Cortical activation pattern during shoulder simple versus vibration exercises: a functional near infrared spectroscopy study
Sung Ho Jang, Sang Seok Yeo, Seung Hyun Lee, Sang Hyun Jin, Mi Young Lee
1 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea
2 Department of Physicalerapy, College of Health Science, Dankook University, Cheonan-si, Republic of Korea
3 Robot System Research Division, Daegu Gyeongbuk Institute of Science & Technology, Daegu, Republic of Korea
4 Department of Physicalerapy, College of Health anderapy, Daegu Haany University, Gyeongsan-si, Republic of Korea
Cortical activation pattern during shoulder simple versus vibration exercises: a functional near infrared spectroscopy study
Sung Ho Jang1, Sang Seok Yeo2, Seung Hyun Lee3, Sang Hyun Jin3, Mi Young Lee4,*
1 Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Republic of Korea
2 Department of Physicalerapy, College of Health Science, Dankook University, Cheonan-si, Republic of Korea
3 Robot System Research Division, Daegu Gyeongbuk Institute of Science & Technology, Daegu, Republic of Korea
4 Department of Physicalerapy, College of Health anderapy, Daegu Haany University, Gyeongsan-si, Republic of Korea
How to cite this article:Jang SH, Yeo SS, Lee SH, Jin SH, Lee MY (2017) Cortical activation pattern during shoulder simple versus vibration exercises: a functional near infrared spectroscopy study. Neural Regen Res 12(8):1294-1298.
To date, the cortical ef f ect of exercise has not been fully elucidated. Using the functional near infrared spectroscopy, we attempted to compare the cortical ef f ect between shoulder vibration exercise and shoulder simple exercise. Eight healthy subjects were recruited for this study. Two dif f erent exercise tasks (shoulder vibration exercise using the fl exible pole and shoulder simple exercise) were performed using a block paradigm. We measured the values of oxygenated hemoglobin in the four regions of interest: the primary sensory-motor cortex (SM1 total, arm somatotopy, and leg and trunk somatotopy), the premotor cortex, the supplementary motor area, and the prefrontal cortex. During shoulder vibration exercise and shoulder simple exercise, cortical activation was observed in SM1 (total, arm somatotopy, and leg and trunk somatotopy), premotor cortex, supplementary motor area, and prefrontal cortex. Higher oxygenated hemoglobin values were also observed in the areas of arm somatotopy of SM1 compared with those of other regions of interest. However, no signif i cant dif f erence in the arm somatotopy of SM1 was observed between the two exercises. By contrast, in the leg and trunk somatotopy of SM1, shoulder vibration exercise led to a signif i cantly higher oxy-hemoglobin value than shoulder simple exercise.ese two exercises may result in cortical activation ef f ects for the motor areas relevant to the shoulder exercise, especially in the arm somatotopy of SM1. However, shoulder vibration exercise has an additional cortical activation ef f ect for the leg and trunk somatotopy of SM1.
nerve regeneration; functional near infrared spectroscopy; cortical activation; shoulder vibration exercise; fl exible pole; neural regeneration
Introduction
It is well known that exercise is necessary for good health (North et al., 1990; Salmon, 2001; Dziedzic et al., 2008; Siddiqui et al., 2010; Fagard, 2011; Weinstein et al., 2013). In general, exercise provides positive physiological effects, such as increased cardiorespiratory fitness and enhanced musculoskeletal function (Dziedzic et al., 2008; Fagard, 2011; Weinstein et al., 2013). In particular, exercise provides positive psychological ef f ects in terms of self-esteem, mood, and relieving stress (North et al., 1990; Salmon, 2001). In addition, many functional neuroimaging studies have reported that various exercises had an effect in the induction of cortical activation (Luet al., 2002; Park et al., 2008; Perrey, 2008; Tashiro et al., 2008; Kim et al., 2011; Lef f et al., 2011); however, this has not been clearly elucidated so far.
There are several kinds of exercises, including, but not limited to, anaerobic exercise, aerobic exercise, stretching exercise, and strengthening exercise (Siddiqui et al., 2010). Strengthening exercise is a type of anaerobic exercise that involves the use of resistance to induce muscular contraction, which can increase motor unit recruitment, synchronization, and muscle fi ber size (Knuttgen, 2007; Siddiqui et al., 2010). It has been performed using various resistance devices, such as elastic tubing, weighted ball, isokinetic machine, dumbbell and so on (Stratton et al., 2004). Recently, a fl exible pole, such as the Bodyblade (Hymanson Inc, Playa del Rey, CA, USA), has been commonly used in strengthening exercises.e purpose of such a device is to hold and shake it to create a vibration that leads to reciprocal muscle contraction to maintain the vibration (Lister et al., 2007; Moreside et al., 2007).erefore, it has been widely used in physical therapy, sports training, and fi tness enhancement (Anders et al., 2008; Leao Almeida et al., 2011). Many studies have reported that exercise using this device has an ef f ect on increasing muscle power, strength, and endurance (Lister et al., 2007; Moreside et al., 2007; Anders et al., 2008; Parry et al., 2012). However, little is known about the cortical ef f ect of this exercise.
To date, there have been many functional neuroimaging studies reporting on the cortical activation pattern induced by various exercises (Luet al., 2002; Park et al., 2008; Perrey, 2008; Tashiro et al., 2008; Kim et al., 2011; Leff et al., 2011). Most of these studies were conducted using functional MRI or positron emission topography (Luet al., 2002; Park et al., 2008; Tashiro et al., 2008; Kim et al., 2011). However,these techniques are sensitive to motion artifact and cannot be used for large movement tasks. By contrast, the functional near infrared spectroscopy (fNIRS), which measures the intensity of scattered near-infrared light to calculate the changes in the concentrations of oxygenated hemoglobin (HbO) and deoxygentated hemoglobin (HbR) in the cerebral cortex, has a unique advantage in the execution of large movements due to less motion artifact (Miyai et al., 2001; Strangman et al., 2002, 2006; Perrey, 2008; Holtzer et al., 2011; Karim et al., 2012; Kurz et al., 2012).
In the current study, using fNIRS, we attempted to investigate the cortical activation pattern between the shoulder vibration exercise (SVE) using the fl exible pole and shoulder simple exercise (SSE).
Participants and Methods
Participants
Eight healthy participants (five males, three females; mean age 29.13 ± 2.70 years, range 26–33 years) with no history of neurological, physical, or psychiatric illness were recruited for this study. All subjects understood the purpose of the study and provided written, informed consent prior to participation. The study protocol was conducted in accordance with the principles of theDeclaration of Helsinkiand approved by the Institutional Review Board of Yeungnam University Hospital (YUMC 2014-01-425).
Shoulder exercise
All subjects were asked to sit comfortably in a chair in an upright position during the experiment. Two dif f erent exercise tasks were performed using a block paradigm (three cycles: resting [20 seconds]-exercise task [20 seconds]-resting [20 seconds]-exercise task [20 seconds]-resting [20 seconds]-exercise task [20 seconds]); SVE: aer receiving brief instructions and participating in a practice session to ensure familiarity in using the fl exible pole [Bodyblade®Classic (length: 48 in weight: 1.5 lb), Bodyblade, Los Angeles, CA, USA], subjects were asked to vibrate the pole.ey were instructed to vibrate the pole in an antero-posterior direction while holding the fl exible pole with the right shoulder at a 90° fl exion. SSE: fl exion-extension movements of the right shoulder were performed at a frequency of 0.5 Hz under the guidance of a metronome (Figure 1). Each exercise task was repeated twice and the sequence of the tasks was randomly assigned.
Functional NIRS
The fNIRS system (FOIRE-3000; Shimadzu, Kyoto, Japan), with continuous wave laser diodes with wavelengths of 780, 805, and 830 nm, was used to record the cortical activity at a sampling rate of 10 Hz; we employed a 49-channel system with 30 optodes (15 light sources and 15 detectors). Based on the modified Beer-Lambert law, we acquired the values for HbO (Baker et al., 2014), following changes in the levels of cortical concentration (Cope and Delpy, 1988). The international 10/20 system, with Cz (cranial vertex) located beneath the 25th channel, was used to position the optodes. A stand-alone application was used for spatial registration ofthe acquired 49 channels on the Montreal Neurological Institute (MNI) brain based on the 25th channel on the Cz (Ye et al., 2009).

Figure 1 Sagittal view for the shoulder simple (le) and vibration exercises (right).

Figure 2 Region of interest and results of functional near infrared spectroscopy (fNIRS) during shoulder simpleversusvibration exercises in healthy participants.
We selected four regions of interest (ROI) based on Brodmann’s area (BA) and anatomical locations of brain areas: primary sensory-motor cortex (SM1) (BA 1, 2, 3, and 4), premotor cortex (PMC) (BA 6, except for the supplementary motor area [SMA]), SMA (anterior boundary: vertical line to the anterior commissure, posterior boundary: anterior margin of M1, medial boundary: midline between the right and left hemispheres, lateral boundary: the line 15 mm lateral from the midline between the right and lehemispheres), and the prefrontal cortex (PFC) (BA 8, 9, 44, 46). In addition, we divided the ROIs of SM1 into two areas: the area of the arm somatotopy (precentral knob) and the area of the leg and trunk somatotopy (medial part to the precentral knob) (Figure 2A) (Dassonville et al., 1998; Mayka et al., 2006; Amiez and Petrides, 2009).e values for HbO were estimated from each channel of the four ROIs during the resting phase and performance of shoulder movements. Subsequently, using NIRS-SPM, the HbO values of each ROI were acquired based on the individual GLM analysis results.
Statistical analysis
SPSS 15.0 software (SPSS, Chicago, IL, USA) was used in performance of data analysis. Data were expressed as the mean ± standard deviation (SD).e Mann-WhitneyUtest was performed to determine the dif f erence of HbO value in each ROI between SVE and SSE.e results were considered signif i cant when thePvalue was < 0.05.
Results
HbO response
In the average HbO value from the result of individual GLM analysis, cortical activation was observed in SM1 (total, arm somatotopy, and leg and trunk somatotopy), PMC, SMA, and PFC during performance of both SVE and SSE. Among these ROIs, the highest HbO values (SVE = 0.013, SSE = 0.011) were observed in the arm somatotopy of SM1 compared with those of other ROIs while performing each exercise. However, no signif i cant dif f erence in the arm somatotopy of SM1 was observed between the two exercises (P> 0.05). By contrast, there was a significantly higher HbO value (0.011) in the leg and trunk somatotopy of SM1 for SVE than for SSE (0.006) (P< 0.05). However, no signif i cant dif f erences in HbO values in the total SM1 (SVE = 0.012, SSE = 0.008), PMC (SVE = 0.008, SSE = 0.007), SMA (SVE = 0.008, SSE = 0.005), and PFC (SVE = 0.005, SSE =0.005) were observed between the two exercises (P>0.05) (Table 1).
SPMt-statistic maps
Discussion
Herein, we investigated the dif f erences of cortical activation patterns between SVE and SSE. We measured the change of HbO in selected ROIs (SM1 [total, arm somatotopy, leg and trunk somatotopy], PMC, SMA, and PFC) that were assumed to have a close association with shoulder exercise. As a result, we observed greater activation in the arm somatotopy of SM1 than in the other ROIs while performing the two types of shoulder exercise. In addition, the leg and trunk somatotopy of SM1 showed a greater activation during performance of SVE than SSE.is result was in accordance with the group analysis.erefore, both exercises have cortical activation ef f ects for the motor areas relevant to shoulder exercises, especially in the arm somatotopy of SM1. However, SVE has an additional cortical activation ef f ect for the leg and trunk somatotopy of SM1.
A number of studies have reported on the physiological effects of vibration exercise in the enhancement of muscle activity or power, cardiovascular function, and increasing corticospinal pathway excitability (Lister et al., 2007; Moreside et al., 2007; Anders et al., 2008; Mileva et al., 2009; Pollock et al., 2010; Cochrane, 2011; Lau et al., 2011; Marconi et al., 2011; Parry et al., 2012). Among these studies, a few studies have demonstrated the effect of vibration exercise using the flexible pole, like the current study (Lister et al., 2007; Moreside et al., 2007; Anders et al., 2008; Parry et al., 2012). Moreside et al. (2007) reported that vibration exercise increased the activation of trunk muscles and spinal stability.erefore, they demonstrated that the use of vibration pole was ef f ective for the recruitment of spinal stabilizers. During the same year, Lister et al. (2007) reported that vibration exercise using the Bodyblade led to a greater scapular stability than exercises using an elastic band or cuf f weight. In a recent study, Parry et al. (2012) reported similar results indicating that shoulder vibration exercise using the Bodyblade induced greater recruitment of shoulder and back muscles than shoulder exercise using dumbbells.erefore, it appears that the results of the aforementioned previous studies usingthe Bodyblade were in agreement with our results showing that SVE using the fl exible pole induced activation of the leg and trunk sommatotopy of the SM1 as well as the arm somatotopy of SM1.
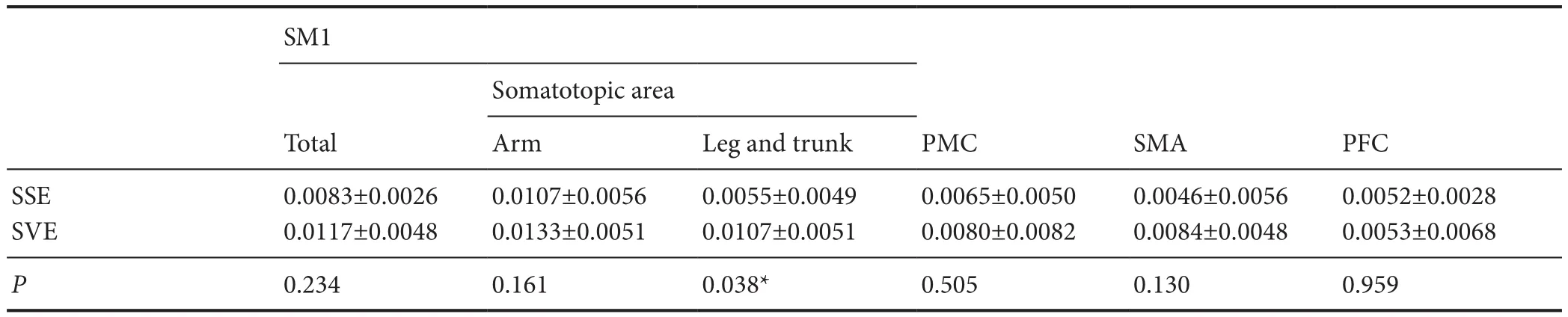
Table 1 Values for oxygenated hemoglobin (M) in the regions of interest during the performance of shoulder simple or vibration exercises
In conclusion, we investigated cortical activation patterns during the performance of SVE versus SSE. According to our fi ndings, SVE induced additional activation in the leg and trunk somatotopy of SM1 as well as the cortical areas relevant to the shoulder exercise. To the best of our knowledge, this is the first study to demonstrate the cortical activation pattern induced by vibration exercise using the fl exible pole. We believe that these fi ndings have an important clinical implication with respect to the brain activation pattern induced by physical exercise. However, the current study has some limitations to consider. The number of the subjects was small and we did not use individual MRI data for the spatial registration of the acquired channels on the MNI brain. In addition, we could not include the whole PFC area due to the limited number of channels in the NIRS system. In order to further investigate the clinical application of vibration exercise for motor recovery in patients with brain injury, further studies are necessary. Moreover, further studies are also necessary to better understand the cortical activation pattern induced by other physical modalities.
Author contributions:MYL designed this study and SSY collected experimental data. SHJ and SHL provided technical assistance and supervised the study. SHJ and MYL wrote the manuscript, provided critical revision of the manuscript for intellectual content. SHJ approved the fi nal version of the paper. All authors approved the fi nal version fof this paper.
Conf l icts of interest:None declared.
Research ethics:
Declaration of participant consent:
Data sharing statement:
Plagiarism check:Checked twice by ienticate.
Peer review:Externally peer reviewed.
Open access statement:
Amiez C, Petrides M (2009) Anatomical organization of the eye fi elds in the human and non-human primate frontal cortex. Prog Neurobiol 89:220-230.
Anders C, Wenzel B, Scholle HC (2008) Activation characteristics of trunk muscles during cyclic upper-body perturbations caused by an oscillating pole. Arch Phys Med Rehabil 89:1314-1322.
Baker WB, Parthasarathy AB, Busch DR, Mesquita RC, Greenberg JH, Yodh AG. (2014) Modif i ed Beer-Lambert law for blood fl ow. Biomed Opt Express. 5:4053-75.
Cochrane DJ (2011) Vibration exercise: the potential benefits. Int J Sports Med 32:75-99.
Cope M, Delpy DT (1988) System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med Biol Eng Comput 26:289-294.
Dassonville P, Lewis SM, Zhu XH, Ugurbil K, Kim SG, Ashe J (1998) Ef f ects of movement predictability on cortical motor activation. Neurosci Res 32:65-74.
Dziedzic K, Jordan JL, Foster NE (2008) Land- and water-based exercise therapies for musculoskeletal conditions. Best Pract Res Clin Rheumatol 22:407-418.
Fagard RH (2011) Exercise therapy in hypertensive cardiovascular disease. Prog Cardiovasc Dis 53:404-411.
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66:879-887.
Karim H, Schmidt B, Dart D, Beluk N, Huppert T (2012) Functional near-infrared spectroscopy (fNIRS) of brain function during active balancing using a video game system. Gait Posture 35:367-372.
Kim MJ, Hong JH, Jang SH (2011)e cortical ef f ect of clapping in the human brain: A functional MRI study. NeuroRehabilitation 28:75-79.
Knuttgen HG (2007) Strength training and aerobic exercise: comparison and contrast. J Strength Cond Res 21:973-978.
Kurz MJ, Wilson TW, Arpin DJ (2012) Stride-time variability and sensorimotor cortical activation during walking. Neuroimage 59:1602-1607.
Lau RW, Liao LR, Yu F, Teo T, Chung RC, Pang MY (2011)e ef f ects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin Rehabil 25:975-988.
Leao Almeida GP, De Souza VL, Barbosa G, Santos MB, Saccol MF, Cohen M (2011) Swimmer’s shoulder in young athlete: rehabilitation with emphasis on manual therapy and stabilization of shoulder complex. Maner 16:510-515.
Lef f DR, Orihuela-Espina F, Elwell CE, Athanasiou T, Delpy DT, Darzi AW, Yang GZ (2011) Assessment of the cerebral cortex during motor task behaviours in adults: a systematic review of functional near infrared spectroscopy (fNIRS) studies. Neuroimage 54:2922-2936.
Li H, Tak S, Ye JC (2012) Lipschitz-Killing curvature based expected Euler characteristics for p-value correction in fNIRS. Journal of neuroscience methods 204:61-67.
Lister JL, Del Rossi G, Ma F, Stoutenberg M, Adams JB, Tobkin S, Signorile JF (2007) Scapular stabilizer activity during Bodyblade, cuf f weights, andera-Band use. J Sport Rehabil 16:50-67.
Marconi B, Filippi GM, Koch G, Giacobbe V, Pecchioli C, Versace V, Camerota F, Saraceni VM, Caltagirone C (2011) Long-term ef f ects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair 25:48-60.
Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE (2006)ree-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage 31:1453-1474.
Mileva KN, Bowtell JL, Kossev AR (2009) Effects of low-frequency whole-body vibration on motor-evoked potentials in healthy men. Exp Physiol 94:103-116.
Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K (2001) Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. Neuroimage 14:1186-1192.
Moreside JM, Vera-Garcia FJ, McGill SM (2007) Trunk muscle activation patterns, lumbar compressive forces, and spine stability when using the bodyblade. Physer 87:153-163.
North TC, McCullagh P, Tran ZV (1990) Ef f ect of exercise on depression. Exerc Sport Sci Rev 18:379-415.
Park JW, Kwon YH, Lee MY, Bai D, Nam KS, Cho YW, Lee CH, Jang SH (2008) Brain activation pattern according to exercise complexity: a functional MRI study. NeuroRehabilitation 23:283-288.
Parry JS, Straub R, Cipriani DJ (2012) Shoulder- and back-muscle activation during shoulder abduction and fl exion using a Bodyblade Pro versus dumbbells. J Sport Rehabil 21:266-272.
Perrey S (2008) Non-invasive NIR spectroscopy of human brain function during exercise. Methods 45:289-299.
Pollock RD, Woledge RC, Mills KR, Martin FC, Newham DJ (2010) Muscle activity and acceleration during whole body vibration: ef f ect of frequency and amplitude. Clin Biomech (Bristol, Avon) 25:840-846.
Salmon P (2001) Ef f ects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev 21:33-61.
Siddiqui NI, Nessa A, Hossain MA (2010) Regular physical exercise: way to healthy life. Mymensingh Med J 19:154-158.
Strangman G, Boas DA, Sutton JP (2002) Non-invasive neuroimaging using near-infrared light. Biol Psychiatry 52:679-693.
Strangman G, Goldstein R, Rauch SL, Stein J (2006) Near-infrared spectroscopy and imaging for investigating stroke rehabilitation: test-retest reliability and review of the literature. Arch Phys Med Rehabil 87(12 Suppl 2):S12-S19.
Stratton G, Jones M, Fox KR, Tolfrey K, Harris J, Maffulli N, Lee M, Frostick SP (2004) BASES position statement on guidelines for resistance exercise in young people. J Sports Sci 22:383-390.
Tak S, Yoon SJ, Jang J, Yoo K, Jeong Y, Ye JC (2011) Quantitative analysis of hemodynamic and metabolic changes in subcortical vascular dementia using simultaneous near-infrared spectroscopy and fMRI measurements. Neuroimage 55:176-184.
Tashiro M, Itoh M, Fujimoto T, Masud MM, Watanuki S, Yanai K (2008) Application of positron emission tomography to neuroimaging in sports sciences. Methods 45:300-306.
Weinstein AA, Chin LM, Keyser RE, Kennedy M, Nathan SD, Woolstenhulme JG, Connors G, Chan L (2013) Ef f ect of aerobic exercise training on fatigue and physical activity in patients with pulmonary arterial hypertension. Respir Med 107:778-784.
Ye JC, Tak S, Jang KE, Jung J, Jang J (2009) NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. Neuroimage 44:428-447.
Copyedited by Li CH, Song LP, Zhao M
*< class="emphasis_italic">Correspondence to: Mi Young Lee, P.T., Ph.D., mykawai@hanmail.net.
Mi Young Lee, P.T., Ph.D., mykawai@hanmail.net.
orcid: 0000-0002-8858-9360 (Mi Young Lee)
10.4103/1673-5374.213549
Accepted: 2017-07-17
- 中国神经再生研究(英文版)的其它文章
- Transcriptional inhibition in Schwann cell development and nerve regeneration
- A progressive compression model of thoracic spinal cord injury in mice: function assessment and pathological changes in spinal cord
- Effects of estrogen receptor modulators on cytoskeletal proteins in the central nervous system
- Optogenetics and its application in neural degeneration and regeneration
- Live-cell imaging: new avenues to investigate retinal regeneration
- Neurotrophic factors and corneal nerve regeneration