Pressure-induced phase transition of B-type Y2O3∗
Qian Zhang(张倩),Xiang Wu(巫翔),and Shan Qin(秦善)
1 Gemological Institute,China University of Geosciences,Wuhan 430074,China
2 State Key Laboratory of Geological Processes and Mineral Resources,China University of Geosciences,Wuhan 430074,China
3 Key Laboratory of Orogenic Belts and Crustal Evolution,Ministry of Educationamp;School of Earth and Space Sciences, Peking University,Beijing 100871,China
Pressure-induced phase transition of B-type Y2O3∗
Qian Zhang(张倩)1,†,Xiang Wu(巫翔)2,and Shan Qin(秦善)3
1 Gemological Institute,China University of Geosciences,Wuhan 430074,China
2 State Key Laboratory of Geological Processes and Mineral Resources,China University of Geosciences,Wuhan 430074,China
3 Key Laboratory of Orogenic Belts and Crustal Evolution,Ministry of Educationamp;School of Earth and Space Sciences, Peking University,Beijing 100871,China
The synthesized monoclinic(B-type)phase of Y2O3has been investigated by in situ angle-dispersive x-ray diffraction in a diamond anvil cell up to 44 GPa at room temperature.A phase transition occurs from monoclinic(B-type)to hexagonal (A-type)phase at 23.5 GPa and these two phases coexist even at the highest pressure.Parameters of isothermal equation of state are V0=69.0(1)˚A3,K0=159(3)GPa,=4(fixed)for the B-type phase and V0=67.8(2)˚A3,K0=156(3)GPa,=4(fixed)for the A-type phase.The structural anisotropy increases with increasing pressure for both phases.
Y2O3,x-ray diffraction,pressure-induced phase transition,equation of state
1.Introduction
Sesquioxides are very important materials which possess a wide range of physical and chemical properties and can be used for different technological applications.For example,they play a vital role in the grain growth inhibitor,[1]are used as additives of ceramics[2]and active catalysts for organic reactions,[3,4]and have potential use in nuclear engineering.[5,6]In addition,sesquioxides can adopt perovskite(Pv),post-perovskite(PPv),and post-PPv phases at extreme conditions,which has significant implications for the mantle of giant extrasolar silicate planets.For instance,Al2O3undergoes a series of pressure-induced phase transitions from corundum structure to Rh2O3(II)-type structure,then to the PPv-type structure,and finally to the post-PPv phase(U2S3-type,Pnma and Z=4)above 370 GPa.[7]Gd2S3-type(Pnma and Z=4)and Th2S3-type(Pnma and Z=4)structures, which have the same space group and cationic coordination numbers with the U2S3-type structure,have been observed in Sc2O3[8]and Ti2O3[9]at high pressure,respectively.
The high-pressure behavior of rare-earth sesquioxides has been widely investigated.So far,five polymorphic forms have been identified in rare-earth sesquioxides.Hexagonal A (P¯3m1)in which cations are in seven-fold coordination,monoclinic B(C2/m)in which cations are mixed with six or sevenfold coordination,and cubic Cwith six-coordinated cations are commonly observed at room temperature and ambient pressure.[10]The two other phases denominated as H (hexagonal,P63/mmc)and X(cubic,)are formed at high temperature.[11]Cations in Gd2S3,Th2S3,and U2S3occupy seven and eight oxygen coordinations,which are higher than those in C,B,A,Pv,and PPv structures.
Scandium and yttrium elements with the chemically similar lanthanide elements are often known to belong to the lanthanide family as the rare-earth metal elements.The optical properties of trivalent rare earth ion-doped nanocrystalline materials have been investigated extensively.Y2O3nanocrystals doped with trivalent rare earth ion have attracted considerable interest because of the high chemical durability and thermal stability.Eu-doped Y2O3is an important commercial luminescent material,it has been widely used in fluorescent lamps,projection television tubes,field emission displays,etc.[12]Y2O3crystallized into the C-type structure under ambient conditions.Lots of attempts have been made to understand its high-pressure behavior,but the high-pressure phase transition sequences show some inconsistencies.As for the pure Y2O3,the C→B,[13,14]C→B→A,[15–17]C→A,[18]and C→B→Gd2S3[19,20]phase sequences have been observed in experiments or theoretical calculations.The in situ highpressure luminescence spectra indicated that the C-type bulk Eu-doped Y2O3transformed into the B-type phase at 15 GPa, while 20 nm-sized nanocrystals did not.[21]
As we all know,the experimental results are influenced by experimental conditions,such as non-hydrostaticity of pressure and regime of temperature treatment.In the static high pressure experiment at 2.5 GPa and 1273 K,the C-type Y2O3transformed to the B-type phase which was also found above 12 GPa in a shock-compression experiment,while the A-type phase was proposed to be the favorable stable high pressure phase.[14]In some quasi-hydrostatic compression experiments at room temperature,Y2O3followed the phase sequence of C→B→A.[15–17]A high-pressure Raman experiment reported two phase transitions,viz,C→B and B→Aat 12 GPa and 19 GPa respectively.[16]However,a high pressure x-ray experiment showed that pure Y2O3exhibited a direct transition to A structure at 12.1 GPa and room temperature,whereas the C→B→A transition was observed in Eu-doped Y2O3.[18]Considering the temperature effect,a sequence of structural phase transitions C→B→A observed in the room-temperature compression did not coincide with the phase transition sequence C→B→Gd2S3under high pressure and high temperature.[19]Moreover,there are some inconsistencies among the room-temperature data collected with different internal pressure standards under conditions close to hydrostatic environment by noble gases like He or Ne media and by solid or liquid media such as KBr and silicone oil.[22]The previous pressure-transmitting media in the experiments on Y2O3were silicone oil,[18]a mixture of methanol and ethanol,[23]or KBr powder.[16]
To the best of our knowledge,starting Y2O3samples used in the former work were characterized by the C-type structure.The B-type polycrystalline Y2O3has been synthesized successfully using multi-anvil press in our previous work.[24]High-pressure x-ray diffraction experiments provide us information about high-pressure phase transitions and physical properties.[25–27]In this study,we carry out the high pressure experiment of B-type Y2O3as the starting material up to 44 GPa by in situ x-ray diffraction(XRD)in diamond anvil cell(DAC).
2.Experiment
The starting sample of the synthesized B-type Y2O3was described in detail in Ref.[24].In our XRD experiment, we generated high pressures by a symmetric type DAC with 300µm-diameter culets.A 150µm-diameter hole was drilled in the pre-indented to∼30µm-thickness rhenium gasket.Pt was loaded in the chamber as pressure standard based on its well-known equation of state.[22]and Ne was used as the pressure medium.The in situ high-pressure XRD experiment at room temperature was conducted at 13IDD at Advanced Photon Source(APS).Diffraction patterns were collected using a MarCCD detector.The monochromatic x-ray which was focused to 5µm×5µm on the sample surface has a wavelength of 0.3344˚A.Collection time for each pattern was 20 s. Diffraction images were integrated to one-dimensional spectra using the Fit2D program.[28]Lattice parameters were obtained using Unitcell.[29]Some x-ray diffraction patterns were fitted by the Le Bail method implemented in the GSAS+EXPGUI software.[30]
3.Results and discussion
The XRD pattern at ambient conditions gives lattice parameters a=13.892(7)˚A,b=3.494(1)˚A,c=8.614(4)˚A, β=100.22(4),and V=411.4(2)˚A3from a full profile model refinement.In situ high-pressure XRD data were collected up to 44 GPa at room temperature.Some selected XRD patterns are shown in Fig.1,where all peaks can be indexed into the B-type Y2O3and minor impurities up to 23.5 GPa.All peaks shifted to higher angles of 2θ with increasing pressure.As shown in Fig.1,the peaks began to change at 23.5 GPa,indicating the appearance of a new phase.According to the previous studies,[19]the new phase would be either A-or Gd2S3-type.By performing the x-ray profile fitting analyses and comparing the d values of the characteristic peaks with the previous experimental data,[19]we confirmed that the new phase adopted the A-type structureUpon compression,the B-type phase was found to obviously coexist throughout the transition process from the B-to A-type phase.The B-type phase still existed at the highest pressure,implying that the transition may be kinetically sluggish.In Sc2O3,the B to A transition at 77 GPa was predicted by abinitio calculation,[31]but the Gd2S3-type phase as the post-B phase was observed above 18 GPa atthe high temperature experiment.[8]Considering the similarity of Sc2O3and Y2O3,Sc2O3would also have the C→B→A phase transition sequence in the high-pressure experiment at room temperature.As previously mentioned,it is not a common phenomenon that the high-pressure structure sequence of Y2O3observed in the room temperature compression does not coincide with the phase transition sequence at high temperature.Generally speaking,the temperature effect is always considered in pressure-induced phase-transition experiments in order to obtain clear high-pressure structural information,because high temperature relaxes the differential stress and overcomes the potential kinetic effects on phase transition.Therefore,the laser heating usually promotes the phase transition to happen at lower pressure than that of the room temperature compression experiment.By analyzing the crystal structures of B-and A-type phases of Y2O3at high pressure,the B-type structure was found to be equivalent to the A-type structure.[19]This is a possible reason why the B to A transition was observed under compression at room temperature.In our synthesis experiment,we tried to synthesize the Gd2S3-type phase of Y2O3in a multi-anvil apparatus at HPHT conditions(20 GPa and 1800°C),but the B-type phase was reserved.[24]Ovsyannikov et al.tried to obtain the B-type Sc2O3at 14 GPa and 1600°C,but only the C-type phase was found in the recovered sample.[32]These results show that the HP-HT behaviors of Y2O3and Sc2O3are complex and further investigations of their P–T phase diagrams are needed.
The B-and A-type unit-cell volumes showed a smooth decrease with the increasing pressure.The unit-cell volume data as a function of pressure is plotted in Fig.2 and analyzed by the second-order Birch–Murnaghan equation of state (B–M EoS).[33]The EoS parameters we obtained are listed in Table 1,which also includes the available experimental and theoretical data for comparison.The equilibrium volume V0obtained from the theoretical computation is underestimated compared with the experimental results,which is typical for the LDA computations.The isothermal bulk moduli of B-and A-type Y2O3compounds are approximate.Our result is consistent with the theoretical simulation data,[11]which shows that the difference between the bulk moduli of B-and A-type rare-earth sesquioxides is considerably small.

Fig.1.(color online)X-ray diffraction profiles of the Y2 O3 sample under room temperature compression.The tick marks indicate the calculated positions of the diffraction peaks of B-and A-type phases with the LeBail method (GSAS).Solid line,symbols,and solid line at the bottom represent the calculated and the observed patterns and their differences at ambient conditions, respectively.Other solid lines represent the observed patterns at high pressure.
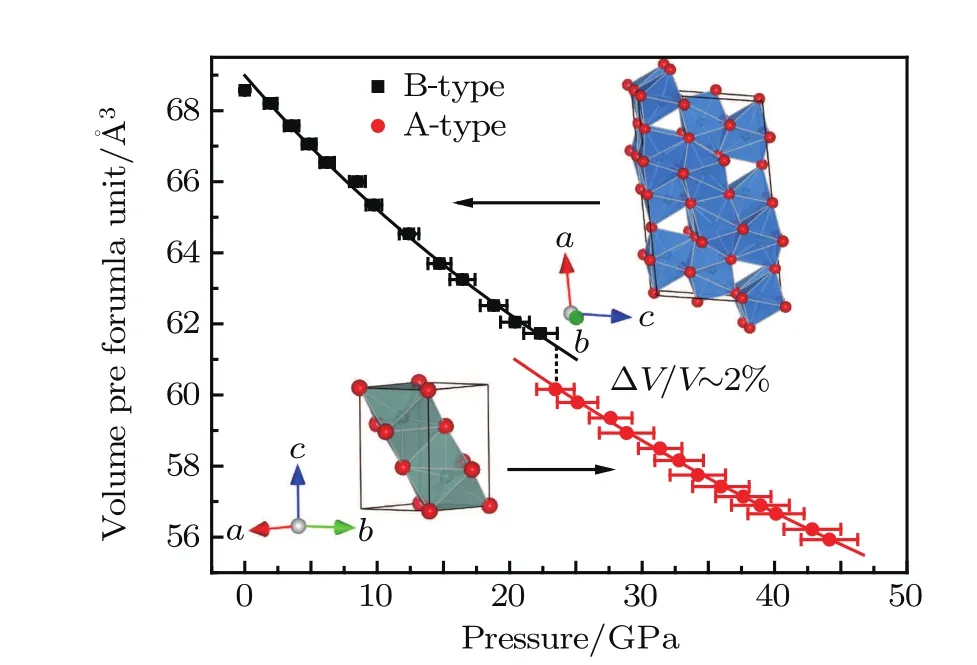
Fig.2.(color online)The volume per formula of B-and A-type phases of Y2O3 as a function of pressure.The solid lines correspond to the second-order B–M EoS fitting to the experimental data.The volume collapse in the phase transition is about 2%at 23.5 GPa.The crystal structures are also shown. Red spheres are oxygen and dark green spheres at the center of polyhedra are yttrium.
The phase transition from B-to A-type Y2O3is followed by a volume collapse of 2%at 23.5 GPa(Fig.2),which is at the same level as that of Sm2O3.[34]This transformation involves only a slight deformation.Compared to this B→A transition,the C→B first-order phase transition is accompanied by a more significant volume decrease(8%,[16]12.5%[15]).
Table 1.Equation of state parameters for the B-and A-type polymorphs of Y2O3.V0,B0,andare the volume per formula unit,the bulk modulus,and its pressure derivative at zero pressure,respectively.
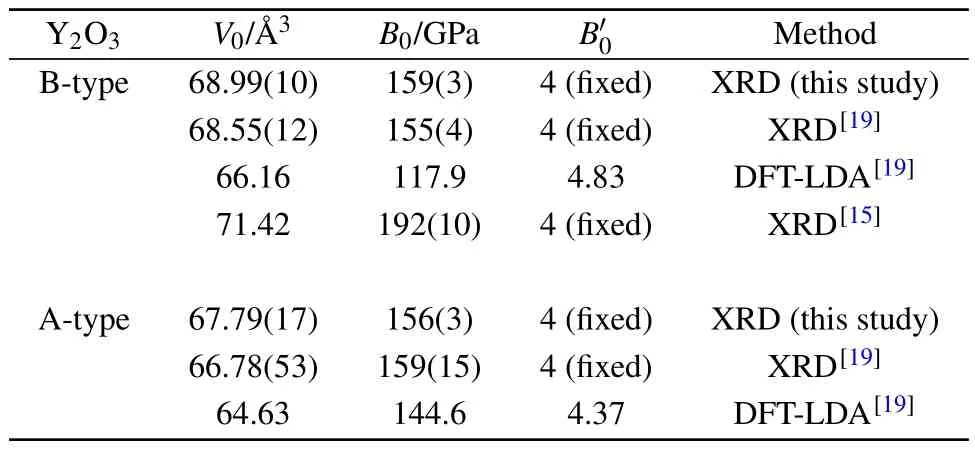
Table 1.Equation of state parameters for the B-and A-type polymorphs of Y2O3.V0,B0,andare the volume per formula unit,the bulk modulus,and its pressure derivative at zero pressure,respectively.
The coordination number of Yincreases from six or seven in B-type structure,to seven in the A-type one during the B→A transition.In addition,the B(C2/m)and Ahave a group-subgroup relationship.[35]Contrary to the C→B reconstructive transition,the B→A transition is inferred to be displacive,which is also suggested in other studies.[17,19]
Theoretical analysis of pressure-induced B to A-type phase transitions shows a linear correlation between bulk modulus,transition pressures,and the ionic radius of the cation.[11]The suggested transition pressure and bulk modulus for Y2O3are at the same level as those of our experimental results. The difference is mainly due to the GGA exchange correlation energy,which gives a larger V0and a smaller B0.The first single-crystal study of Sc2O3exhibited that the denser B-type phase is a bit more compressible than the C-type one.[32]This result did not confirm the other experimental studies on powdery Sc2O3.[8]The previous studies on powders of lanthanide sesquioxides did not reveal a noticeable difference in the bulk moduli of C-,B-,and A-type phases,e.g.,Ho2O3and Sm2O3.[34,36]Whether there exist noticeable bulk modulus differences among the C-,B-,and A-type rare-earth sesquioxides requires more experimental and theoretical investigations.
During the past few decades,the rare-earth sesquioxides have been studied by numerous researchers to investigate the phase relationships among the C,B,and A phases.Early in 1966,Hoekstra found that the effect of ionic radius is much greater than temperature or pressure in shifting the C↔B equilibrium line.[37]Moreover,compression experiments and theoretical results exhibited that higher pressure would be needed to stabilize the A-type phase in rare-earth sesquioxides with smaller cationic radius.[11,34]However,this systematics of the C→B→A phase sequence may not be applicable to the rare-earth sesquioxides only according to their cationic radii.The comparative crystallography in rare-earth sesquioxides has been summarized in other study.[8]Sc2O3(Sc3+;0.745˚A), In2O3(In3+;0.800˚A),and Y2O3(Y3+;0.900˚A),which adopt the C-type structure at ambient conditions,crystallize into the Gd2S3structure at high pressure after laser heating.[8,19,38]It is possible that the Gd2S3structure would be found as a post B-type structure in other rare-earth sesquioxides at high temperature.
The pressure evolution of the lattice parameters of the B-and A-type phases is shown in Fig.3.Regarding the unit-cell compressibilities,the a axis is the most compressible and the b axis is the least compressible for the B-type phase.As for the A-type Y2O3,the c axis is more compressible than the a axis mainly because of the large intervals between the layers in the c axial orientation.It indicates that the axial compressiblities of B-and A-type phases are anisotropic.
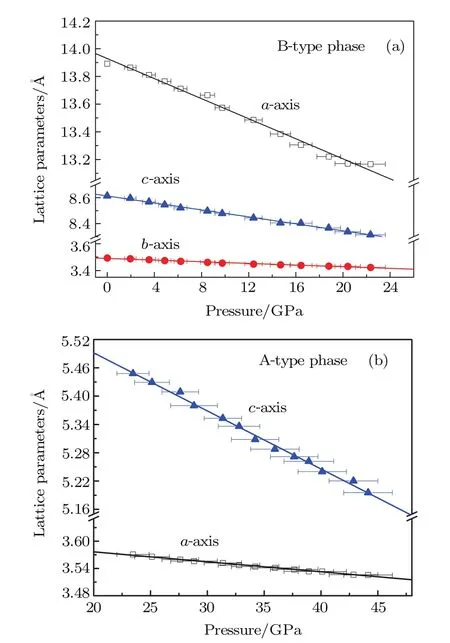
Fig.3.(color online)Pressure-induced variations in the lattice parameters of the B-type(a)and A-type(b)phases of Y2O3.The solid lines are linear fittings of the experimental data.
4.Conclusion
The structural properties of B-type Y2O3under compression have been investigated by synchrotron radiation x-ray diffraction experiment with neon as the pressure-transmitting medium at room temperature up to 44 GPa.We observed a sluggish phase transition from B-to A-type phase at 23.5 GPa. The isothermal P–V relationship of Y2O3was described by the second-order Birch–Murnaghan equation of state with B0= 159(3)GPa for the B-type phase,and 156(3)GPa for the A-type phase.Note that the high pressure behavior of rare-earth sesquioxides is apparently complex,and there are lots of unanswered questions in their condensed matter physics.Further studies will bring exciting results on their high-pressure properties.
Acknowledgements
The authors are deeply grateful to Sergey V.Ovsyannikov and Leonid S.Dubrovinsky for synthesizing the sample.High-pressure experiments were performed at GeoSoil Enviro CARS of the APS,ANL.GeoSoil EnviroCARS operations are supported by the National Science Foundation-Earth Sciences(EAR-1128799)and the Department of Energy Geosciences(DE-FG02-94ER14466).APS is supported by DOEBES,under Contract No.DE-AC02-06CH11357.
[1]Aktas B,Tekeli S and Kucuktuvek M 2014 Int.J.Mater.Res.105 208
[2]Scott H 1975 J.Mater.Sci.10 1527
[3]Hussein G A 1996 J.Anal.Appl.Pyrolysis 37 111
[4]Dedov A,Loktev A,Moiseev I,Aboukais A,Lamonier J F and Filimonov I 2003 Applied Catalysis A:General 245 209
[5]Shikama T,Toh K,Nagata S,Tsuchiya B,Yamauchi M,Nishitani T, Suzuki T,Okamoto K and Kubo N 2006 Nucl.Fusion 46 46
[6]Weber W J,Ewing R C,Catlow C R A,de la Rubia T D,Hobbs L W, Kinoshita C,Matzke H,Motta A T,Nastasi M,Salje E K H,Vance E R and Zinkle S J 1998 J.Mater.Res.13 1434
[7]Umemoto K and Wentzcovitch R M 2008 Proc.Natl.Acad.Sci.USA 105 6526
[8]Yusa H,Tsuchiya T,Sata N and Ohishi Y 2009 Inorg.Chem.48 7537
[9]Nishio-Hamane D,Katagiri M,Niwa K,Sano-Furukawa A,Okada T and Yagi T 2009 High Press.Res.29 379
[10]Zinkevich M 2007 Prog.Mater.Sci.52 597
[11]Wu B,Zinkevich M,Aldinger F,Wen D and Chen L 2007 J.Solid State Chem.180 3280
[12]Wang H,Uehara M,Nakamura H,Miyazaki M and Maeda H 2005 Adv. Mater.17 2506
[13]Hoekstra H R and Gingerich K A 1964 Science 146 1163
[14]Atou T,Kusaba K,Fukuoka K,Kikuchi M and Syono Y 1990 J.Solid State Chem.89 378
[15]Halevy I,Carmon R,Winterrose M L,Yeheskel O,Tiferet E and Ghose S 2010 J.Phys.Conf.Ser.215 012003
[16]Husson E,Proust C,Gillet P and Itie J 1999 Mater.Res.Bull.34 2085
[17]Bose P P,Gupta M,Mittal R,Rols S,Achary S,Tyagi A and Chaplot S 2011 Phys.Rev.B 84 094301
[18]Wang L,Pan Y,Ding Y,Yang W,Mao W L,Sinogeikin S V,Meng Y, Shen G and Mao H 2009 Appl.Phys.Lett.94 061921
[19]Yusa H,Tsuchiya T,Sata N and Ohishi Y 2010 Inorg.Chem.49 4478
[20]Umemoto K and Wentzcovitch R M 2011 Phys.Chem.Miner.38 387
[21]Bai X,Song H,Liu B,Hou Y,Pan G and Ren X 2008 J.Nanosci. Nanotechnol.8 1404
[22]Fei Y,Ricolleau A,Frank M,Mibe K,Shen G and Prakapenka V 2007 Proc.Natl.Acad.Sci.USA 104 9182
[23]Zhang J,Cui H,Zhu P,Ma C,Wu X,Zhu H,Ma Y and Cui Q 2014 J. Appl.Phys.115 023502
[24]Zhang Q,Wu X,Ovsyannikov S V,Dong J,Qin S,Dubrovinsky L S and Chen D 2016 Chem.Res.Chin.Univ.32 545
[25]Tang S X,Zhu H Y,Jiang J R,Wu X X,Dong Y X,Zhang J,Yang D P and Cui Q L 2015 Chin.Phys.B 24 096101
[26]Li N N,Li Y,Li H,Tang R L,Zhao Y S,Han D D,Ma Y M,Cui Q L, Zhu P W and Wang X 2014 Chin.Phys.B 23 069101
[27]Yang S W,Peng F,Li W T,Hu Q W,Yan X Z,Lei L,Li X D and He D W 2016 Chin.Phys.B 25 076101
[28]Hammersley A,Svensson S,Hanfland M,Fitch A and Hausermann D 1996 High Press.Res.14 235
[29]Holland T and Redfern S 1997 Mineral.Mag.61 65
[30]Toby B H 2001 J.Appl.Crystallogr.34 210
[31]Liu D,Lei W,Li Y,Ma Y,Hao J,Chen X,Jin Y,Yu S and Cui Q 2009 Inorg.Chem.48 8251
[32]Ovsyannikov S V,Bykova E,Bykov M,Wenz M D,Pakhomova A S, Glazyrin K,Liermann H P and Dubrovinsky L 2015 J.Appl.Phys.118 165901
[33]Birch F 1952 J.Geophys.Res.57 227
[34]Jiang S,Liu J,Lin C,Li X and Li Y 2013 J.Appl.Phys.113 113502
[35]Hahn T 2002 International Table for Crystallography,A(5th edn.) (Dordrecht:Kluwer)pp.540,541
[36]Jiang S,Liu J,Li X,Bai L,Xiao W,Zhang Y,Lin C,Li Y and Tang L 2011 J.Appl.Phys.110 013526
[37]Hoekstra H R 1966 Inorg.Chem.5 754
[38]Yusa H,Tsuchiya T,Tsuchiya J,Sata N and Ohishi Y 2008 Phys.Rev. B 78 092107
3 May 2017;revised manuscript
15 May 2017;published online 18 July 2017)
10.1088/1674-1056/26/9/090703
∗Project supported by the National Natural Science Foundation of China(Grant Nos.U1232204 and 41502029)and China Postdoctoral Science Foundation (Grant No.2015M580679).
†Corresponding author.E-mail:qianzhang@cug.edu.cn
©2017 Chinese Physical Society and IOP Publishing Ltd http://iopscience.iop.org/cpb http://cpb.iphy.ac.cn
- Chinese Physics B的其它文章
- Relationship measurement between ac-Stark shift of 40Ca+clock transition and laser polarization direction∗
- Air breakdown induced by the microwave with two mutually orthogonal and heterophase electric field components∗
- Collective motion of active particles in environmental noise∗
- Temperature dependence of heat conduction coefficient in nanotube/nanowire networks∗
- Analysis of dynamic features in intersecting pedestrian flows∗
- Heat transfer enhancement in MOSFET mounted on different FR4 substrates by thermal transient measurement∗

