性氨基聚酯烘漆的合成研究[J].林产化学与工业,2000,20(4):1-5. SHANG S B,XIE H,HUANG H,et al.Study on amino polyester baking varnish modified by tung oil[J].Chemistry and Industry of Forest Products,2000,20(4):1-5. [10]黄坤,夏建陵.桐油及其衍生物的改性在高分子材料中的应用进展[J].化工进展,2008,27(10):1588-1592. HUANG K,XIA J L.Progress of modification of tung oil and its derivatives in the application of polymer materials[J].Chemical Industry and Engineering Progress,2008,27(10):1588-1592. [11]BIJWE J,MAJUMDAR N,SATAPATHY B K.Influence of modified phenolic resins on the fade and recovery behavior of friction materials[J].Wear,2005,259(7):1068-1078. [12]SHIBATA M,NAKAI K.Preparation and properties of biocomposites composed of bio-based epoxy resin,tannic acid,and microfibrillated cellulose[J].Journal of Polymer Science Part B:Polymer Physics,2010,48(4):425-433. [13]WANG C C,JONES F.Stability and film properties of tung oil modified soybean alkyd emulsion[J].Journal of Applied Polymer Science,2000,78(9):1698-1706. [14]WEN Z A,YU X H,TU S T,et al.Biodiesel production from waste cooking oil catalyzed by TiO2-MgO mixed oxides[J].Bioresource Technology,2010,101(24):9570-9576. [15]GUNER F S,YAGCI Y,ERCIYES A T.Polymers from triglyceride oils[J].Progress in Polymer Science,2006,31(7):633-670. [16]IX-ISO.ISO 1066-1975 Analysis of soaps;Determination of glycerol content;Titrimetric method[S].1975. [17]DORADO M P,BALLESTEROS E,LPEZ F J.Optimization of alkali-catalyzed transesterification ofBrassicacarinataoil for biodiesel production[J].Energy & Fuels,2004,18(1):77-83. [18]郭峰.新型固体碱催化剂的制备及其在生物柴油合成中的应用[D].大连:大连理工大学博士学位论文,2010. GUO F.Preparation of novel solid acid and base catalysts and their application in biodiesel synthesis[D].Dalian: Doctoral Dissertation of Dalian University of Technology,2010. [19]国家质量技术监督局,GB252-2000,轻柴油[S].北京:中国标准出版社,2000. The State Bureau of Quality and Technical supervision. GB252-2000Light diesel fuels[S].Beijing: Standard Press of Chica, 2000. [20]SHANG Q,JIANG W,LU H F,et al.Properties of tung oil biodiesel and its blends with 0#diesel[J].Bioresource Technology,2010,101(2):826-828. [21]连奕新,王会芳,方维平,等.载体焙烧温度对 Co-Mo/MgO-Al2O3变换催化剂性能的影响[J].催化学报,2009,30(6):549-554. LIAN Y X,WANG H F,FANG W P,et al.Effect of calcination temperature of support on the performance of Co-Mo/MgO-Al2O3water-gas shift catalyst[J].Chinese Journal of Catalysis,2009,30(6):549-554. 本刊信息 2016- 12- 22 湖南省科技厅科技计划项目(2012FJ4474) 刘文柱(1987— ), 男, 河北保定人,硕士生,主要从事生物质材料研究工作 刘文柱,唐克华,李硕.桐壳灰固体碱催化剂的制备及其催化桐油甲酯化(英文)[J].林产化学与工业,2017,37(4):73-80. 桐壳灰固体碱催化剂的制备及其催化桐油甲酯化 刘文柱1, 唐克华1, 2, 李 硕1 (1.吉首大学 林产化学加工工程湖南省重点实验室,湖南 张家界 427000;2.张家界桐发科技有限公司,湖南 张家界 427000) 以KOH和Al2O3联合桐壳灰制备了固体碱催化剂(KOH-TSA-Al2O3),并将其用于催化桐油甲酯化。采用单因素及正交试验,以桐油甲酯化过程中桐油的转化率为指标,考察了催化剂KOH用量、烧结温度及桐壳灰添加量对甲酯化的影响, 结果表明:当KOH用量为6 g、Al2O3用量为4 g、桐壳灰分添加量为3 g,烧结温度700 ℃下煅烧10 h制备的固体碱催化剂催化桐油甲酯化效率最高,为76.34%。对得到的固体碱催化剂进行碱量、SEM及XRD分析,结果显示该固体碱催化剂表面为树状多枝结构;加入桐壳灰后,该催化剂产生CaO、MgO和SiO2等新相。与0#柴油相比,该催化剂催化下制备的生物柴油具有更优良的性能。 固体碱;桐壳灰分;甲酯化;桐油 *通讯作者:唐克华(1965— ), 男,硕士生导师, 主要从事天然产物研究与开发, E-mail: tkhthllh@126.com。 10.3969/j.issn.0253-2417.2017.04.011 TQ35 Document code:A Article ID:0253-2417(2017)04-0073-08

3 Conclusions


