Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
Julia J. Segura-Uribe, Rodolfo Pinto-Almazán, Angélica Coyoy-Salgado, Claudia E. Fuentes-Venado, Christian Guerra-Araiza,
1 Unidad de Investigación Médica en Enfermedades Neurológicas, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City, Mexico
2 Sección de Estudios de Posgrado e Investigación, Escuela Superior de Medicina, Instituto Politécnico Nacional, Mexico City, Mexico
3 Unidad de Investigación Hospital Regional de Alta Especialidad Ixtapaluca, Ixtapaluca, Mexico
4 Institute for the Developing Mind, Children’s Hospital Los Angeles, Los Angeles, CA, USA
5 Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
6 Consejo Nacional de Ciencia y Tecnología, Mexico City, Mexico
7 Clínica de Trastornos del Sueño, Universidad Autónoma Metropolitana-Iztapalapa, Mexico City, Mexico
8 Servicio de Medicina Física y Rehabilitacion, Hospital General de Zona No. 197, Texcoco, Mexico
9 Unidad de Investigación Médica en Farmacología, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City, Mexico
Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
Julia J. Segura-Uribe1,2, Rodolfo Pinto-Almazán3,4,5, Angélica Coyoy-Salgado1,6, Claudia E. Fuentes-Venado7,8,9, Christian Guerra-Araiza9,*
1 Unidad de Investigación Médica en Enfermedades Neurológicas, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City, Mexico
2 Sección de Estudios de Posgrado e Investigación, Escuela Superior de Medicina, Instituto Politécnico Nacional, Mexico City, Mexico
3 Unidad de Investigación Hospital Regional de Alta Especialidad Ixtapaluca, Ixtapaluca, Mexico
4 Institute for the Developing Mind, Children’s Hospital Los Angeles, Los Angeles, CA, USA
5 Department of Pediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
6 Consejo Nacional de Ciencia y Tecnología, Mexico City, Mexico
7 Clínica de Trastornos del Sueño, Universidad Autónoma Metropolitana-Iztapalapa, Mexico City, Mexico
8 Servicio de Medicina Física y Rehabilitacion, Hospital General de Zona No. 197, Texcoco, Mexico
9 Unidad de Investigación Médica en Farmacología, Hospital de Especialidades, Centro Médico Nacional Siglo XXI, Instituto Mexicano del Seguro Social, Mexico City, Mexico
How to cite this article:Segura-Uribe JJ, Pinto-Almazán R, Coyoy-Salgado A, Fuentes-Venado CE, Guerra-Araiza C (2017) Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system. Neural Regen Res 12(8):1231-1240.
Estrogen receptor modulators are compounds of interest because of their estrogenic agonistic/antagonistic Effects and tissue specif i city.ese compounds have many clinical applications, particularly for breast cancer treatment and osteoporosis in postmenopausal women, as well as for the treatment of climacteric symptoms. Similar to estrogens, neuroprotective effects of estrogen receptor modulators have been described in different models. However, the mechanisms of action of these compounds in the central nervous system have not been fully described. We conducted a systematic search to investigate the Effects of estrogen receptor modulators in the central nervous system, focusing on the modulation of cytoskeletal proteins. We found that raloxifene, tamoxifen, and tibolone modulate some cytoskeletal proteins such as tau, microtuble-associated protein 1 (MAP1), MAP2, neurof i lament 38 (NF38) by dif f erent mechanisms of action and at dif f erent levels: neuronal microf i laments, intermediate fi laments, and microtubule-associated proteins. Finally, we emphasize the importance of the study of these compounds in the treatment of neurodegenerative diseases since they present the benef i ts of estrogens without their side Effects.
estrogen receptor modulators; selective estrogen receptor modulators; microtubules; neurof i laments; tibolone; tamoxifen; raloxifene
Introduction
In mammals, endogenous estrogens are involved in the regulation of many processes ranging from tissue growth maintenance to reproduction.eir action is mediated by estrogen receptors (ERs): estrogen receptor alpha (ERα) and estrogen receptor β (ERβ), which are located in the cell nucleus where they act as nuclear transcription factors. Both subtypes of estrogenic receptors are markedly expressed in the central nervous system (CNS). The activation of these nuclear receptors is responsible for the well-known genomic events produced by estrogens since they regulate the transcription of target genes through binding to specif i c DNA sequences. The tissue-specific and pleiotropic actions of estrogens are inf l uenced by ER subtypes dif f erential expression and their coregulatory proteins (Farooq, 2015; Farzaneh and Zarghi, 2016).
Estrogen receptor modulators (ERMs) constitute a group of compounds with a chemical structure that gives them an af finity to bind to estrogen receptors depending on the target tissue where this binding is performed (Pérez-Edo, 2004). Within the ERMs, selective estrogen receptor modulators (SERMs) (Pérez-Edo, 2004) and selective tissue estrogenic activity regulators (STEARs) (Reed and Kloosterboer, 2004) are found. SERMs induce estrogen agonistic (bone tissue, cardiovascular system, liver, brain) and antagonistic (breast, endometrium) Effects in contrast to the purely agonistic Effects of estrogens (Pérez-Edo, 2004).e term STEAR focuses on the estrogenic activity, which is particularly expressed in a tissue selective manner; moreover, steroid metabolism plays an essential role in establishing the availability of the ligand for the receptor (Reed and Kloosterboer, 2004).
A neuroprotective ef f ect of SERMs and STEARs has been described in dif f erent models of damage, such as epilepsy (Velisek et al., 2013), focal cerebral ischemia (Zhang et al., 2005), traumatic brain injury (Kokiko et al., 2006), ozone exposure (Farfán-García et al., 2014; Pinto-Almazán et al., 2014), aging (Neri-Gomez et al., 2017) and Parkinson’s disease (Morissette et al., 2008). One of the mechanisms by which SERMs and STEARs exert this neuroprotective ef f ect is through the modulation of the expression of cytoskeletal proteins (Barreto et al., 2009; Pinto-Almazán et al., 2014).
In this work, a systematic review of the Effects of SERMs and STEARs on the modulation of cytoskeletal proteins in the CNS was performed. This manuscript included the search of controlled clinical trials from MEDLINE (viaPubMed), LILACS (viaBIREME), Ovid Global Health, SCOPUS, Scielo and Web of Science. Language restriction was applied to English. All searches were performed from 1990 to January 2017 and included the controlled vocabulary indexed on databases as well as keywords. Terms used on Medical Subject Heading (MeSH) were “tamoxifen”, “clomiphene”, “toremifene”, “GW 5638”, “raloxifene”, “arzoxifene”,“lasofoxifene”, “basedoxifene”, “tibolone”, “tubulin”, “actin”,“MAP”, “MAP1”, “MAP2”, “tau”, “GFAP”, “ketarin”, “nestin”,“neurofilament”, “vimentin”, “brain” and “central nervous system”.e Boolean operator “AND” was used to perform a broad range of combinations in databases and fi nd all relevant studies.
An Overview of ERMs
As mentioned before, ERMs are compounds that lack the steroid structure of estrogens but selectively bind to ERs, depending on their chemical structure as well as their tissue specif i city.ey exhibit diverse agonist and antagonist characteristics (biocharacter) in a given tissue. Furthermore,in vitroexperiments have shown that individual SERMs can exhibit distinct activities in the same cell type (Dutertre and Smith, 2000). Due to their structure, ERMs also present antioxidant activity (Yu et al., 2007; Farfán-García et al., 2014).
Classif i cation of ERMs
According to their chemical structure, SERMs have been classified into five groups: triphenylethylenes, benzothiophenes, tetrahydronaphtylenes, indoles and benzopyrans (Diez-Perez, 2006). In contrast, only one STEAR has been described so far: tibolone (Reed and Kloosterboer, 2004) (Figure 1).
Mechanism of action of ERMs
Estrogens and SERMs also activate other pathways that are non-genomic or rapid acting, such as those dependent upon nitric oxide (NO): vasodilatation, ischemic myocardial damage, response to endothelial damage and coronary artery relaxation, among others (Navarro-Despaigne, 2001; Diez-Perez, 2006).
Tissue-selective effects of STEARs result from metabolism, enzyme regulation and receptor activation, which are dif f erent depending on the type of tissue. Tibolone is rapidly converted in the organism into three metabolites: 3α- and 3β-hydroxy-tibolone, with estrogenic Effects, and Δ(4)-isomer, with progestogenic and androgenic Effects (Kloosterboer, 2004).
Clinical use of ERMs
As these drugs can act as estrogen agonists and antagonists depending on the target tissue, they are used as a treatment for dif f erent conditions. For example, clomiphene has been used in the management of infertility (Goldstein et al., 2000), whereas tamoxifen, toremifene and droloxifene have been used as adjuvant therapy in breast cancer (Osborne et al., 2000). Raloxifene has been used to prevent osteoporosis in postmenopausal women (Kulak et al., 2010; Maximov et al., 2013) and it has been suggested that it may exert cardioprotective Effects (Goldstein et al., 2000). Ospemifene has been used to treat postmenopausal dyspareunia associated with vaginal atrophy (Pinkerton andomas, 2014). Tibolone has been used to diminish climacteric symptoms in menopausal women (Pinto-Almazán et al., 2017).
One key difference among the effects of ERMs is breast and endometrial cancer risk safety (Pinkerton andomas, 2014). Many clinical trials examining various SERMs preparations in postmenopausal osteoporotic women showed that SERMs can maintain bone mineral density (BMD) and reduce the incidence of vertebral fractures but did not reduce non-vertebral fracture risk, indicating that their benef i t for fractures is anatomically limited (An, 2016).e study of the mechanisms of action of SERMs has increased the understanding of hormone-receptor regulatory processes. Their development has allowed a certain efficacy profile to avoid some of the side effects of hormone therapy. Their clinical utility relies today mostly on the Effects on breast cancer and BMD (Diez-Perez, 2006).
Many other SERMs such as GW 5638, arzoxifene and pipendoxifene are on preclinical stage trials, and other SERMs like MDL 101,986, SR16234, tetrahydroisoquinoline derivatives and 2-phenylspiroindenes are being developed to prevent and treat osteoporosis, breast cancer and cardiovascular diseases (Terán and Teppa, 2005).
Neuroprotection by ERM
Extensive work has reported the neuroprotective activity of estrogens (Green et al., 1997) as well as ERMs. In a neuronal cell culture, raloxifene was found to be protective against a variety of toxic insults including glutamate, Aβ25–35, and H2O2.e neuroprotective activity of raloxifene in an oxygen-glucose deprivation model was observed to be G protein-coupled receptor 30 (GPR30)-dependent and ER-independent and not mediated by antioxidant effects (Abdelhamid et al., 2011). Raloxifene and tamoxifen reduced microglial activation induced by neuroinf l ammatory stimuli in young and aged rats, which suggests the neuroprotective Effects of these SERMs in brain trauma (Barreto et al., 2014).ey also reversed spine density loss observed in a cerebral ischemia model in ovariectomized adult female rats (Khan et al. 2015). Aer the administration of kainic acid in adult ovariectomized rats, tamoxifen, raloxifene and bazedoxifene prevented hippocampal neuronal loss (Ciriza et al., 2004). In a male mouse model of Parkinson’s disease, raloxifene showed distinct neuroprotective actions similar to those of estradiol and progesterone following a cytotoxic brain insult (Littleton-Kearney et al., 2002). In an ovariectomized rat focal stroke model, azoxifene signif i cantly reduced ischemic infarction volume in the caudoputamen providing some degree of neuroprotection (Littleton-Kearney et al., 2002).
In the brain, SERMs may exert therapeutic potential either by modulating brain neurotransmitter transmission or through neuroprotective activity. The clinical potential for raloxifene in neurodegeneration and cognitive decline is shown by studies in elderly males and postmenopausal women (Cyr et al., 2000). Recent studies hint that raloxifene and arzoxifene are neuroprotective and may preserve some elements of cognitive function. Raloxifene mimics estrogen in the cholinergic system and increases brain-derived neurotrophic factor (BDNF) and nerve growth factor receptors, and may influence cognitive status. In postmenopausal women, raloxifene produced a small but signif i cant improvement in verbal memory scores. Improvements in cognitive function were also observed in postmenopausal women with and without Alzheimer’s disease (AD) (Littleton-Kearney et al., 2002).
Tibolone has been described to present neuroprotective effects and to modulate neuroplasticity in some animal models of oxidative stress and aging (Pinto-Almazán et al., 2014; Neri-Gómez et al., 2017). In postmenopausal women, tibolone improves well-being, cognition, and mood (Genazzani et al., 2006).
Importance of Cytoskeletal Proteins in Neuronal Physiology
The neuronal cytoskeleton is composed of three interconnected types of long chains of protein subunits, each with specif i c properties. Neurof i laments are formed by intermediate fi lament (IF) proteins; microtubules (MT) are cytoskeleton heterodimers comprising protof i laments of α/ β-tubulin; and actin microf i laments (MF), which contain both fi lamentous actin (F-actin) and polymerized globular actin (G-actin) (Hansberg-Pastor et al., 2015; Menon and Gupton, 2016).
Outstanding properties of NF include a long half-life and elastic fi brous nature that allows upholding the distinctively asymmetrical shape of neurons. Whereas the main role of NF is to increase the axonal caliber of myelinated axons, and consequently the velocity of transmission of electrical impulses, they also contribute to the dynamic properties of the axonal cytoskeleton during neuronal differentiation, axon outgrowth (where NFL and NFM subunits are especially important), regeneration and guidance (Perrot et al., 2008; Yuan et al., 2012).
Before the arrival of the electronic microscope, MT could only be described as part of the mitotic spindle and cytoplasm. At present, it has been found that MT are hollow tubes with an approximate diameter of 25 nm and characteristically assembled from 13 laterally associating protof i laments of αβ-tubulin heterodimers.ey constantly alternate between rapid phases of dynamic instability: growth (known as “rescue”) and shrinkage (named “catastrophe”). As a result, MT cytoskeleton remains suitable for swily remodeling according to intracellular cues (Menon and Gupton, 2016; van de Willige et al., 2016).
Evidence in different eukaryotic cells has reported that MT cytoskeleton serves as a primary spatial regulator of cell shape. Its high dynamic properties compel to interact with actin in areas of cellular growth or reorganization during cell division, polarization and migration. With no exception, neurons depend on MT to determine their development for their distinctive morphology (Kaech et al., 2001; Jaworski et al., 2008).
Since early migration stages from the ventricular zone into more remote regions, MT are indispensable to provide the track path. However, ever since mature neurons must remain plastic as a response to their continuous rewiring connections, this development also depends on both the MT and MF, their crosstalk and accessory proteins (van de Willige et al., 2016).
A mature neuron comprises several structures, including the axon, which transmits and propagates electric stimuli, and its dendrites, the receivers of the input from other neurons. From the very beginning, upon the stage of dif f erentiation, neurite formation (protrusions which later become axons and dendrites) is yet powered by MT sliding. As soon as the axon is newly formed, it also relies on stable MT tracks for the transport of proteins, vesicles and organelles necessary for the formation of new axonal segments (van de Willige et al., 2016).
MTs also play a key role when participating in DNA segregation during mitosis, cell migration and maintenance of cell polarity; therefore, they are involved in neuronal plasticity (van de Willige et al., 2016; Brandt and Bakota, 2017).
In combination, MF and MT work to guide and support the growth and differentiation of axons and dendrites. Though dynamic actin filaments, they lead the course of growth cones and MT stabilize the structure of the new process. The axon outgrowth direction is defined by the growth cone, a specialized cytoskeletal-based motile structure, which responds to extracellular cues to guide the axon toward postsynaptic partners. Despite the fact that dynamic MT play only a minor role in neurite outgrowth, their role is crucial for axon polarization, pathf i nding and branching (Kaech et al., 2001; Menon and Gupton, 2016; van de Willige et al., 2016).
The ultimate elements of the cellular cytoskeleton to be endorsed are MF. It should be reminded that actin is one of the most prominent proteins in neurons as well as in muscle in cells.e main components of the actin skeleton are bundles and networks of fi lamentous actin (F-actin). Moreover, actin is also present as a monomer (G-actin; globular actin) in living cells. In vivo, G-actin-binding proteins (e.g., thymosin β4) are responsible for assembly/disassembly and higher-order organization of actin fi laments, isolate G-actin and prevent the assembly of G-actin into F-actin. Additionally, G-actin/F-actin equilibrium in dendritic spines also depends on the presence of a sequestered G-actin pool, which allows site-directed F-actin polymerization in response to synaptic activity (Sekino et al., 2007).
An actin filament is a double helix of actin protomers decorated with binding proteins. A cohort of actin-binding proteins determines the particular organization of F-actin, either in bundles or networks, providing each F-actin to have unique physical and biochemical properties according to its binding proteins. In neurons, F-actin networks are found in spine heads whereas straight bundles are present in spine necks. However, the subcellular localization of actin-binding proteins can be changed by extracellular stimulation. Many actin-binding proteins identified in dendritic spines, such as Arp2/3, cortactin, ADF/cof i lin, prof i lin, gelsolin, drebrin and neurabin (Sekino et al., 2007; Shirao and González-Billault, 2013) have been described.
Actin fi laments possess polarity. Also, actin fi laments keep treadmilling reaction when their ends are not covered by actin-capping proteins, which perform as fi ne regulators of actin polymerization. According to the treadmilling reaction, actin protomers are continuously polymerized at the barbed end and depolymerized at the pointed end. As some studies suggest, treadmilling reaction of actin filament occurs in dendritic spines; however, it does not generate the force to change spine morphology (Shirao and González-Billault, 2013).
A comprehensive approach to cytoskeletal proteins allows deepening knowledge beyond form and structure of cells. Particularly in neurons, these proteins have associated and specific functions merging on the cytoskeleton, which enable a model for both physiological and pathological cellular conditions from which pharmacological tests might develop.
Ef f ect of ERMs on Neuronal Microf i laments
NFs or intermediate filaments are the most resistant elements of the cytoskeleton. NFs are heteropolymers, which are abundant in neuronal axons, with extremely elastic fibrous properties that help to maintain the asymmetrical shape of the neuronal cell and regulate the axon diameter and growth (Yuan et al., 2012). NFs are composed of three distinct polypeptides with a molecular weight of 200, 160 and 68 kDa.
Brain injury produces reactive gliosis (Williams et al., 2006), causing a glial scar to avoid the propagation of inf l ammation and damage. Astrocytes and NG2 cells participate in glial scar formation (Alonso, 2005).
A reactive phenotype characterized by a series of morphological and molecular modifications, including the expression of the cytoskeletal protein vimentin, is acquired by astrocytes.
In the rat brain, tamoxifen exhibited an antagonist action on ER (Zhao et al., 2005). Furthermore, it had a signif i cant effect on reducing reactive astrocytes after brain injury (Barreto et al., 2009) and reduced the increased number of astrocytes, perhaps as a consequence of emigration from the injury zone or death (Arevalo et al., 2012). Tamoxifen could favor brain repair by promoting neuron survival, adjusting glial cell number and recover adequate neural communication (Franco-Rodríguez et al., 2013).
Other SERMs, like bazedoxifene, tamoxifen and raloxifene, present different dose-dependent neuroprotective effects. In the hippocampus of adult ovariectomized rats, the administration of kainic acid induced the expression of vimentin in reactive astroglia and a signif i cant neuronal loss in the hilus. At dif f erent optimal doses, bazedoxifene, tamoxifen and raloxifene prevented neuronal loss. These SERMs may act through dif f erent neuroprotective mechanisms. Despite the fact that they were unable to reduce reactive gliosis, these molecules prevented neuronal loss in the hippocampus aer kainic acid excitotoxicity (Ciriza et al., 2004).
In another study, young and aged ovariectomized rats received a stab wound brain injury before the treatment with estradiol, raloxifene or tamoxifen. The results showed that reactive astrogliosis was reduced in all animal groups, including controls.ese fi ndings indicate that SERMs are potential candidates for the control of astrogliosis in individuals and aer a prolonged depletion of ovarian hormones (Barreto et al., 2014).e Effects on astrogliosis could be attributed to the different doses administered, the model used to induce the injury or the age of the animals used in the study.
Tight junction proteins (TJs) are connected to the cortical actin cytoskeletonviamulti-domain scaffolding proteins of the peripheral membrane-associated guanylate kinase (MAGUK) family. ZO-1 is not a transmembrane protein but a cytoplasmic TJ-associated protein, which can determine whether and where claudins are polymerized in an independent manner (Umeda et al., 2006). Claudins are members of a family of transmembrane proteins, which establish the structural and functional features of TJs with tissue-specif i c expression. ZO-1 def i ciency disrupts TJs, and reduced ZO-1 levels are associated with barrier breakdown in many neurological disorders (Katsuno et al., 2008).e immunostaining for ZO-1 in the brain cortex and hippocampus of ovariectomized rats administered with vehicle, tibolone or 17β-estradiol (E2) revealed similar staining patterns. In this study, the authors also evaluated GFAP expression.e staining of GFAP was more intense in tibolone and E2 groups than in the control group (Ceylan et al., 2012).ese results showed that some cytoskeleton-associated proteins are regulated by ERMs, and other are less responsive.
Ef f ect of ERMs on Neuronal Intermediate Filaments
MF are formed by actin filaments. Their polymerization dynamics are associated with the activity of actin-binding proteins like drebrin and the ADF/cof i lin. Drebrin is a protein located in the dendritic spines of the neuron that plays a role in the synaptic plasticity together with actin fi laments. Drebrin binds to and organizes filamentous actin (F-actin) in dendritic spines, the receptive regions of most excitatory synapses that play a crucial role in higher brain functions (Kojima and Shirao, 2007). Moreover, the ADF/cof i lin family comprises small actin-binding proteins that enhance actin dynamics in three ways: by depolymerization (accelerating monomer loss at the pointed end), by severing fi laments into shorter protomers and by directly or indirectly facilitating actin fi lament growth (Bernstein and Bamburg, 2010). ADF and cof i lin-1 are both expressed in the mammalian brain.e genetic deletion of cof i lin in the nervous system reduces neuronal cell proliferation and migration but not neurite formation (Bellenchi et al., 2007). Moreover, the genetic ablation of ADF affects neither the development of the nervous system nor the formation of neurites in particular (Bellenchi et al., 2007).erefore, MF regulate the function of synapses, axonal cone growth and protein traf fi cking (Disanza et al., 2005).
The cytoskeletal rearrangements are controlled by the Rho family of GTPases, which regulate the activity of diverse cytoskeleton-associated proteins such as actin-binding proteins (Gonzalez-Billault et al., 2012).e process of cytoskeleton remodeling including the formation of new MF and their interaction with the plasma membrane depends on the participation of diverse actin-binding proteins (Kim et al., 2006). MF can be modulated by hormones (Arevalo et al., 2010; Ferri et al., 2014). However, to the extent of our knowledge, the effect of MF regulation by SERMs has not been described.
Ef f ect of ERMs on Neuronal Microtubules and Microtubule-Associated Proteins
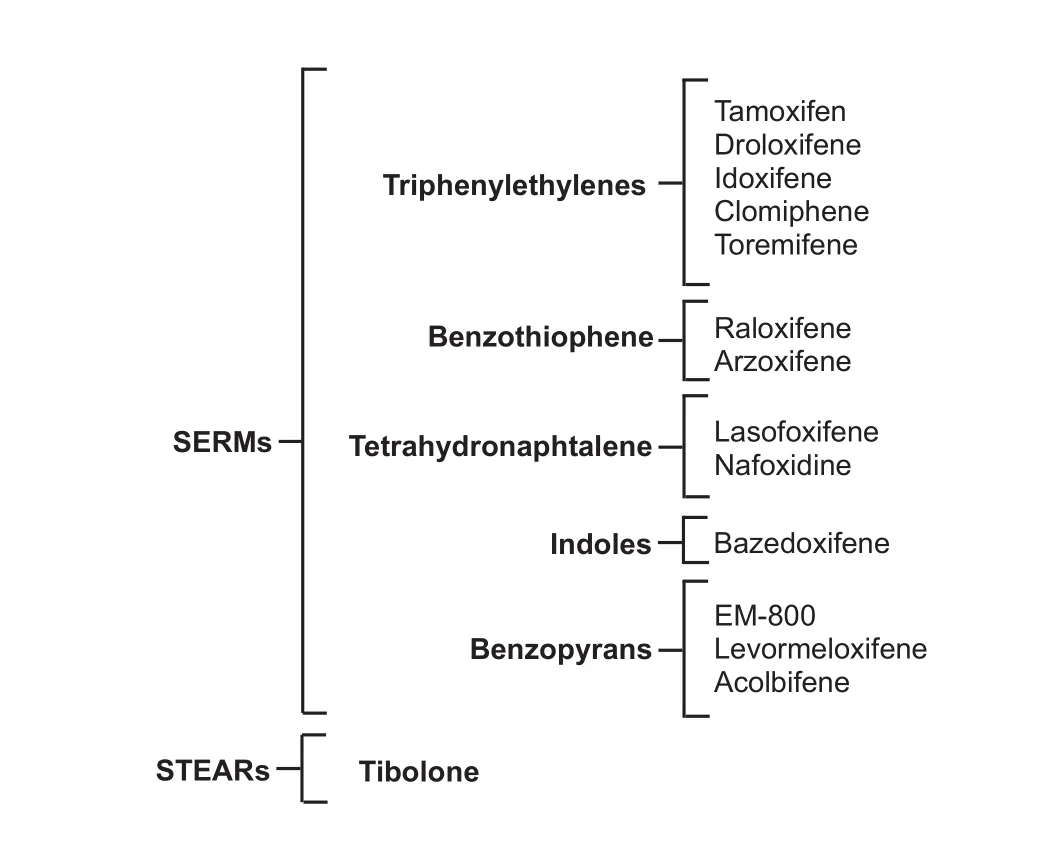
Figure 1 Classif i cation of ERMs.
Microtubules (MT) are highly dynamic polymers of α and β tubulin essential for the growth and maintenance of the shape, movement, signaling and reproduction of cells. Polymerization is indispensable for MT functions, allowing their reorganization depending on the cell necessities (Jordan, 2002). MT nucleation is the process by which soluble αβ-tubulin subunits are arranged parallel to a cylindrical axis and are converted into a growing MT that may be as long as millimeters (Jordan, 2002; Wieczorek et al., 2015). MT arrays in axons and dendrites are necessary for both assembly and transport properties of these neurites (Conde and Cáceres, 2009).
Kinetics of MT assembly and disassembly dynamics can be influenced by non-enzymatic proteins called microtubule-associated proteins (MAPs), being MAP1B and tau the first proteins implicated (Conde and Cáceres, 2009; Wieczorek et al., 2015).
Structural MAPs belong to four dif f erent families: MAP1, MAP2, MAP4 and tau proteins that are dif f erent in type and structure. MAP1, MAP2 and tau are the most important in neurons. The MAP1 family is formed by MAP1A, MAP1B and MAP1S; MAP1A and MAP1B have a key role in the stabilization, guidance and function of axons. MAP1S is important for the regulation of cell division; its expression in neurons is lower when compared with MAP1A and B. MAP1B is essential for the development and maturing of dendritic spines (Conde and Cáceres, 2009; Mohan and John, 2015).
MAP2a, MAP2b, MAP2c and MAP2d, members of the MAP2 family, are formed by alternate splicing. In neurons, the most abundant proteins are those from MAP2 family.ey are part of axons and dendrites in initial stages of neurodevelopment but limited only to dendrites in adults. In addition, MAP2 have been associated with actin in the development of axons and the inside of dendritic spines (Conde and Cáceres, 2009; Mohan and John, 2015).
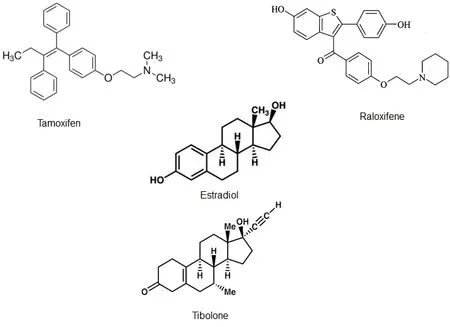
Figure 2 Chemical structure of some estrogen receptor modulators (ERMs) and Effects on cytoskeletal proteins.
Tau family is formed of six dif f erent isoforms produced by alternative splicing and post-translational modif i cations.e length of each isoform depends on the number of repeats (3 or 4) of the microtubule-binding domain at their C-terminus and the number of N-terminal inserts (0-2). Tau proteins are associated with establishing neuronal polarity and axon elongation by controlling the assembly and stabilization of neuronal MT allowing the regulation of intracellular transport, which in turn plays a critical role during myelin formation (Conde and Cáceres, 2009; LoPresti, 2015; Mohan and John, 2015).
The expression of MAPs is differentiated in neurons. MAP1A expression is localized in dendrites and mainly expressed in adult neurons. MAP1B, MAP2c and tau could be differentially found in axons. MAP1B and MAP2c are predominantly expressed in embryonic and neonatal brains. MAP2a, MAP2b and MAP2d are expressed only in adult neuronal cells specif i cally in the soma and dendrites (Mohan and John, 2015). Therefore, MAP2 antibodies are excellent markers in neurons (Zhang et al., 2001; Conde and Cáceres, 2009).
Zhang et al. (2001) studied the mechanisms underlying the neuroprotective effects of estrogens in a neurotoxic β-amyloid peptide model. In this model, Aβ31–35signif i cantly decreased the number of neurons. Furthermore, it was demonstrated that the total number of MAP2 positive cells (MAP2+) decreased. TMX was used for its estrogen receptor antagonist characteristics, abolishing the neuroprotective Effects of E2. However, the total number of MAP2+cells did not change with TMX treatment. Therefore, according to these results, TMX had no Effects on the expression or con-tent of MAP2 (Zhang et al., 2001).
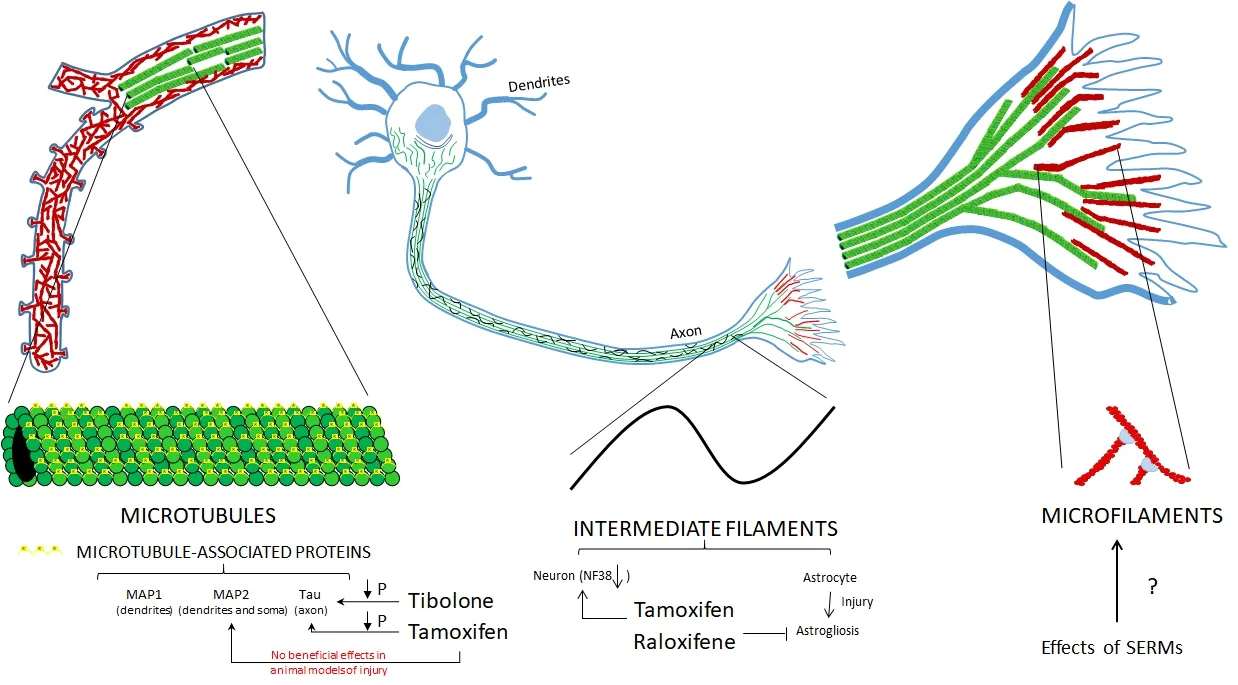
Figure 3 Ef f ect of estrogen receptor modulators (ERMs) on cytoskeletal proteins at dif f erent levels of organization.
Haynes et al. (2003) studied the Effects of E2 in the overall neuronal damage produced by dexamethasone.e authors evaluated the levels of MAP2+in the striatum and hippocampus. They reported that TMX pretreatment prevented estrogen neuroprotection given that they observed similar results in the MAP-2 scores between both vehicle/vehicle/ dexamethasone and TMX/estrogen/dexamethasone groups. Interestingly, the same damage produced by dexamethasone alone (reduction of MAP2+neurons) in the hippocampus was observed for the TMX/vehicle/dexamethasone group; moreover, the damage was increased in the striatum (Haynes et al., 2003).
At the present day, only the Effects of TMX treatment on MAPs have been studied.e results on animal models have shown that TMX does not exert neuroprotective Effects and even increases the damage produced by dexamethasone.
Modulation of Cytoskeletal Proteins by ERMs in Neurodegenerative Diseases
MT can display multiple functions due to their fl exibility. Tubulin assembly can accomplish several functions depending on their binding partners, generating different physiological or pathological microtubule structures (Oláh et al., 2013).
Some neurodegenerative diseases known as tauopathies present a pathological aggregation of tau called neurof i brillary tangles. Abnormal phosphorylation of tau decreases its capability for stabilizing microtubules, generating cytoskeleton destabilization and perturbation of axonal transport. In af f ected neurons, hyperphosphorylation of tau followed by neurof i brillary tangles, which aggregate into paired helical filaments (PHFs), have been reported (Alvarez-de-la-Rosa et al., 2005; Pinto-Almazán et al., 2012; Corbel et al., 2015).
Several studies have been designed with the idea of using sex hormones and analogs as therapeutic strategies for tauopathies and dementia. Neuroprotective effects of ERMs have been evaluated on tau phosphorylation, and also their role as ER antagonists (Alvarez-de-la-Rosa et al., 2005; Pinto-Almazán et al., 2012; Corbel et al., 2015; Lo-Presti, 2015).
Corbel et al. (2015) screened over 1,760 compounds that could inhibit the activity of CDK5, one of the major tau kinases, from which they identif i ed TMX as a prospect.ey performed BRET-based screening assays, cellular and western blots studies and determined that TMX inhibits CDK5/ p25 protein-protein interaction by preventing the increase of CDK5 kinase activity and the increase of tau phosphorylation produced by glutamate.
In concordance with these fi ndings, Alvarez-de-la-Rosa et al. (2005) treated neuroblastoma and female neuronal cells for 24 hours with E2 or E2 + TMX to study the Effects of E2 on tau phosphorylation. Western blot analyses were performed to analyze tau dephosphorylation (Tau-1 epitope), tau hyper-phosphorylation (12E8Site) and total tau.e treatments with E2 alone decreased okadaic acid-induced tau hyperphosphorylation (12E8Site) and increased tau dephosphorylation (Tau-1 epitope) and total tau expression. These E2 effects were blocked with E2 + TMX treatment. Therefore, TMX alone demonstrated to increase Tau-1 and total tau expression without an ef f ect on 12E8Site epitope.
As the studies performed with TMX, the Effects of tibolone on tau phosphorylation also have been evaluated. Pinto-Almazán et al. (2012) reported that chronic treatment with 0.5 mg/kg of tibolone decreased tau hyperphosphorylation, increased tau dephosphorylation and correlated with an increased phosphorylation of GSK3 in Ser9, which is the inactive form of this kinase in the hippocampus and cerebellum of ovariectomized adult rats.
Interestingly, other pharmacological properties of TMX have helped in the understanding of the pathophysiological process of tauopathies and dementia (LoPresti, 2015; Wang et al., 2015). Wang et al. (2015) produced Akt cTKO mice to understand the pathological process of tauopathies and the importance of Akt contribution. First, they created viable Akt1f/f; Akt2−/−; Akt3−/−; CAG-CreER mice that became Akt cTKO aer the treatment with TMX. In this novel Akt cTKO mouse model, tau hyperphosphorylation was reported without signif i cant changes in the total number of TUNEL+cells or NeuN+cells and also unchanged levels of GSK3β, CDK5, ERK and p38 in their active forms.
Furthermore, LoPresti designed a truncated tau (ΔTau) inducible expression model in oligodendrocytes (OLGs) for studying the Effects of this non-microtubule-associated tau in neurodegenerative diseases. She generated a Floxed LacZ-STOP/EGFP–ΔTau founder transgenic mice, which expressed EGFP-ΔTau and Cre transgenes.e Cre recombinases system activated by administration of TMX has been an important tool for inducingin vivogene activity in space- and time-dependent manner. In this study, she injected TMX to the 12-day-old offspring mice for three days and observed gait abnormalities, such as stumbles and loss of balance, in the p18 mice, which were correlated with myelin decrease.
Unlike other studies on MAPs, not only the Effects of TMX but also the Effects of TIB on tau protein have been evaluated. Both treatments demonstrated their neuroprotective effects by increasing the dephosphorylated form of tau (Tau-1). Moreover, other pharmacological properties of TMX have been used to produce different models of neurodegeneration, useful for a better understanding of the mechanisms of tauopathies.
Perspectives
Some ERMs have an ef f ect on the modulation of cytoskeletal proteins (Figure 2) through several mechanisms, mainly on filaments and MAPs (Figure 3). Because of the diverse functions of these proteins, ERMs can modulate cell motility, astrocyte proliferation, the integrity of the blood-brain barrier, myelination and neuronal plasticity. Hence, they can exert a neuroprotective ef f ect in dif f erent models of neuronal damage, as well as in neurodegenerative diseases such as AD in which these proteins participate.
An important aspect to be considered in the observed Effects of ERMs is the route of administration.roughout the review, it was observed that several routes of administration of the ERMs were used in the dif f erent studies, which included intraperitoneal, esophageal, ICV and the addition to the means of culture in the case of cell lines. It was also observed that depending on the route of administration the administered dose varied because through both intraperitoneal and esophageal routes ERMs can be metabolized in the liver. Also, a higher concentration was required to cross the blood-brain barrier and observe an ef f ect in the CNS. In contrast, ICV administration and drug addition to cell cultures had a more direct and powerful action. For this reason, a smaller dose was required to observe an ef f ect. Hence, it is important to carry out more studies in which the Effects of dif f erent doses of ERMs are evaluated, as well as the routes of administration used.
Finally, it is necessary to continue the search for an ideal ERM that has all the benef i ts of estrogens, even the neuroprotective Effects, but without the risk of side Effects, such as breast or endometrial cancer and cardiovascular risks and stroke.
Author contributions:CGA, RPA, JJSU conceptualized and designed the study; RPA, CEFV acquired the data; RPA, JJSU, ACS analyzed and interpreted the data. CGA, RPA, JJSU, CEFV and ACS draed and revised the manuscript critically for important intellectual content; All authors have read and approved the fi nal version of the manuscript to be published.
Conf l icts of interest:None declared.
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Open peer review reports:
Reviewer 1:Sarah L. Ferri, University of Iowa, USA.
Comments to authors:
Reviewer 2:Arash Abdolmaleki, Ferdowsi University of Mashhad, Iran.
Abdelhamid R, Luo J, Vandevrede L, Kundu I, Michalsen B, Litosh VA, Schiefer IT, Gherezghiher T, Yao P, Qin Z,atcher GR (2011) Benzothiophene selective estrogen receptor modulators provide neuroprotection by a novel GPR30-dependent mechanism. ACS Chem Neurosci 2:256-268.
Alonso G (2005) NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia 49:318-338.
Alvarez-de-la-Rosa M, Silva I, Nilsen J, Pérez MM, García-Segura LM, Ávila J, Naolin F (2005) Estradiol prevents neural Tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann N Y Acad Sci 1052:210-224.
An KC (2016) Selective estrogen receptor modulators. Asian Spine J 10:787-791.
Arevalo MA, Diz-Chaves Y, Santos-Galindo M, Bellini MJ, García-Segura LM (2012) Selective oestrogen receptor modulators decrease the inf l ammatory response of glial cells. J Neuroendocrinol 24:183-190.
Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM (2010) Actions of estrogens on glial cells: Implications for neuroprotection. Biochim Biophys Acta 1800:1106-1112.
Barreto G, Santos-Galindo M, Diz-Chaves Y, Pernia O, Carrero P, Azcoitia I, García-Segura LM (2009) Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: Effects of aging and prolonged depletion of ovarian hormones. Endocrinology 150:5010-5015.
Barreto GE, Santos-Galindo M, Garcia-Segura LM (2014) Selective estrogen receptor modulators regulate reactive microglia aer penetrating brain injury. Front Aging Neurosci 20:132.
Bellenchi GC, Gurniak CB, Perlas E, Middei S, Ammassari-Teule M, Witke W (2007) N-cofilin is associated with neuronal migration disorders and cell cycle control in the cerebral cortex. Genes Dev 21:2347-2357.
Brandt R, Bakota L (2017) Microtubule dynamics and the neurodegenerative triad of Alzheimer’s disease: the hidden connection. J Neurochem doi:10.1111/jnc.14011.
Ceylan U, Akhan SE, Bastu E, Gungor-Ugurlucan F, Iyibozkurt AC, Topuz S (2012) Comparison of tibolone and 17beta-estradiol administration on the expression of zonula occludens-1, occludin, glial fi brillary acidic protein and c-Fos levels in the brain cortex and hippocampus of female rats. Neurol Endocrinol Lett 33:505-510.
Ciriza I, Carrero P, Azcoitia I, Lundeen SG, García-Segura LM (2004) Selective estrogen receptor modulators protect hippocampal neurons from kainic acid excitotoxicity: dif f erences with the ef f ect of estradiol. J Neurobiol 61:209-221.
Conde C, Cáceres A (2009) Microtubule assembly, organization and dynamics in axons and dendrites. Nat Rev Neurosci 10:319-332.
Corbel C, Zhang B, Le Parc A, Baratte B, Colas P, Couturier C, Kosik KS, Landrieu I, Le Tilly V, Bach S (2015) Tamoxifen inhibits CDK5 kinase activity by interacting with p35/p25 and modulates the pattern of tau phosphorylation. Chem Biol 22:472-482.
Cyr M, Calon F, Morissette M, Garndbois M, Callier S, Di Paolo T (2000) Drugs with estrogen-like potency and brain activity: potential therapeutic applications for the CNS. Curr Pharm Des 6:1287-1312.
Day JR, Laping NJ, Lampert-Etchells M, Brown SA, O’Callaghan JP, McNeill TH, Finch CE (1993) Gonadal steroids regulate the expression of glial fi brillary acidic protein in the adult male rat hippocampus. Neuroscience 55:435-443.
Diez-Perez A (2006) Selective estrogen receptor modulators (SERMS). Arq Bras Endocrinol Metab 50:720-734.
Disanza A, Stef f en A, Hertzog M, Frittoli E, Rottner K, Scita G (2005) Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement. Cell Mol Life Sci 62:955-970.
Dutertre M, Smith CL (2000) Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther 295:431-437.
Eddleston M, Mucke L (1993) Molecular prof i le of reactive astrocytes–implications for their role in neurologic disease. Neuroscience 54:15-36.
Elobeid A, Bongcam-Rudlof f E, Westermark B, Nister M (2000) Effects of inducible glial fi brillary acidic protein on glioma cell motility and proliferation. J Neurosci Res 60:245-256.
Farooq A (2015) Structural and functional diversity of estrogen receptor ligands. Curr Top Med Chem 15:1372-1384.
Farfán-García ED, Castillo-Hernández MC, Pinto-Almazán R, Rivas-Arancibia S, Gallardo JM, Guerra-Araiza C (2014) Tibolone prevents oxidation and ameliorates cholinergic def i cit induced by ozone exposure in the male rat hippocampus. Neurochem Res 39:1776-1786.
Farzaneh S, Zarghi A (2016) Estrogen receptor ligands: a review (2013-2015). Sci Pharm 84:409-427.
Ferri SL, Hildebrand PF, Way SE, Flanagan-Cato LM (2014) Estradiol regulates markers of synaptic plasticity in the hypothalamic ventromedial nucleus and amygdala of female rats. Horm Behav 66:409-420.
Fiordelisio T, Hernández-Cruz A (2002) Oestrogen regulates neurof i lament expression in a subset of anterior pituitary cells of the adult female rat. J Neuroendocrinol 14:411-424.
Fracy AJM, van de Klundert JMH, Bloemendal H (1993) Intermediate fi laments: regulation of gene expression and assembly. Eur J Biochem 214:351-366.
Franco-Rodríguez NE, Dueñas-Jiménez JM, De la Torre-Valdovinosa B, López-Ruiza JR, Hernández-Hernández L, Dueñas-Jiménez SH (2013) Tamoxifen favoured the rat sensorial cortex regeneration aer a penetrating brain injury. Brain Res Bull 98:64-75.
Genazzani AR, Pluchino N, Bernardi F, Centofani M, Luisi M (2006) Benef i cial ef f ect of tibolone on mood, cognition, well-being, and sexuality in menopausal women. Neuropsychiatr Dis Treat 2:299-307.
Goldstein SR, Siddhanti S, Ciaccia AV, Plouf f e Jr L (2000) A pharmaceutical review of selective oestrogen receptor modulators. Hum Reprod Update 6:212-224.
Gonzalez-Billault C, Muñoz-Llancao P, Henriquez DR, Wojnacki J, Conde C, Caceres A (2012)e role of small GTPases in neuronal morphogenesis and polarity. Cytoskeleton (Hoboken) 69:464-485.
Green PS, Bishop J, Simpkins JW (1997) 17α-Estradiol exerts neuroprotective Effects on SK-N-SH cells. J Neurosci 17:511-515.
Hansberg-Pastor V, González-Arenas A, Piña-Medina AG, Camacho-Arroyo I (2015) Sex hormones regulate cytoskeletal proteins involved in brain plasticity. Front Psychiatry 6:165.
Haynes LE, Lendon CL, Barber DJ, Mitchell IJ (2003) 17 Beta-oestradiol attenuates dexamethasone-induced lethal and sublethal neuronal damage in the striatum and hippocampus. Neuroscience 120:799-806.
Jaworski J, Kapitein LC, Gouveia SM, Dortland BR, Wulf PS, Grigoriev I, Camera P, Spangler SA, Di Stefano P, Demmers J, Krugers H, Def i lippi P, Akhmanova A, Hoogenraad CC (2009) Dynamic microtubules regulate dendritic spine morphology and synaptic plasticity. Neuron 61:85-100.
Jordan MA (2002). Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2:1-17.
Julien JP, Grosveld F (1991) Structure and expression of neurof i lament genes. In:e Neuronal Cytoskeleton (Burgoyne R, ed), pp215-231. New York: Wiley-Liss.
Kaech S, Parmar H, Roelandse M, Bornmann C, Matus A (2001) Cytoskeletal microdifferentiation: a mechanism for organizing morphological plasticity in dendrites. Proc Natl Acad Sci U S A 98:7086-7092.
Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima Y, Noda T, Tsukita S (2008) Def i ciency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell 19:2465-2475.
Khan MM, Wakade C, de Sevilla L, Brann DW (2015) Selective estrogen modulators (SERMs) enhance neurogenesis and spine density following focal cerebral ischemia. J Steroid Biochem Mol Biol 146:38-47.
Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho Ryu S, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P (2006) Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442:814-817.
Kloosterboer HJ (2004) Tissue-selectivity: the mechanism of action of tibolone. Maturitas 48:S30-S40.
Kojima N, Shirao T (2007) Synaptic dysfunction and disruption of postsynaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive def i cits. Neurosci Res 58:1-5.
Kokiko ON, Murashov AK, Hoane MR (2006) Administration of raloxifene reduces sensorimotor and working memory def i cits following traumatic brain injury. Behav Brain Res 170:233-240.
Kulak Júnior J, Kulak CA, Taylor HS (2010) SERMs in the prevention and treatment of postmenopausal osteoporosis: an update. Arq Bras Endocrinol Metabol 54:200-205.
Liedtke W, Edelmann W, Bieri PL, Chiu FC, Cowan NJ, Kucherlapati R, Raine CS (1996) GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron 17:607-615.
Littleton-Kearney MT, Ostrowski NL, Cox DA, Rossberg MI, Hurn PD (2002) Selective estrogen receptor modulators: tissue actions and potential for CNS protection. CNS Drug Rev 8:309-330.
LoPresti P (2015) Inducible expression of a truncated form of Tau in oligodendrocytes elicits gait abnormalities and a decrease in myelin: implications for selective CNS degenerative diseases. Neurochem Res 40:2188-2199.
Maximov PY, Lee TM, Jordan VC (2013)e discovery and development of selective estrogen receptor modulators (SERMs) for clinical practice. Curr Clin Pharmacol 8:135-155.
Menon S, Gupton SL (2016) Building blocks of functioning brain: cytoskeletal dynamics in neuronal development. Int Rev Cell Mol Biol 322:183-245.
Mohan R, John A (2015) Microtubule-associated proteins as direct crosslinkers of actin fi laments and microtubules. IUBMB Life 67:395-403.
Morissette M, Al Sweidi S, Callier S, Di Paolo T (2008) Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol 290:60-69
Navarro-Despaigne DA (2001) Selective estrogen receptor modulators: their benefit in postmenopausal women. Rev Cubana Endocrinol 12:124-127.
Neri-Gómez T, Espinosa-Raya J, Díaz-Cintra S, Segura-Uribe J, Orozco-Suárez S, Gallardo JM, Guerra-Araiza C (2017) Tibolone modulates neural plasticity through regulating Tau, GSK3β/Akt/PI3K pathway and CDK5 p35/p25 complexes in the hippocampus of aged male mice. Neural Regen Res 12:588-595.
Oláh J, Tőkési N, Lehotzky A, Orosz F, Ovádi J (2013) Moonlighting microtubule-associated proteins: regulatory functions by day and pathological functions at night. Cytoskeleton (Hoboken) 70:677-685.
Osborne CK, Zhao H, Fuqua SA (2000) Selective estrogen receptor modulators: structure, function, and clinical use. J Clin Oncol 18:3172-3186.
Otani N, Nawashiro H, Fukui S, Ooigawa H, Ohsumi A, Toyooka T, Shima K, Gomi H, Brenner M (2006) Enhanced hippocampal neurodegeneration aer traumatic or kainate excitotoxicity in GFAP-null mice. J Clin Neurosci 13:934-938.
Pérez-Edo L (2004) Selective estrogen receotor modulators (SERM). Rev Esp Reumatol 31:13-17.
Perrot R, Berges R, Bocquet A, Eyer J (2008) Review of the multiple aspects of neurof i lament functions, and their possible contribution to neurodegeneration. Mol Neurobiol 38:27-65.
Pinkerton JV, Thomas S (2014) Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 142:142-154.
Pinto-Almazán R, Calzada-Mendoza CC, Campos-Lara MG, Guerra-Araiza C (2012) Effect of chronic administration of estradiol, progesterone, and tibolone on the expression and phosphorylation of glycogen synthase kinase- 3β and the microtubule-associated protein tau in the hippocampus and cerebellum of female rat. J Neurosci Res 90:878-886.
Pinto-Almazán R, Rivas-Arancibia S, Farfán-García ED, Rodríguez-Martínez E, Guerra-Araiza C (2014) Neuroprotective effects of tibolone against oxidative stress induced by ozone exposure. Rev Neurol 58:441-448.
Pinto-Almazán R, Segura-Uribe J, Farfán-García ED, Guerra-Araiza C (2017) Effects of tibolone on the central nervous system: clinical and experimental approaches. Biomed Res Int 2017:8630764.
Pollard TD, Borisy GG (2003) Cellular motility driven by assembly and disassembly of actin fi laments. Cell 112:453-465.
Reed MJ, Kloosterboer HJ (2004) Tibolone: a selective tissue estrogenic activity regulator (STEAR). Maturitas 48:S4-S6.
Sekino Y, Kojima N, Shirao T (2007) Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem Int 51:92-104.
Shirao T, González-Billault C (2013) Actin fi laments and microtubules in dendritic spines. J Neurochem 126:155-164.
Terán-Dávila J, Teppa-Garrán AJ (2005) Selective estrogen receptor modulators (SERMs): biochemistry, pharmacology and clinical application in gynecology. Ginecol Obstet Mex 73:424-435.
Toda M, Miura M, Asou H, Sugiyama I, Kawase T, Uyemura K (1999). Suppression of glial tumor growth by expression of glial fibrillary acidic protein. Neurochem Res 24:339-343.
Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M (2006) ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell 126:741-754.
van de Willige D, Hoogenraad CC, Akhmanova A (2016) Microtubule plus-end tracking proteins in neuronal development. Cell Mol Life Sci 73:2053-2077.
Velisek L, Nebieridze N, Chachua T, Veliskova J (2013) Anti-seizure medications and estradiol for neuroprotection in epilepsy: the 2013 update. Recent Pat CNS Drug Discov 8:24-41.
Wang L, Cheng S, Yin Z, Xu C, Lu S, Hou J, Yu T, Zhu X, Zou X, Peng Y, Xu Y, Yang Z, Chen G (2015) Conditional inactivation of Akt three isoforms causes tau hyperphosphorylation in the brain. Mol Neurodegener 10:33.
Wieczorek M, Bechstedt S, Chaaban S, Brouhard GJ (2015) Microtubule-associated proteins control the kinetics of microtubule nucleation. Nat Cell Biol 17:913-916.
Williams AJ, Hartings JA, Lu XC, Rolli ML, Tortella FC (2006) Penetrating ballistic-like brain injury in the rat: dif f erential time courses of hemorrhage, cell death, inf l ammation, and remote degeneration. J Neurotrauma 23:1828-1846.
Yu B, Dietz BM, Dunlap T, Kastrati I, Lantvit DD, Overk CR, Yao P, Qin Z, Bolton JL,atcher GR (2007) Structural modulation of reactivity/activity in design of improved benzothiophene selective estrogen receptor modulators: induction of chemopreventive mechanisms. Mol Cancerer 6:2418-2428.
Yuan A, Rao MV, Veeranna, Nixon RA (2012) Neurofilaments at a glance. J Cell Sci 125:3257-3263.
Zhang L, Rubinow DR, Xaing G, Li B, Chang YH, Maric D, Barker JL, Ma W (2001) Estrogen protects against β-amyloid-induced neurotoxicity in rat hippocampal neurons by activation of Akt. NeuroReport 12:1919-1923.
Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK. (2005) Behavioral and histological neuroprotection by tamoxifen aer reversible focal cerebral ischemia. Exp Neurol 196:41-46.
Zhao L, O’Neill K, Diaz Brinton R (2005) Selective estrogen receptor modulators (SERMs) for the brain: current status and remaining challenges for developing NeuroSERMs. Brain Res Brain Res Rev 49:472-493.
*< class="emphasis_italic">Correspondence to: Christian Guerra-Araiza, Ph.D., christianguerra2001@gmail.com.
Christian Guerra-Araiza, Ph.D., christianguerra2001@gmail.com.
orcid: 0000-0002-7164-4116 (Christian Guerra-Araiza)
10.4103/1673-5374.213536
2017-07-17
- 中国神经再生研究(英文版)的其它文章
- LINGO-1 and AMIGO3, potential therapeutic targets for neurological and dysmyelinating disorders?
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Transcriptional inhibition in Schwann cell development and nerve regeneration
- Optogenetics and its application in neural degeneration and regeneration
- Live-cell imaging: new avenues to investigate retinal regeneration
- Neurotrophic factors and corneal nerve regeneration