Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair
Angelina Angelova, Borislav Angelov
1 Institut Galien Paris-Sud, CNRS UMR 8612, University of Paris-Sud, Université Paris-Saclay, LabEx LERMIT, Châtenay-Malabry cedex, France
2 Institute of Physics, ELI Beamlines, Academy of Sciences of the Czech Republic, Prague, Czech Republic
Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair
Angelina Angelova1,*, Borislav Angelov2
1 Institut Galien Paris-Sud, CNRS UMR 8612, University of Paris-Sud, Université Paris-Saclay, LabEx LERMIT, Châtenay-Malabry cedex, France
2 Institute of Physics, ELI Beamlines, Academy of Sciences of the Czech Republic, Prague, Czech Republic
Among the macromolecular drug targets in neurodegenerative disorders, the neurotrophin brain-derived neurotrophic factor (BDNF) and its high-affinity tropomyosin-related kinase receptor (TrkB) present strong interest for nanomedicine development aiming at neuronal and synaptic repair. Currently, BDNF is regarded as the neurotrophic factor of highest therapeutic significance. However, BDNF has delivery problems as a protein drug.e enhanced activation of the transcription factor CREB (cAMP response element-binding protein) has been evidenced to increase the BDNF gene expression and hence the production of endogenous BDNF. We assume that BDNF delivery by nanocarriers and mitochondrial protection may provide high potential for therapeutic amelioration of the neuroregenerative strategies. Beneficial therapeutic outcomes may be expected for synergistic dual or multi-drug action aiming at (i) neurotrophic protein regulation in the central and peripheral nervous systems, and (ii) diminishment of the production of reactive oxygen species (ROS) and the oxidative damage in mitochondria. Our research strategy is based on a nanoarchitectonics approach for the design of nanomedicine assemblies by hierarchical self-assembly. We explore nanoarchitectonics concepts in so-matter nanotechnology towards preparation of biodegradable self-assembled lipid nanostructures for safe neuro-therapeutic applications of multi-target nanomedicines.
BDNF delivery; neuroprotective lipid nanocarriers; neurotrophic factor; CREB; nanomedicine; macromolecular drugs; combination therapy
Introduction
Restorative therapies of neurological disorders require new technologies and strategies for neurorepair and stimulation of neurogenesis against aging. Neurodegenerative and neuropsychiatric disorders (amyotrophic lateral sclerosis (ALS), Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s (HD) diseases, Rett syndrome, multiple sclerosis, stroke, hearing disorder, anxiety, depression, bipolar disorder, epilepsy, schizophrenia, and addiction) are complex pathologies provoked by genetic mutations and environmental stressor conditions, which lead to neuronal loss and dysfunction (Re et al., 2012). Targeting of a single pathway mechanism in consequent therapies has oen yielded insuf ficient regenerative outcome owing to the fact that the neurodegenerative diseases are multi-factorial and are caused by several risk factors (Liu et al., 2016). For example, the target mechanisms in AD include β-amyloid fibril formation, protein hyperphosphorylation (p-tau), neurotrophin deficiency, oxidative stress, mitochondrial damage and dysfunction, apolipoprotein E4 (ApoE4) genetic risk factor, impaired transcription and altered gene expression, disruptions in metal ion homeostasis, altered lipid metabolism, and neuroinflammation. Genetic mutations, neurotrophin deficiency, oxidative stress, mitochondrial dysfunction, altered mitochondrial morphology, mtDNA deletions, inflammation, and apoptosis appear to be risk factors for the pathogenesis of ALS and PD as well (Viral and Mira, 2016).
BDNF in Neuronal Regeneration
Macromolecular therapeutics (e.g., recombinant proteins, synthetic peptides, monoclonal antibodies, plasmid DNA, siRNA, and CRISPR/Cas 9 therapeutics) have considerable potential for use in neuroregenerative nanomedicine structure-based design (Angelov et al., 2017). The majority of them are expected to reach higher success rates in clinical development as compared to the conventional small organic molecule drugs. Among the macromolecular drug targets, the neurotrophin brain-derived neurotrophic factor (BDNF) and its high-affinity tropomyosin-related kinase receptor (TrkB) present strong interest for nanomedicine improvement aiming at neuronal and synaptic repair (Géral et al., 2013). BDNF participates in the development and maintenance of neuronal populations through activation of the TrkB receptor signaling that is responsible for differentiation, proliferation, plasticity, growth, and survival of neurons in the central and peripheral nervous systems (Chao, 2003). This neurotrophin stimulates the process of neurogenesis. BDNF supports also the formation of developing excitatory and inhibitory synapses. Increased neurotrophin levels favour the strengthening of the existing synapsesvialong-term potentiation (Lu et al., 2013). Therefore, the protein BDNF is very important for the neuro-cognitive function. In addition, BDNF activates the translational machinery and stimulates de novo protein synthesis in neurons (Zhang et al., 2013).
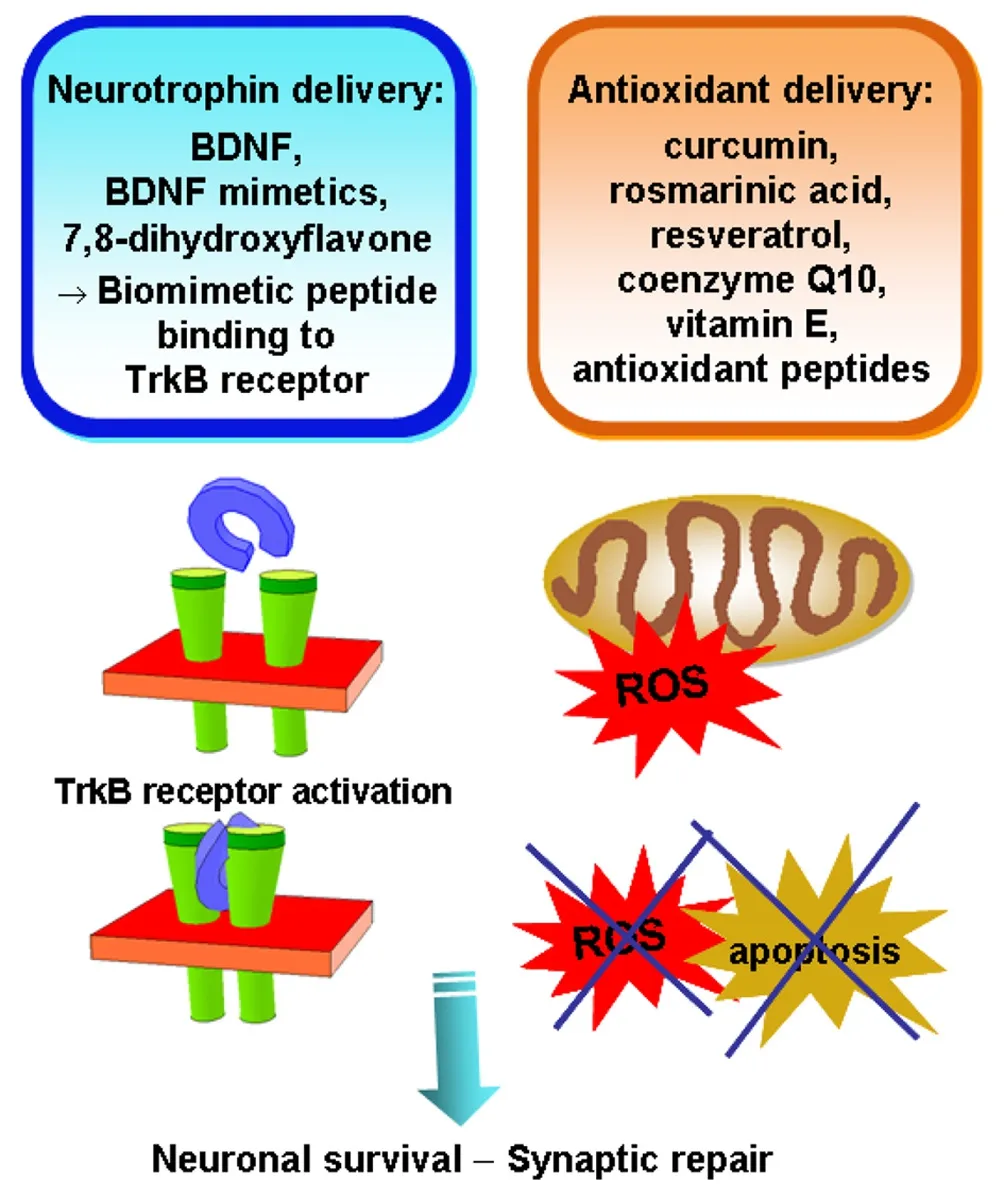
Figure 1 Perspective for combination therapy of neurodegenerative disorders requiring dual or multi-drug delivery in order to target a combination of signaling pathways in the disease mechanisms.
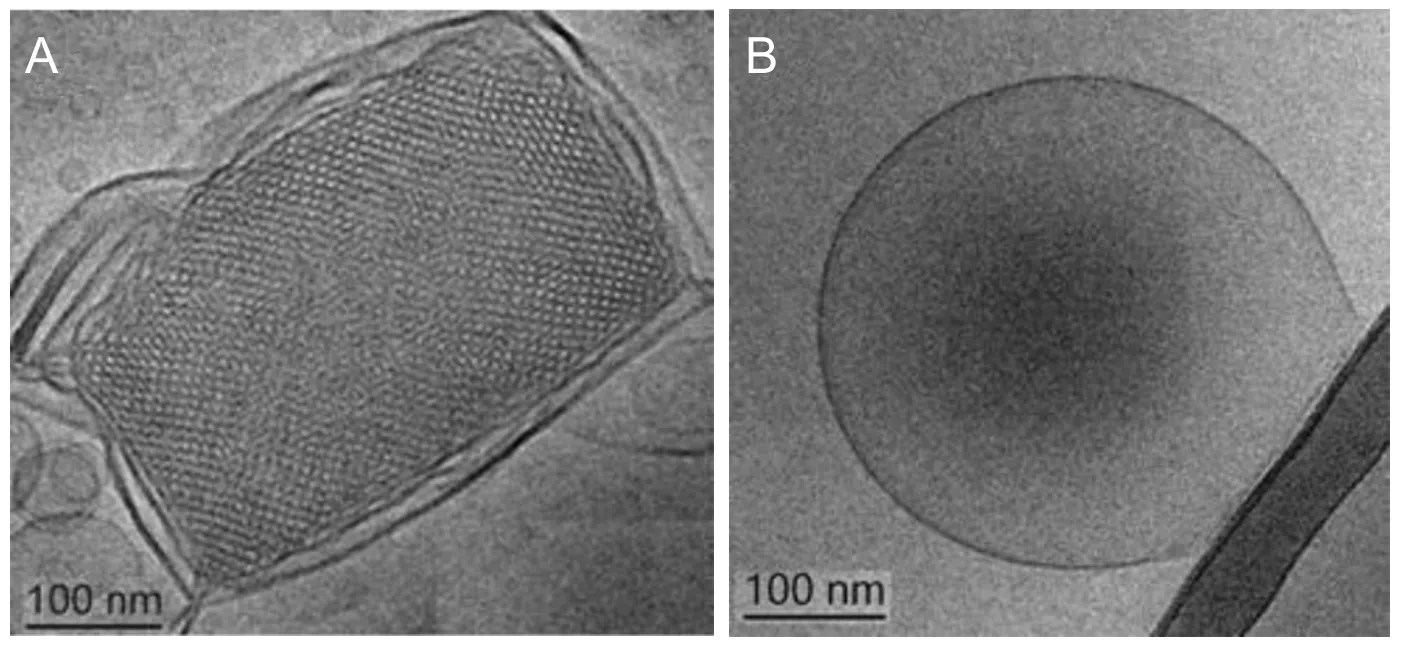
Figure 2 Synthetic self-assembled nanoparticle systems for combination nanodrug delivery in neuroregenerative therapies.
The neurotrophin BDNF, along with its affinity for the TrkB receptor, can regulate processes in the mitochondrial organelles (Geisler et al., 2017).is crosstalk suggests that mitochondria may modulate the intracellular signaling, which controls the neurite growth towards neuro-regeneration. Indeed, BDNF has been shown to increase the brain mitochondrial respiratory coupling at complexi(Markham et al., 2004). On the other hand, the neurotrophin BDNF stimulates the mitochondrial biogenesis (Cheng et al., 2012). The developmental organization of the mitochondria in neurites during growth has been insuf ficiently studied so far. It has been hypothesized that mitochondrial dynamics regulates the neurite growth rate and guidance and that optimal mitochondrial dynamics supports enhanced neurite growth (Todorova et al., 2017).
Evidence exists that BDNF can control the mitochondrial transport and distribution at synapses, where high energy and Ca2+ion buffering are required (Su et al., 2014). It has been shown that the delivery of BDNF for 15 minutes to hippocampal neurons decreases the percentage of moving mitochondria along the axons (Su et al., 2014). As a consequence, the BDNF-induced mitochondrial arrest provokes increased accumulation of mitochondria at presynaptic sites.e number of moving mitochondria along the axons is thus controlled through TrkB receptor-mediated downstream PI3K and phospholipase-Cγ signaling pathways.e accumulation of more mitochondria at presynaptic sites has favored the synaptic transmission (Su et al., 2014).
Nanotechnology for Neurodegenerative Disorders
Figure 1presents the concept of neuroregenerative therapy that promotes neuronal survival through combined nanodrug delivery and targeting of complementary signaling mechanisms. Beneficial therapeutic outcomes may be expected for synergistic dual or multi-drug action aiming at (i) neurotrophic protein regulation in the central and peripheral nervous systems (Figure 1left panel), and (ii) diminishment of the production of reactive oxygen species (ROS) and the oxidative damage in mitochondria (Figure 1right panel).
Due to the low bioavailability of the insoluble neuroprotective antioxidant compounds and the instability of the protein drugs (e.g., neurotrophin BDNF) that should be administered to treat severe neurological disorders, nanotechnology approaches for drug delivery have gained increasing recent interest (Ingallina et al., 2016). Nanoparticle formulations may significantly increase the concentration of the therapeutic agents at the target sites. The application of nanomedicine in neurology is still at early stage of development (Re et al., 2012). Neuro-nanomedicine development needs to consider the neurodegenerative diseases as network states, for which multimaterial systems for multicomponent drug delivery will be required. One possibility comes from the administration of multi-target hybrid neurotherapeutic molecules. In parallel, novel nanotechnologies reveal the next-generation candidate nanobuilding blocks for the design of multi-target nanomedicines to treat neurodegenerative disorders (Angelov et al., 2014). Taking into account the potential cross-talk between the involved biological intracellular signaling cascades, such smart nanoarchitectures may enable the dynamic modulation of target protein networks through co-delivery of genes and/or peptide drugs with small-molecular-weight substances acting through ligand-receptor binding, membrane receptor cross-activation or organelle-specific mechanisms.
Amphiphile Nanoarchitectonics Approach
Our research strategy is based on a nanoarchitectonics approach for the design of nanomedicine assemblies by hierarchical self-assembly (Angelova et al., 2015). We explore nanoarchitectonics concepts in so-matter nanotechnology towards the development of biodegradable self-assembled lipid nanostructures and multifunctional material scaffolds for safe biomedical applications (Angelov et al., 2014).is strategy focuses on nanoformulation and delivery of therapeutic genes, proteins, and peptides, which do not cross the biological barriers and therefore could not exert their therapeutic potential in clinics yet.
Nanocarriers prepared by self-assembly of lyotropic lipids and amphiphilic molecules provide reservoirs for combined encapsulation of multiple bioactive molecules (Figure 2). Thus, the concept for synergistic or additive drug action upon nanoparticle-mediated co-delivery of therapeutic agents can be tested.e supramolecular organization of the nanoparticles and their internal structure, determined by the chosen amphiphilic composition, is crucial for the drug delivery outcome.Figure 2shows two examples of lipid-based nanocarriers with different topologies that result from the self-assembly process underlying the nanoparticles preparation (Angelov et al., 2015).
We have recently demonstrated the advantage of combined drug delivery of a lipophilic phytochemical antioxidant compound (curcumin) and ω-3 polyunsaturated fatty acid DHA (docosahexaenoic acid) for stimulation of the BDNF-TrkB signaling pathway (Guerzoni et al., 2017).e neuritogenic properties of the nanoformulations have been investigatedin vitrowith human neuroblastoma SH-SY5Y cells, which express the neurotrophin receptor TrkB upon differentiation. We have examined the BDNF biosynthesis in designed neuronal cell culture treatment schemes. Neurite outgrowth hasbeen monitored as a criterion of arresting the induced neurodegeneration and reversing the process towards neuroregeneration. Synergistic effects of curcumin, DHA and BDNF have been efficient in preventing the neuronal damageviamodulation of the TrkB receptor signaling towards promotion of neuronal cell survival, proliferation, and growth.e expression of the phosphorylated protein CREB, detected by the anti-phospho CREB (Ser133) monoclonal antibody in the intracellular domain of multi-drug treated SH-SY5Y cells, has been evidenced by flow cytometry for several therapeutic conditions.e sequential chronic treatment scheme has shown potential towards neuronal survival and neurorepair by multidrug formulations fulfilling the activities indicated in Figure 1.e obtained results have evidenced that neuronal cell proliferation, survival, and regeneration may be promoted through additive or synergistic effects exerted by the studied multi-drug compositions (Guerzoni et al., 2017).
In perspective, nanoparticle-based controlled release formulations may be investigated for their ef ficiency in the induction of neuroregeneration processes towards the repair of neuronal damage. To that aim, various technical challenges for fabrication of nanodelivery systems for macromolecular drugs need to be urgently addressed.
Author contributions:AA and BA framed the concept of the review, wrote the paper, and approved the final manuscript.
Conflicts of interest:None declared.
Open access statement:
Contributor agreement:A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check:This paper has been checked twice with duplication-checking soware ienticate.
Peer review:A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Angelov B, Angelova A, Drechsler M, Garamus VM, Mutafchieva R, Lesieur S (2015) Identification of large channels in cationic PEGylated cubosome nanoparticles by synchrotron radiation SAXS and Cryo-TEM imaging. SoMatter 11:3686-3692.
Angelov B, Angelova A, Filippov SK, Drechsler M, Štěpánek P, Lesieur S (2014) Multicompartment lipid cubic nanoparticles with high protein upload: Millisecond dynamics of formation. ACS Nano 8:5216-5226.
Angelov B, Garamus, VM, Drechsler M, Angelova, A (2017) Structural analysis of nanoparticulate carriers for encapsulation of macromolecular drugs. J Mol Liq 235:83-89.
Angelova A, Angelov B, Drechsler M, Lesieur S (2013) Neurotrophin delivery using nanotechnology. Drug Discovery Today 18:1263-1271.
Angelova A, Angelov B, Mutafchieva R, Lesieur S (2015) Biocompatible mesoporous and sonanoarchitectures. J Inorg Organomet Polym 25:214-232.
Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4:299-309.
Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP (2012) Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun 3:1250.
Geisler JG, Marosi K, Halpern J, Mattson MP (2017) DNP, mitochondrial uncoupling, and neuroprotection: A little dab’ll do ya. Alzheimers Dement 13:582-591.
Géral C, Angelova A, Lesieur S (2013) From molecular to nanotechnology strategies for delivery of neurotrophins: emphasis on brain-derived neurotrophic factor (BDNF). Pharmaceutics 5:127-167.
Guerzoni LPB, Nicolas V, Angelova A (2017) In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm Res 34:492-505.
Ingallina C, Rinaldi F, Bogni A, Ponti J, Passeri D, Reggente M, Rossi M, Kinsner-Ovaskainen A, Mehn D, Rossi F, Botta B, Carafa M, Marianecci C (2016) Niosomal approach to brain delivery: Development, characterization and in vitro toxicological studies. Int J Pharm 511:969-982.
Liu Y, An S, Li J, Kuang Y, He X, Guo Y, Ma H, Zhang Y, Ji B, Jiang C (2016) Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 80:33-45.
Lu B, Nagappan G, Guan X, Nathan PJ, Wren P (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14:401-416.
Markham A, Cameron I, Franklin P, Spedding M (2004) BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci 20:1189-1196.
Re F, Gregori Ma, Masserini M (2012) Nanotechnology for neurodegenerative disorders. Nanomedicine 8:S51-58.
Su B, Ji YS, Sun XL, Liu XH, Chen ZY (2014) Brain-derived neurotrophic factor (BDNF)-induced mitochondrial motility arrest and presynaptic docking contribute to BDNF-enhanced synaptic transmission. J Biol Chem 289:1213-1226.
Todorova V, Blokland A (2017) Mitochondria and synaptic plasticity in the mature and aging nervous system. Curr Pharmacol 15:166-173.
Viral M, Mira H (2016) Regulation of neurogenesis by neurotrophins during adulthood: Expected and unexpected roles. Front Neurosci 10:26.
Zhang G, Bowling H, Hom N, Kirshenbaum K, Klann E, Chao MV, Neubert TA (2014) In-depth quantitative proteomic analysis of de novo protein synthesis induced by brain-derived neurotrophic factor. J Proteome Res 13:5707-5714.
Copyedited by Jackson C, Li HF, Liu ZFSong LP, Liu WJ, Zhao M
How to cite this article: Angelova A, Angelov B (2017) Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regen Res 12(6):886-889.
Funding: AA is supported by CNRS. BA is supported by the Czech Science Foundation Grant GACR 17-00973S and the projects ELI - Extreme Light Infrastructure – phase 2 (CZ.02.1.01/0.0/0.0/15_008/0000162) and ELIBIO (CZ.02.1.01/0.0/0.0/15_003/0000447) from the European Regional Development Fund.
*Correspondence to:
Angelina Angelova, Ph.D.,
angelina.angelova@u-psud.fr.
orcid:
0000-0002-0285-0637
(Angelina Angelova)
10.4103/1673-5374.208546
Accepted: 2017-05-22
- 中国神经再生研究(英文版)的其它文章
- Synaptosomal-associated protein 25 may be an intervention target for improving sensory and locomotor functions after spinal cord contusion
- On the role of endogenous neurotoxins and neuroprotection in Parkinson’s disease
- Interfacing peripheral nerve with macro-sieve electrodes following spinal cord injury
- Neuroprotective effects of ganoderma lucidum polysaccharides against oxidative stress-induced neuronal apoptosis
- Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats
- Galantamine protects against beta amyloid peptide-induced DNA damage in a model for Alzheimer’s disease

