Relationship of nocturnal concentrations of melatonin, gamma-aminobutyric acid and total antioxidants in peripheral blood with insomnia after stroke: study protocol for a prospective non-randomized controlled trial
Wei Zhang, Fang Li, Tong Zhang,
1 Capital Medical University School of Rehabilitation Medicine, Beijing, China
2 Neurorehabilitation Center, Beijing Bo’ai Hospital, China Rehabilitation Research Center, Beijing, China
Relationship of nocturnal concentrations of melatonin, gamma-aminobutyric acid and total antioxidants in peripheral blood with insomnia after stroke: study protocol for a prospective non-randomized controlled trial
Wei Zhang1,2, Fang Li1,2, Tong Zhang1,2,*
1 Capital Medical University School of Rehabilitation Medicine, Beijing, China
2 Neurorehabilitation Center, Beijing Bo’ai Hospital, China Rehabilitation Research Center, Beijing, China
How to cite this article:Zhang W, Li F, Zhang T (2017) Relationship of nocturnal concentrations of melatonin, gamma-aminobutyric acid and total antioxidants in peripheral blood with insomnia aer stroke: study protocol for a prospective non-randomized controlled trial. Neural Regen Res 12(8):1299-1307.

Graphical Abstract
Melatonin and gamma-aminobutyric acid (GABA) have been shown to regulate sleep.e nocturnal concentrations of melatonin, GABA and total antioxidants may relate to insomnia in stroke patients. In this prospective single-center non-randomized controlled clinical trial performed in the China Rehabilitation Research Center, we analyzed the relationship of nocturnal concentrations of melatonin, GABA and total antioxidants with insomnia aer stroke. Patients during rehabilitation of stroke were recruited and assigned to the insomnia group or non-insomnia group. Simultaneously, persons without stroke or insomnia served as normal controls. Each group contained 25 cases.e primary outcome was nocturnal concentrations of melatonin, GABA and total antioxidants in peripheral blood.e secondary outcomes were Pittsburgh Sleep Quality Index, Insomnia Severity Index, Epworth Sleepiness Scale, Fatigue Severity Scale, Morningness-Eveningness Questionnaire (Chinese version), and National Institute of Health Stroke Scale.e relationship of nocturnal concentrations of melatonin, GABA and total antioxidants with insomnia aer stroke was analyzed and showed that they were lower in the insomnia group than in the non-insomnia group.e severity of stroke was higher in the insomnia group than in the non-insomnia group. Correlation analysis demonstrated that the nocturnal concentrations of melatonin and GABA were associated with insomnia aer stroke.is trial was registered at ClinicalTrials.gov, identif i er: NCT03202121.
nerve regeneration; stroke; insomnia; melatonin; γ-aminobutyric acid; total antioxidants; sleep-related scales; National Institute of Health Stroke Scale; neural regeneration
Introduction
Sleep disturbance, especially insomnia, is a common complication aer ischemic stroke for patients during the rehabilitation of cerebral infarction (Leppävuori et al., 2002; Suh et al., 2014; Kim et al., 2015). More than half of ischemic stroke patients have insomnia complaints (Leppävuori et al., 2002) and poor quality of sleep may greatly impede stroke rehabilitation and induce other complications.us, it is of importance tostudy causes of insomnia in post-stroke patients, especially during rehabilitation of cerebral infarction.
Melatonin is a pineal hormone with a peak nocturnal secretion (Claustrat et al., 2005). Melatonin typically acts in coordination with circadian rhythms to regulate sleep function (Hajak et al., 1996; Rodenbeck et al., 1999; Micic et al., 2015).e peak of melatonin secretion is around 12:00 midnight to 3:00 a.m. (Claustrat et al., 2005; Atanassova et al., 2009). Along with other antioxidants, melatonin also functions as an effective neuroprotective enzyme against neurodegeneration and ischemic brain injury (Wang, 2009; Shekleton et al., 2010; Andrabi et al., 2015; Milanlioglu et al., 2016). Thus, melatonin has an important role in acute ischemic stroke, where its rhythm is impaired and it undergoes nocturnal decrease (Fiorina et al., 1996; Beloosesky et al., 2002; Atanassova et al., 2009; Ritzenthaler et al., 2009). Gamma-aminobutyric acid (GABA) is likewise a strong sleep regulator that may activate GABA receptors as well as inhibitors of waking processes (Gottesmann, 2002; Harrison, 2007). It is known that GABA levels in humans are strongly associated with the impairment of patients after acute ischemic stroke (Paik and Yang, 2014; Blicher et al., 2015). Antioxidants are important for the balance of oxidation by scavenging free radicals and are important markers of insomnia in post-stroke patients. However, to our knowledge, there has been no report of the simultaneous measurement of levels of melatonin, GABA and antioxidants in the blood of patients during convalescence from ischemic stroke or their association with insomnia complications in post-stroke patients.
Therefore, this prospective single-center randomized controlled clinical trial was designed to investigate the relationship between the nocturnal concentrations of melatonin, γ-aminobutyric acid and total antioxidants with insomnia after stroke by comparing levels in stroke patients with or without insomnia and normal controls.
Design and Methods
Study design
Study participants
We screened stroke patients who had treatment at the China Rehabilitation Research Center from January 2012 to June 2014 and were in a period of rehabilitation based on electronic medical records.
(1) Stroke patients with sleep disorders:
Inclusion criteria: patients presenting with all of the following criteria were considered for study inclusion:
• Infarction occurred in the middle cerebral artery blood supply area (identified by medical record, magnetic resonance imaging, magnetic resonance angiography, computed tomography or computed tomography angiogram)
• Diagnostic criteria for insomnia according to the Diagnostic and Statistical Manual of Mental Disorders (4thedition) (American Psychiatric Association, 1994)
• Course of disease ≥ 3 months
• Mini-Mental State Examination (Pangman et al., 2000) >27
• Age range from 50 to 70 years old
• Right handedness
Exclusion criteria: Patients with one or more of the following conditions were excluded from this study:
• Cognitive and language disorders
• History of rheumatism, cancer, severe liver and kidney dysfunction, benign prostatic hyperplasia, severe cardiac insuf fi ciency
• High-risk sleep apnea,i.e., STOP-Bang Questionnaire scores (Nagappa et al., 2015) ≥ 3
• Unexplained limb pain, getting up in the night many times to urinate or restless legs syndrome
• Frequency of application of sleeping drugs > once/week or the use of psychotropic drugs, such as anti-anxiety and depression drugs, and antipsychotic drugs
• Frequency of drinking cof f ee and other stimulating drinks> three times/week
• Drug or alcohol abuse
• Insomnia caused by poor sleeping conditions, such as noise, light, and bedmate interference
• Insomnia before stroke
• Hamilton Depression Scale scores (Hamilton, 1960) > 20 or Hamilton Anxiety Scale scores (Hamilton, 1959) > 14
• Participation in other clinical trials
(2) Stroke patients with normal sleep:
Patients did not have insomnia symptoms,i.e., not in accordance with the Diagnostic criteria for insomnia of the Diagnostic and Statistical Manual of Mental Disorders (4th edition) (American Psychiatric Association, 1994). The remaining screening criteria were the same as those with stroke sleep disorders.
Control group: Patients who had treatment at the China Rehabilitation Research Center from January 2012 to June 2014were used as controls because they did not suf f er from stroke or insomnia.
Inclusion criteria: patients presenting with all of the following criteria were considered for study inclusion
• Mini-Mental State Examination (Pangman et al., 2000) >27
• Age range from 50 to 70 years old
• Right handedness
Exclusion criteria: patients with one or more of the following conditions were excluded from this study:
• Stroke patients
• Diagnostic criteria for insomnia according to the Diagnostic and Statistical Manual of Mental Disorders (4thedition) (American Psychiatric Association, 1994)
• Cognitive and language disorders
• High-risk sleep apnea,i.e., STOP-Bang Questionnaire scores (Nagappa et al., 2015) ≥ 3
• Unexplained limb pain, getting up many times in the night to urinate or restless legs syndrome
• Frequency of application of sleeping pill > once/week or the use of psychotropic drugs, such as anti-anxiety and depression drugs, and antipsychotic drugs
• Frequency of drinking cof f ee and other stimulating drinks> three times/week
• Drug or alcohol abuse
• Hamilton Depression Scale scores (Hamilton, 1960) > 20 or Hamilton Anxiety Scale scores (Hamilton, 1959) > 14
• Participation in other clinical trials
Recruitment
We screened stroke patients who had treatment at the China Rehabilitation Research Center from January 2012 to June 2014 and were in a period of rehabilitation based on electronic medical records. We contacted patients directly for the purpose of the trial and recruited patients to participate in the trial. Aer providing informed consent, these potential participants were screened using the inclusion and exclusion criteria.
Sample size and allocation
Nocturnal concentrations of melatonin in persons aged 60 years old was 40 pg/mL on average (Zhao et al., 2003). Concentrations of melatonin were 12 pg/mL and 35 pg/mL in cerebral infarction persons with and without sleeping disorders. Standard deviation was estimated at 30. Takingβ= 0.1 and power = 90% with a signif i cance level ofα= 0.05, a fi nal sample size ofn= 30 per group was calculated using PASS 11.0 software (PASS, UT, USA). After screening according to the inclusion and exclusion criteria, 25 patients per group were included in the trial. Simultaneously, 25 normal controls were included. The sample size results were analyzed according to the intention-to-treat principle.
Blinding
Patients and physicians were not blinded to group information because insomnia involved subjective judgment and required medical diagnosis. The assessors were blinded to group information.
Outcome measures
Primary outcome measure
Nocturnal concentrations of melatonin, GABA and total antioxidants in peripheral blood
Two weeks before blood collection, patients avoided the use of alcohol, drugs, sleeping pills or antipsychotic drugs.ree days before measurement, patients were admitted to standard sleep laboratories to acclimatize to the environment. Blood samples from the ulnar vein were collected at 3:00 a.m. Patients were required to wear an eye mask before blood taking, and the laboratory remained in dim light to avoid af f ecting the secretion of melatonin.e collected blood was centrifuged immediately, and stored at −80°C.
High-performance liquid chromatography-mass spectrometry (HPLC-MS) was used to determine melatonin and GABA levels. (1) Standard concentration curves of melatonin and GABA. (2) Detection of melatonin using HPLC-MS: melatonin concentrations in the blood samples were measured with an ACQUITY UPLC H-Class system (Waters Corporation, Milford, MA, USA), which was connected to a Waters TQ-S mass spectrometer (Waters Corporation). A reversed-phased C18 BEH column (100 mm × 2.1 mm, 1.7 µm; Waters Corporation) was installed and the column temperature was maintained at 25°C. The flow rate was controlled at 0.35 mL/min. Acetonitrile was used as mobile phase A and 0.1% ammonium hydroxide aqueous solution was used as mobile phase B. A gradient elution was used with an initial ratio of mobile phase A:B = 30:70; at 1.5 minutes, adjusting the ratio to A:B = 90:10; and at 2.1 minutes, adjusting the ratio back to A:B = 90:10. Prior to the operation, the mobile phase was saturated with nitrogen to remove bubbles and dissolved air. The MS system (Waters Corporation) was running in the MRM mode. Ionization condition settings were desolvation at 400°C, source block at 150°C, cone voltage of 20 V, and capillary voltage of 2.7 kV. Eight melatonin standard solutions were tested to cover the range of melatonin concentrations in the blood samples. (3) Detection of GABA using HPLC-MS: GABA concentrations in the blood samples were separated on an ACQUITY UPLC H-Class system, and identif i ed and quantif i ed with a Waters TQ-S mass spectrometer. A Kinetex HILIC column (100 × 4.60 mm, 2.6 µm; Phenomenex, Torrance, CA, USA) was installed, and the column temperature was controlled at 40°C.e fl ow rate was maintained at 1.0 mL/min. A gradient elution model was used with acetonitrile as mobile phase A and aqueous solution containing 10 mM ammonium acetate + 0.1% formic acid as mobile phase B.e initial ratio of mobile phase A:B was controlled at 80:20, and at 4 minutes, this ratio was changed to A:B = 65:35.e mobile phase was saturated with nitrogen to remove the air bubbles and dissolved air. The MS system was running in MRM cation scanning mode.e parameters of ion sources were as follows: CUR: 25.00, IS: 5,000.0, TEM: 600.00, GS1: 55.00, GS2: 60.00, CAD: High. Six GABA dilution series were tested against a standard curve to calibrate GABA levels by weighted least-square regression mode.
Determination of total antioxidant concentrations using colorimetry: antioxidant levels were assessed with a colorimetric Antioxidant Assay Kit (Sigma-Aldrich, St. Louis, MO, USA). Trolox Standards were prepared for obtaining a standard curve. ABTS substrate working solution was prepared by adding 25 mL of 3% hydrogen peroxide solution to 10 mL of ABTS substrate solution and vortexed.e solution was used within 30 minutes.e assays were prepared in a 96-well plate. For the Trolox standard curve, 10 mL of a Trolox Standard was added, followed by 20 mL of Myoglobin working solution. For the test samples, 10 mL of test sample was added, followed by 20 mL of Myoglobin working solution. Afterwards, 150 mL of ABTS substrate working solution was added to each well. Aer the plate was incubated for 5 minutes at room temperature, 100 mL of stop solution was added to each well.e endpoint absorbance values at 405 nm were measured using a plate reader.e plate was read within one hour.e assays were performed in duplicate and the average was used to determine the fi nal value.
Secondary outcome measures
• Sleep-related scales included the Pittsburgh Sleep Quality Index, Insomnia Severity Index, Epworth Sleepiness Scale, Fatigue Severity Scale, and Morningness-Eveningness Questionnaire (Chinese version).
Pittsburgh Sleep Quality Index: an effective instrument used to measure the quality and patterns of sleep in adults established by Buysse et al. (1989).e measure consists of 19 individual items. Overall scores range from 0 to 21, where lower scores denote a healthier sleep quality.
Insomnia Severity Index: a brief self-reporting instrument measuring the patient’s perception of nocturnal and diurnal symptoms of insomnia. It comprises seven items. Each item contains five grades with a total score of 28. A high score represents severe insomnia (Morin et al., 2011).
Epworth Sleepiness Scale: a scale intended to measure daytime sleepiness by use of a very short questionnaire. It was introduced by Dr. Murray Johns of Epworth Hospital in Melbourne, Australia. It contains eight items.e full score is 24. A high score represents obvious sleepiness tendency.e score of a normal person is 7.6 (Johns, 1991).
Fatigue Severity Scale: a 9–item questionnaire with questions related to how fatigue interferes with certain activities that rates severity. The items are scored on a 7 point scale with 1 = strongly disagree and 7 = strongly agree.is scale was developed by Krupp et al. (1989) for the treatment of systemic lupus erythematosus and multiple sclerosis. A higher score indicates greater fatigue severity.
Morningness-Eveningness Questionnaire (Chinese version): a self-rating scale for assessing circadian rhythm. It contains 19 items.e total score is 16–86.e tendency of the circadian rhythm of 163 healthy subjects was assessed by Zhang et al. (2006) from Guangdong General Hospital of China using the Morningness-Eveningness Questionnaire (Chinese version). Zhang et al. recorded sleeping habits through 2-week sleep record table.e reliability and validity of the scale were tested and the demarcation point was sought. Nineteen items were identif i ed as two factors: sleep phase factor and best performance time factor. Demarcation point in Chinese version: absolute morning type, 70–86; moderate morning type, 63–69; intermediate type, 50–62; moderate evening type, 43–49; absolute evening type, 16–42.e Cronbach coef fi cient (Chinese version) was 0.701–0.738; the Spearman-Brown split-half reliability was 0.584–0.697; and for Retest reliability the data reached an acceptable level of psychometrics.e Morningness-Eveningness Questionnaire (Chinese version) has good psychometric characteristics.e new demarcation points can distinguish the morning and night types ef f ectively.
• National Institute of Health Stroke Scale: a tool used to quantify stroke severity. The full score is 42. A high score suggests severe nerve injury (Spilker et al., 1997; Roth et al., 1998).
Data management
Clinical researchers fi lled out the complete clinical trial observation form accurately and in a timely manner. Data were recorded electronically by data managers using a double-data entry strategy.e electronic database was locked by the project manager aer checking. All data were analyzed statistically by professional statisticians. Anonymized trial data will be published at www.f i gshare.com.
Statistical analysis
Data were presented as the mean ± standard deviation for normally distributed variables, or median values (P25, P75) for non-normally distributed variables. Student’st-tests or nonparametric Mann-WhitneyU-tests were performed to compare the dif f erences between normally distributed variables or non-normally distributed variables. For the analysis of biochemical test results, data were transferred to normal distribution and Hotelling’s T2 tests were performed. Before entering variables into the regression model, centering predictor variables were performed to avoid nonessential collinearity. Binary logistic regression analysis was conducted to identify the association between variables or variable interactions and insomnia diagnosis aer infarction. Multiple linear regression analysis was carried out to determine the correlation between variables or variable interactions and sleep-related scores, such as Epworth Sleepiness Scale scores, Pittsburgh Sleep Quality Index scores, Insomnia Severity Index scores, Morningness-Eveningness Questionnaire (Chinese version) scores and Fatigue Severity Scale scores by using the backward method.Pvalues < 0.05 were considered statistically significant. SPSS 22.0 software (IBM, Armork, NY, USA) was used for statistical analyses.
Conf i dentiality
Clinical trial observation forms and informed consents were password-protected in the China Rehabilitation Research Center.e patient’s identity will not be disclosed unless the law requires it.e fi ndings will be published for scientif i c purposes without disclosing the patient’s identity.

Figure 1 Clinical trial fl ow chart.
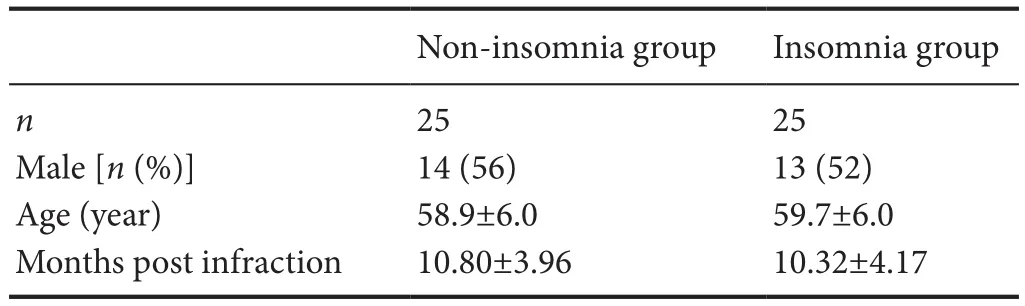
Table 1 Clinical information in the non-insomnia and insomnia groups
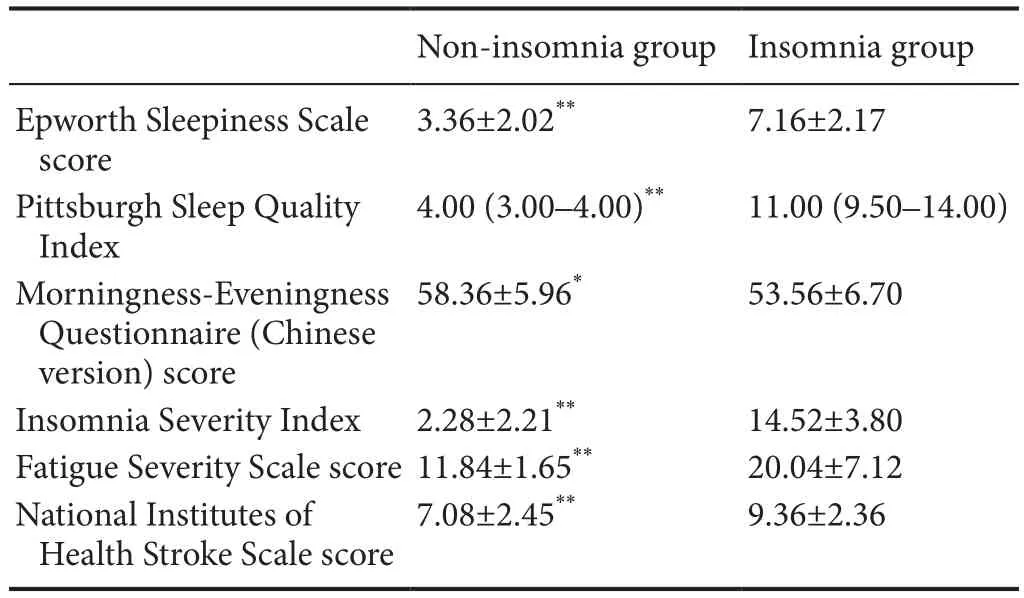
Table 2 Comparison of sleep-related scales and National Institute of Health Stroke Scale scores between the non-insomnia and insomnia groups

Figure 2 Nocturnal concentrations of melatonin, γ-aminobutyric acid (GABA) and total antioxidants in peripheral blood.
Ethical requirements
The study protocol was approved by the Ethics Committee of China Rehabilitation Research Center on 20 March 2012. All protocols were performed in accordance with the Ethical Principles for Medical Research Involving Human Subjects in theDeclaration of Helsinki(2013), formulated by the World Medical Association.e writing and editing of the article were performed in accordance with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Additional fi le 1).is trial was registered at ClinicalTrials.gov (identifier: NCT03202121). Written informed consents were provided by a legal representative of each patient aer they indicated that they fully understood the treatment plan.
Results
Patient recruitment and data collection of the insomnia and non-insomnia groups is fi nished.
Clinical information of patients in the insomnia group and non-insomnia group
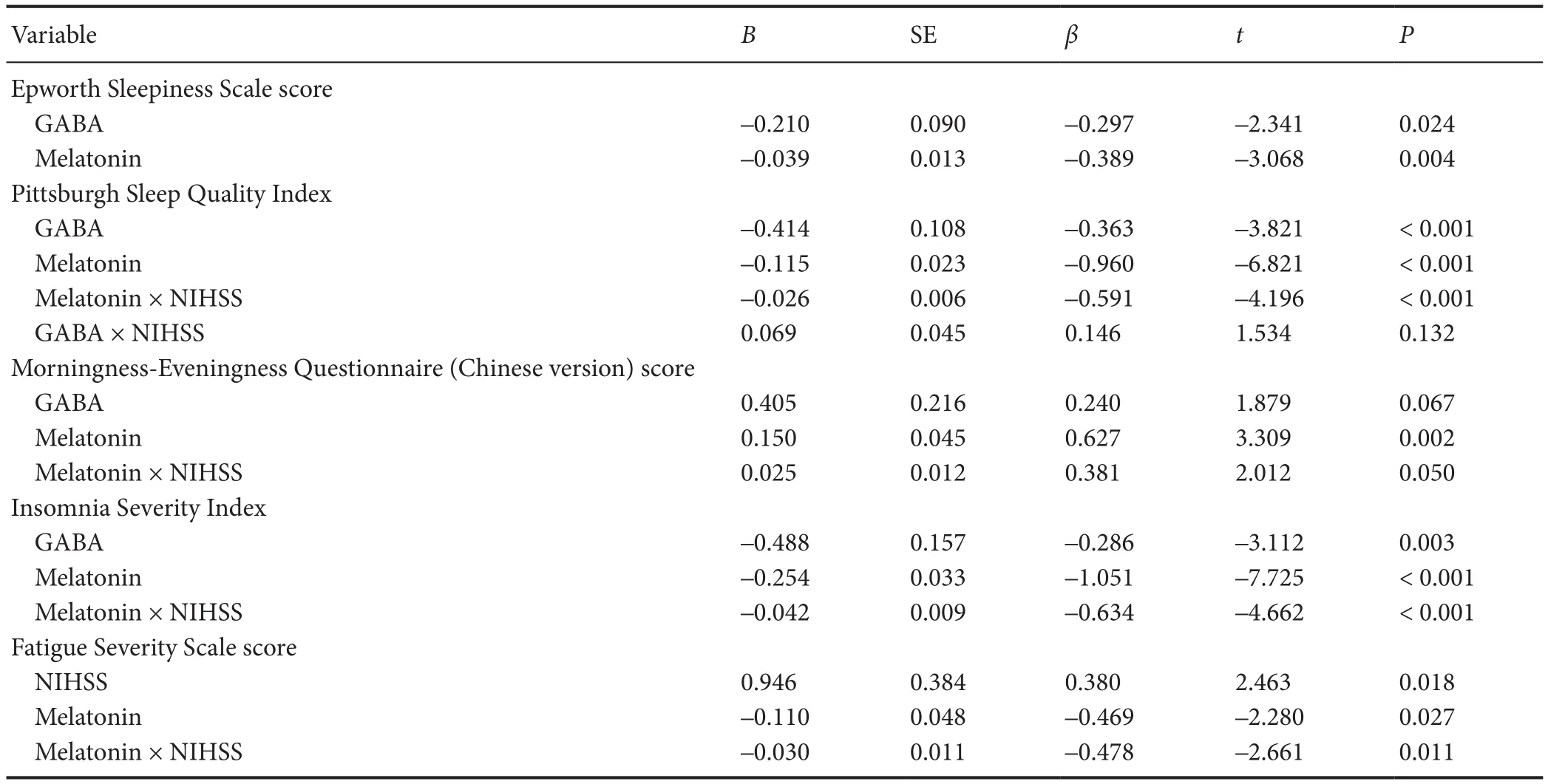
Table 4 Linear regression analysis of the relationship of GABA, antioxidants, melatonin, NHISS and factor interaction with sleep-related scale
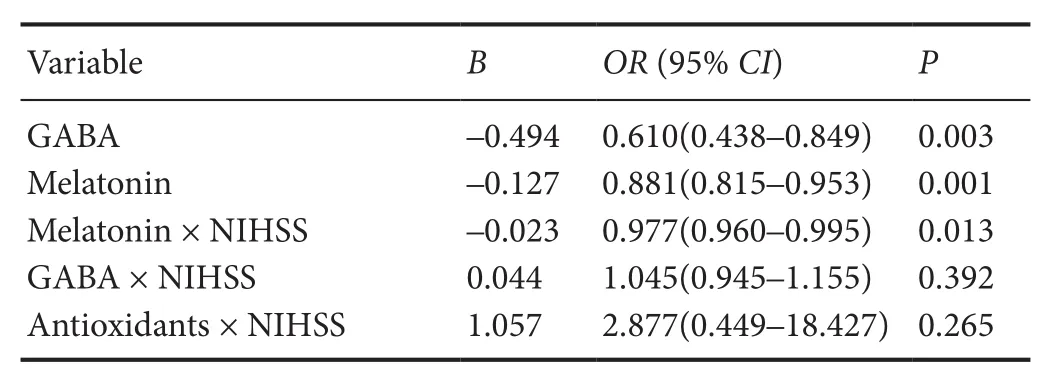
Table 3 Binary logistic regression for the relationship of biochemical factors, NIHSS and factor interaction with insomnia
Dif f erences in nocturnal concentrations of melatonin, GABA and total antioxidants in the peripheral blood of patients in the insomnia and non-insomnia groups
Results of UPLC-MS and colorimetric antioxidant assay showed that the nocturnal blood concentrations of melatonin, GABA, and total antioxidants in the insomnia group were lower than that in the non-insomnia group (P< 0.01; Figure 2).
Dif f erences in sleep status of patients in the insomnia and non-insomnia groups
As shown in Table 2, patients in the insomnia group had signif i cantly worse status assessed by related sleep scales. Of note, the Epworth Sleepiness Scale, Pittsburgh Sleep Quality Index, Insomnia Severity Index and Fatigue Severity Scale scores in the insomnia group were higher than in the non-insomnia group (P< 0.01). Additionally, patients in the insomnia and non-insomnia groups displayed significant differences in Morningness-Eveningness Questionnaire (Chinese version) scores, implying that the patients in the insomnia group can be represented as moderate evening types, whereas patients in the non-insomnia group can be represented as moderate morning types.
Relationship of nocturnal concentrations of melatonin, GABA and total antioxidants in peripheral blood of patients with insomnia aer stroke
Results of UPLC-MS and colorimetric antioxidant assay showed that the nocturnal blood concentrations of melatonin, GABA, and total antioxidants in the insomnia group were lower than in the non-insomnia group (P< 0.01). Strikingly, the current data also showed an elevated NIHSS score in the insomnia group compared with the non-insomnia group (P< 0.01), indicating more severe infarction impairment in the insomnia group (Table 2).
Based on the sleep-related scale scores, multiple linear regressions were achieved (Table 4). Interaction factors, suchas melatonin, GABA, and total antioxidants with NHISS, infl uenced the insomnia patients from di ff erent aspects. Both melatonin and GABA had negative correlations with ESS (P= 0.024,P= 0.004), PSQI (P< 0.001,P< 0.001) and ISI (P= 0.003,P< 0.001). Melatonin also had a positive correlation with the Morningness-Eveningness Questionnaire (Chinese version) score and a negative correlation with the Fatigue Severity Scale score (P= 0.002 andP= 0.018). In addition, regression analysis indicated that the melatonin × NIHSS interaction was signi fi cantly associated with the Pittsburgh Sleep Quality Index score (P< 0.001), Morningness-Eveningness Questionnaire (Chinese version) score (P= 0.05), Insomnia Severity Index score (P< 0.001) and Fatigue Severity Scale score (P= 0.011), but not with the Epworth Sleepiness Scale score (P> 0.05).
Discussion
Melatonin, an indole derived from serotonin, is a rhythmically secreted neurohormone produced mainly by the pineal gland (Claustrat et al., 2005; Atanassova et al., 2009; Lu et al., 2015).is feature makes melatonin an important sleep regulator that regulates circadian sleep and waking. Here, we collected blood samples from patients aer middle cerebral artery infarction and directly measured the concentrations of melatonin via highly sensitive UPLC-MS.ere was a signif i cant decrease in nocturnal melatonin levels in the blood samples from insomnia patients. Regression analysis indicated a negative relationship between nocturnal melatonin levels and insomnia for post middle cerebral artery infarction patients.e analysis also suggested an inf l uence of nocturnal melatonin levels on other aspects of insomnia, including the quality and patterns of sleep, circadian rhythm, severity of insomnia and fatigue levels.
All these results indicate that melatonin disturbance is an important factor for middle cerebral artery infarction patients who suffer from insomnia. Middle cerebral artery infarction is the most common subtype of stroke and is characterized by a poor prognosis (Ng et al., 2007). Furthermore, middle cerebral artery infarction has a clear blood supply area and its ef f ects on sleep and other biochemicals are uniform without significant interference from other factors. Although the blood supply area of the posterior cerebral artery has a close relationship with the pineal gland, its main melatonin secreting region and blood supply area is regarded to be complicated because of the inclusion of the ascending reticular active system. In addition, the posterior cerebral artery infarction impacts the consciousness of patients, and may af f ect a patient’s ability on fi nishing sleep-related scale assessments. As a result, the middle cerebral artery was explored as a starting point in the current study, which may allow us to exclude interference by other factors. Further studies of the posterior cerebral artery patients are required.
According to early reports, acute ischemic stroke patients, including anterior circulation stroke, extensive cortical stroke, and deep and lacunar strokes, show a significant decrease in nocturnal urinary melatonin excretion at day 3 post ischemic stroke, caused by melatonin rhythm disturbance or peak delay at the acute stage of stroke (Fiorina et al., 1996; Beloosesky et al., 2002; Atanassova et al., 2009). It was also reported that melatonin secretion pattern disturbances can be reverted to a normal pattern within 10 days post stroke (Beloosesky et al., 2002). However, there is still lack of reports on the dysregulation of melatonin or other neurotransmitters in infarction patients, especially those in the chronic rehabilitation stage.
Following the downregulation of melatonin levels in the blood of patients, sustained melatonin levels may partially contribute to the onset of insomnia in the rehabilitation phase of stroke patients. In addition, because melatonin is partially produced from serotonin in the brain, decreased melatonin levels in the blood of patient shown in the current study suggest decreased serotonin levels in the patients, which will be followed up in future studies. Our data also showed that middle cerebral artery infarction patients with insomnia complained of significantly higher NIHSS values compared with patients without insomnia.e NIHSS value is a reliable systematic tool to assess neurologic deficits in stroke-related patients (Spilker et al., 1997; Roth et al., 1998; Kasner, 2006), so an increased NIHSS value in insomnia patients may suggest a worse neurologic def i cit induced by the middle cerebral artery infarction.
However, regression analysis in our study indicated that NIHSS itself and its synergic interaction with disturbed melatonin levels in the blood were markedly correlated with insomnia in these infarction patients.e interaction factor of NIHSS × melatonin level was negatively correlated with the quality of sleep (Pittsburgh Sleep Quality Index scores), severity of insomnia (Insomnia Severity Index scores), and fatigue level of patients (Fatigue Severity Scale scores), but positively correlated with the Morningness and Eveningness Questionnaire (Chinese version) score, indicating a change in sleep type aer cerebral infarction may be a cause of insomnia aer stroke.is change may be associated with the secretion of melatonin aer cerebral infarction. To the best of our knowledge, this is the first demonstration of a clear association between the NIHSS × melatonin interactions and insomnia occurrence for post infarction rehabilitation of patients.
NIHSS is highly correlated with the size of ischemic area and severity of ischemic impairment in stroke patients (Spilker et al., 1997; Meuli, 2004; Kasner, 2006).ese neurologic deficits may involve serious impairment of endogenous melatonin secretion from specif i c neurons, resulting in a decreased recovery of melatonin levels in rehabilitation patients and def i cient quality of sleep or insomnia. However, early studies reported that melatonin in the blood was protective in a middle cerebral artery stroke rodent model (Sinha et al., 2001; Pei et al., 2003; Pei and Cheung, 2004).us, sustaining low blood levels of melatonin for middle cerebral artery infarction patients probably has low protection against ischemia injury. However, this study only reported limited observations, and the mechanism of such an underlying interaction are unknown. Currently, we are working on the etiology of insomnia for infarction patients to reveal thisinteraction mechanism.
GABA is an inhibitory neurotransmitter and an important regulator that activates unused synapses and promotes the recovery and rehabilitation of patients aer ischemic stroke (Morgan et al., 2012; Paik and Yang, 2014; Blicher et al., 2015). In this study, a functional decrease in GABA levels in the blood of patients aer stroke ref l ected a disinhibition of synaptic activity and promotion of brain recovery. GABA levels in the blood of patients were decreased after subcortical lesion stroke, suggesting a decreased regulation of GABA to boost the recovery of brain function (Blicher et al., 2015). In this case, because patients in the insomnia group expressed a higher NIHSS, suggesting a worse impairment by cerebral infarction, the GABA level in the blood samples may result in a signif i cant functional downregulation in the insomnia patients, which might assist stroke rehabilitation. A functional decrease of GABA in the blood may not only af f ect the sleep of patients but also be the cause of insomnia. However, it is only a partial explanation for the signif i cance of GABA and insomnia post infarction.
We also studied the effect of antioxidants that have a critical role in the balance of oxidation by scavenging free radicals and limiting oxidative stress during neurological damage caused by ischemic stroke (Allen and Bayraktutan, 2009). Previous studies reported inconsistent results regarding changes in antioxidants in patients with acute ischemic stroke (Zimmermann et al., 2004; Aygul et al., 2006). According to our data, total antioxidant levels in the blood were decreased in patients of the insomnia group during the recovery stage post infarction.is suggests that patients in the acute ischemic stage and rehabilitation stage may have different regulatory mechanisms (Shekleton et al., 2010). In addition, melatonin contributes to antioxidant responses in ischemic stroke (Ritzenthaler et al., 2013); therefore, the decreased melatonin levels in the blood observed in this study may partially contribute to the decrease of total antioxidants in insomnia patients. Regression analysis indicated no association between the total antioxidant concentration and insomnia or sleep-related scores; therefore, a decrease in total antioxidants in the blood may be ascribed to the decrease of melatonin.
Although clinical evaluation dominates the fi nal insomnia diagnosis, these valid and brief self-reports can expedite the evaluation of insomnia and promote lengthy routine clinical assessment (Morin et al., 2011).ese self-report questionnaires provide a multidimensional measurement system to assess insomnia status. Based on the sleep-related scales, our data demonstrated that melatonin, GABA, and NIHSS as well as interactions between them af f ected insomnia symptoms. They also provide insight into the multidimensional aspects of insomnia, such as the quality and patterns of sleep, circadian rhythm, severity of insomnia and fatigue level of patients. Although our observations do not allow a conclusion regarding the cause-and-effect relationship, the data in this study demonstrate for the fi rst time that melatonin, GABA, and total antioxidants are directly correlated with insomnia and other sleep disorders in patients with post middle cerebral artery infarction.
Recruitment of normal controls is ongoing, so it cannot completely explain the changes in nocturnal concentrations of melatonin, GABA and total antioxidants in the peripheral blood after stroke. Therefore, further data collection will provide an experimental basis for exploring the risk factors of sleep disorders aer stroke.
Acknowledgments:We appreciate the contribution of all colleagues participating in this research, as well as the patients who consented to be enrolled in this study.
Author contributions:WZ and TZ conceived and designed the experiments, and wrote the paper. WZ and FL performed the study. WZ, FL and TZ analyzed the data. All authors approved the fi nal version of the paper.
Conf l icts of interest:None declared.
Research ethics:
Declaration of patient consent:The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal.e patients understand that their names and initials will not be published and due ef f orts will be made to conceal their identity, but anonymity cannot be guaranteed.
Data sharing statement:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Additional fi le:
Additional Table 1: SPIRIT checklist.
Allen CL, Bayraktutan U (2009) Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke 4:461-470.
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders.
Andrabi SS, Parvez S, Tabassum H (2015) Melatonin and ischemic stroke: mechanistic roles and action. Adv Pharmacol Sci 2015:384750.
Atanassova PA, Terzieva DD, Dimitrov BD (2009) Impaired nocturnal melatonin in acute phase of ischaemic stroke: cross-sectional matched case-control analysis. J Neuroendocrinol 21:657-663.
Aygul R, Kotan D, Demirbas F, Ulvi H, Deniz O (2006) Plasma oxidants and antioxidants in acute ischaemic stroke. J Int Med Res 34:413-418.
Beloosesky Y, Grinblat J, Laudon M, Grosman B, Streif l er JY, Zisapel N (2002) Melatonin rhythms in stroke patients. Neurosci Lett 319:103-106.
Blicher JU, Near J, Næss-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Østergaard L, Ho YC (2015) GABA levels are decreased aer stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil Neural Repair 29:278-286.
Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ (1989)e Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193-213.
Claustrat B, Brun J, Chazot G (2005)e basic physiology and pathophysiology of melatonin. Sleep Med Rev 9:11-24.
Fiorina P, Lattuada G, Ponari O, Silvestrini C, DallAglio P (1996) Impaired nocturnal melatonin excretion and changes of immunological status in ischaemic stroke patients. Lancet 347:692-693.
Gottesmann C (2002) GABA mechanisms and sleep. Neuroscience 111:231-239.
Hajak G, Rodenbeck A, Adler L, Huether G, Bandelow B, Herrendorf G, Staedt J, Rüther E (1996) Nocturnal melatonin secretion and sleep aer doxepin administration in chronic primary insomnia. Pharmacopsychiatry 29:187-192.
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56-62.
Harrison NL (2007) Mechanisms of sleep induction by GABA(A) receptor agonists. J Clin Psychiatry 68 Suppl 5:6-12.
Johns MW (1991) A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14:540-545.
Kasner SE (2006) Clinical interpretation and use of stroke scales. Lancet Neurol 5:603-612.
Kim J, Kim Y, Yang KI, Kim DE, Kim SA (2015)e relationship between sleep disturbance and functional status in mild stroke patients. Ann Rehabil Med 39:545-552.
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989)e fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121-1123.
Leppävuori A, Pohjasvaara T, Vataja R, Kaste M, Erkinjuntti T (2002) Insomnia in ischemic stroke patients. Cerebrovasc Dis 14:90-97.
Lu T, Wei N, Zhang C, Dong YZ (2015) Osteogenic dif f erentiation of adipose-derived stem cells and the ef f ect of melatonin on the bio-viability of differentiated cells. Zhongguo Zuzhi Gongcheng Yanjiu 19:8072-8076.
Meuli RA (2004) Imaging viable brain tissue with CT scan during acute stroke. Cerebrovasc Dis 17 Suppl 3:28-34.
Micic G, Lovato N, Gradisar M, Burgess HJ, Ferguson SA, Kennaway DJ, Lack L (2015) Nocturnal melatonin prof i les in patients with delayed sleep-wake phase disorder and control sleepers. J Biol Rhythms 30:437-448.
Milanlioglu A, Aslan M, Ozkol H, Cilingir V, Nuri Aydin M, Karadas S (2016) Serum antioxidant enzymes activities and oxidative stress levels in patients with acute ischemic stroke: inf l uence on neurological status and outcome. Wien Klin Wochenschr 128:169-174.
Morgan PT, Pace-Schott EF, Mason GF, Forselius E, Fasula M, Valentine GW, Sanacora G (2012) Cortical GABA levels in primary insomnia. Sleep 35:807-814.
Morin CM, Belleville G, Belanger L, Ivers H (2011)e Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 34:601-608.
Nagappa M, Liao P, Wong J, Auckley D, Ramachandran SK, Memtsoudis S, Mokhlesi B, Chung F (2015) Validation of the STOP-Bang Questionnaire as a screening tool for obstructive sleep apnea among dif f erent populations: a systematic review and meta-analysis. PLoS One 10:e0143697.
Ng YS, Stein J, Ning M, Black-Schaf f er RM (2007) Comparison of clinical characteristics and functional outcomes of ischemic stroke in dif f erent vascular territories. Stroke 38:2309-2314.
Paik NJ, Yang E (2014) Role of GABA plasticity in stroke recovery. Neural Regen Res 9:2026-2028.
Pangman VC, Sloan J, Guse L (2000) An examination of psychometric properties of the mini-mental state examination and the standardized mini-mental state examination: implications for clinical practice. Appl Nurs Res 13:209-213.
Pei Z, Cheung RT (2004) Pretreatment with melatonin exerts anti-infl ammatory e ff ects against ischemia/reperfusion injury in a rat middle cerebral artery occlusion stroke model. J Pineal Res 37:85-91.
Pei Z, Pang SF, Cheung RT (2003) Administration of melatonin aer onset of ischemia reduces the volume of cerebral infarction in a rat middle cerebral artery occlusion stroke model. Stroke 34:770-775.
Ritzenthaler T, Lhommeau I, Douillard S, Cho TH, Brun J, Patrice T, Nighoghossian N, Claustrat B (2013) Dynamics of oxidative stress and urinary excretion of melatonin and its metabolites during acute ischemic stroke. Neurosci Lett 544:1-4.
Ritzenthaler T, Nighoghossian N, Berthiller J, Schott AM, Cho TH, Derex L, Brun J, Trouillas P, Claustrat B (2009) Nocturnal urine melatonin and 6-sulphatoxymelatonin excretion at the acute stage of ischaemic stroke. J Pineal Res 46:349-352.
Rodenbeck A, Huether G, Rüther E, Hajak G (1999) Nocturnal melatonin secretion and its modif i cation by treatment in patients with sleep disorders. Adv Exp Med Biol 467:89-93.
Roth EJ, Heinemann AW, Lovell LL, Harvey RL, McGuire JR, Diaz S (1998) Impairment and disability: their relation during stroke rehabilitation. Arch Phys Med Rehabil 79:329-335.
Shekleton JA, Parcell DL, Redman JR, Phipps-Nelson J, Ponsford JL, Rajaratnam SM (2010) Sleep disturbance and melatonin levels following traumatic brain injury. Neurology 74:1732-1738.
Sinha K, Degaonkar MN, Jagannathan NR, Gupta YK (2001) Ef f ect of melatonin on ischemia reperfusion injury induced by middle cerebral artery occlusion in rats. Eur J Pharmacol 428:185-192.
Spilker J, Kongable G, Barch C, Braimah J, Brattina P, Daley S, Donnarumma R, Rapp K, Sailor S (1997) Using the NIH Stroke Scale to assess stroke patients.e NINDS rt-PA Stroke Study Group. J Neurosci Nurs 29:384-392.
Suh M, Choi-Kwon S, Kim JS (2014) Sleep disturbances aer cerebral infarction: role of depression and fatigue. J Stroke Cerebrovasc Dis 23:1949-1955.
Zhang B, Hao YL, Rong RG (2006)e reliability and validity of Chinese version morningness/eveningness questionnaire. Zhongguo Xingwei Yixue Kexue 15:856-858.
Zhao ZY, Lu FH, Luan Y, Xie Y, Fu YR, Liu JP, Han K, Zhang XQ, Touitou Y (2003) Study on variation of serum melatonin level in Chinese elders. Zhonghua Laonian Xinxueguanbing Zazhi 5:156-158.
Zimmermann C, Winnefeld K, Streck S, Roskos M, Haberl RL (2004) Antioxidant status in acute stroke patients and patients at stroke risk. Eur Neurol 51:157-161.
Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Tong Zhang, M.D., Ph.D., zt61611@sohu.com.
Tong Zhang, M.D., Ph.D., zt61611@sohu.com.
orcid: 0000-0001-8245-0029 (Tong Zhang)
10.4103/1673-5374.213550
Accepted: 2017-05-26
- 中国神经再生研究(英文版)的其它文章
- Transcriptional inhibition in Schwann cell development and nerve regeneration
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
- Optogenetics and its application in neural degeneration and regeneration
- Live-cell imaging: new avenues to investigate retinal regeneration
- Neurotrophic factors and corneal nerve regeneration