Current status and challenges in sentinel node navigation surgery for early gastric cancer
Bang Wool Eom, Young-Il Kim, Hong Man Yoon, Soo-Jeong Cho, Jong Yeul Lee, Chan Gyoo Kim, Soo Jin Kim, Ji Yoon Rho, Seok Ki Kim, Myeong-Cherl Kook, Young-Woo Kim, Keun Won Ryu
1Center for Gastric Cancer;2Center for Diagnostic Oncology, National Cancer Center, Republic of Korea
Current status and challenges in sentinel node navigation surgery for early gastric cancer
Bang Wool Eom1, Young-Il Kim1, Hong Man Yoon1, Soo-Jeong Cho1, Jong Yeul Lee1, Chan Gyoo Kim1, Soo Jin Kim1, Ji Yoon Rho1, Seok Ki Kim2, Myeong-Cherl Kook1, Young-Woo Kim1, Keun Won Ryu1
1Center for Gastric Cancer;2Center for Diagnostic Oncology, National Cancer Center, Republic of Korea
Although a number of feasibility studies for sentinel node (SN) concepts in gastric cancer have been conducted since 2000, there remains a debate regarding detailed detection techniques and oncological safety. Two important multicenter phase II clinical trials were performed in Japan that used different methods and reached different conclusions; one confirmed acceptable results with a false-negative rate of 7%, and the other showed an unacceptably high false-negative rate of 46.4%. The Sentinel Node Oriented Tailored Approach (SENORITA) trial is a multicenter randomized controlled phase III trial being performed in Korea. Patient enrollment is now complete and the long-term results are currently awaited. Recently, an image-guided SN mapping technique using infrared ray/fluorescence was introduced. This method might be a promising technology because it allows the clear visualization of SNs. With regard to the primary tumor, the non-exposed endoscopic wall-inversion surgery technique and non-exposure endolaparoscopic full-thickness resection with simple suturing technique have been reported. These methods prevent abdominal infection and tumor seeding and can be good alternatives to conventional laparoscopic gastric wedge resection. For indications, SN navigation surgery can be extended to patients who underwent non-curative endoscopic resection. Although a few studies have been performed on these patients, sentinel concepts may be beneficial to patients as they omit the need for additional gastrectomy. SN navigation surgery can lead to actual organ-preserving surgery and plays a key role in improving the quality of life of patients with early gastric cancer in the future.
Sentinel node navigation surgery; early gastric cancer; SENORITA
View this article at:https://doi.org/10.21147/j.issn.1000-9604.2017.02.01
Introduction
Sentinel nodes (SNs) are the first possible sites of metastasis via lymphatic drainage from a primary tumor. The absence of metastasis in SNs is thought to be correlated with the absence of metastasis in downstream lymph nodes, allowing unnecessary prophylactic lymphadenectomy to be avoided. This concept was applied to melanoma and breast cancer, and studies showed that SN biopsy was a safe and accurate method to predict metastatic lymph nodes (1,2). Subsequently, the SN concepts were extended to other solid tumors including gastric cancer.
To date, a number of feasibility studies for SN concepts in gastric cancer have been conducted (3,4). Because the proportion of early gastric cancer among all gastric cancer has been increasing in East Asia and the incidence of lymph node metastasis was reported to be 8.0%–20.0% in these early gastric cancer patients, SN navigation surgery has been noted as a new minimally invasive approach (5-8). This surgery not only reduces the extent of lymph nodedissection but also enables stomach-preserving surgery and improves the quality of life in patients with negative SN metastasis. Most previous studies for SN in gastric cancer showed a high detection rate and acceptable accuracy of SN mapping (3,4,9). However, there is still debate about SN concepts regarding detailed detection techniques and oncological safety, and SN navigation surgery is not yet clinically used. This review aimed to evaluate the current status of SN navigation surgery for gastric cancer and discuss several emerging issues (10).
From feasibility studies to a multicenter randomized controlled study for SN navigation surgery in gastric cancer
SN biopsy has been performed using various methods in more than 50 institutions for more than a decade. Each study proved the feasibility of SN biopsy with a high detection rate, whereas the studies were different in their indications (only early gastric cancer or including advanced gastric cancer), approach (openvs. laparoscopic), method of biopsy (pick upvs. basin dissection), tracer (singlevs. dual), injection site (submucosavs. subserosa), and histological examination [hematoxylin and eosin stain onlyvs. including immunohistochemistry or real-time polymerase chain reaction (RT-PCR)] (3). A meta-analysis study by Ryuet al. showed a significant inter-heterogeneity (P<0.001) among the studies and suggested that SN biopsy is not clinically applicable. However, another meta-analysis by Wanget al. reached a positive conclusion for SN biopsy with a similar detection rate and sensitivity (4). The authors commented that SN biopsy was considered to be technically feasible and acceptable, and they also evaluated several factors to improve the sensitivity or detection rate.
Two important multicenter phase II clinical trials were performed in Japan and the results were recently published (11,12). These two studies used different methods of SN biopsy and consequently obtained different results. In the study by Kitagawaet al. (11), SN mapping was performed using a dual tracer (99mTc-Tin colloid and isosulfan blue) endoscopic submucosal injection technique. They confirmed acceptable results with a false-negative rate of 7%. However, the open subserosal injection technique using a single tracer [indocyanine green (ICG)] was performed in the JCOG0302 trial, and an unacceptably high false-negative rate of 46.4% was revealed. This JCOG0302 trial showed the limitations of utilizing a single tracer and intraoperative histological examination using only one plane (13).
In Korea, the long-term outcomes of a phase II clinical trial on laparoscopic SN navigation surgery were recently reported (14). In this study, the false-negative rate of an intraoperative pathological examination was 15.4% (2/13) compared with permanent pathology, and patients who underwent SN navigation surgery had a better quality of life than those who underwent conventional laparoscopic distal gastrectomy. The 3-year relapse-free and overall survival rates for all patients were 96% and 98%, respectively. This study demonstrated that laparoscopic SN navigation surgery was feasible and safe.
Although the results of multicenter clinical trials were reported, the clinical application of SN navigation surgery as a routine practice remains controversial. Further steps should be performed to provide sufficient evidence of oncological safety compared with conventional surgery. For this purpose, the Sentinel Node Oriented Tailored Approach (SENORITA) trial was launched in January, 2013. The SENORITA trial is an investigator-initiated, open-label, parallel-assigned, multicenter randomized controlled phase III trial (15). This study aims to prove the non-inferiority of laparoscopic sentinel basin dissection with stomach-preserving surgery compared with the standard laparoscopic gastrectomy in terms of long-term recurrence and survival. Eligible criteria included patients with a single early gastric cancer of less than 3 cm and a clinical stage of T1N0M0 according to the American Joint Committee for Cancer (AJCC) 7th edition (Figure 1) (16).
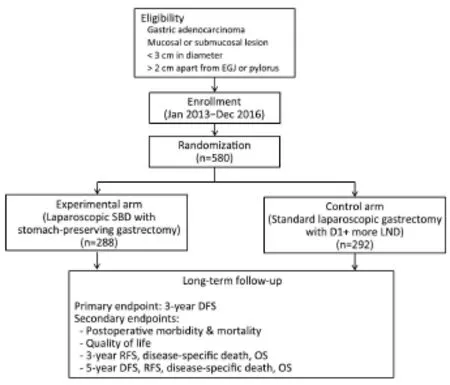
Figure 1Study flow of the Sentinel Node Oriented Tailored Approach (SENORITA) trial. SBD, sentinel basin dissection; LND, lymph node dissection; DFS, disease-free survival; RFS, recurrence-free survival; OS, overall survival.
Moreover, the lesion should be more than 2 cm apart from the esophagogastric junction or pylorus. In the laparoscopic sentinel basin dissection group, the endoscopic submucosal injection technique with dual tracer (99mTc-human serum albumin and ICG) was performed, and then stomachpreserving surgery was performed when the SNs were negative following the frozen section evaluation (17). Stomach-preserving surgery includes endoscopic submucosal dissection, endoscopic full-thickness resection (EFTR), laparoscopic wedge resection, and laparoscopic segmental resection (8). A total of 7 Korean institutions participated in this study after an initial quality control study, and the planned sample size, calculated by a 5% margin of non-inferiority, was 290 patients in each group (580 patients in total) (18). The enrollment was completed in December, 2016, and regular follow-up and monitoring is currently being conducted. We are awaiting the longterm results of the SENORITA.
SN mapping using infrared ray/fluorescence imaging
Except for the conventional dual tracer, image-guided SN mapping techniques have already been introduced (19-23). The infrared ray system has advantages in terms of the highly sensitive detection of not only lymph nodes but also the lymphatic vessels, in addition to safety and convenience, and could be another option in SN navigation surgery (24). Initially, Nimuraet al. reported that the combination of ICG staining with an infrared ray system enhanced the sensitivity of detection of SN (100%vs. 50% in the infrared ray and ordinary light, respectively) (19). Recently, a multicenter prospective study for ICG plus infrared ray was performed (25). Although the sample size was too small to obtain statistical significance (n=44), this method highlighted the new possibility of SN biopsy with a detection rate of 100% and a false-negative rate of 0%.
The ICG fluorescence imaging method was recently developed and is performed using an infrared camera system with a specific light source and detector; the light source is a light-emitting diode that emits light at a wavelength of 760 nm, while the detector is a chargecoupled device (CCD) camera with a cut filter that filters light with a wavelength below 820 nm (22). So far, the outcomes of ICG fluorescence imaging were comparable with those of conventional radio-guided methods [detection rate of more than 94% (94.7%–97.3%) and a false-negative rate of less than 25% (14.3%–25%)] (22,23).
However, this new method has the great advantage of the clear visualization of SN and was recently used in not only gastric cancer but also other solid tumors as a promising technology (26). Future studies are necessary to better understand the spreading speed of ICG particles and detection timing (27).
Non-exposed full-thickness resection after SN mapping
During SN navigation surgery, the approach for the primary tumor is an issue as important as the SN mapping method. Theoretically, stomach-preserving gastrectomy, including EFTR, wedge resection, or segmental gastrectomy, can be performed after confirmation of negative SN metastasis (8). Huret al. reported 13 cases of laparoscopy-assisted EFTR with sentinel basin dissection and 9 patients successfully underwent the procedure without conversion (28). However, during this EFTR or wedge resection, opening of the gastric wall is inevitable because a surgeon should check the tumor location and margin. As such, these procedures could be criticized regarding intra-abdominal infection due to leakage of gastric fluid or tumor implantation (29).
Non-exposed endoscopic wall-inversion surgery (NEWS) was developed to solve the problem of transluminal communication in EFTR (30-32). In the NEWS, markings are made in both the mucosal and the serosal sides and then laparoscopic seromuscular dissection and suture are conducted. Finally, the lesion is dissected using a conventional endoscopic submucosal dissection (ESD) technique (31). However, the laparoscopic circumferential seromuscular incisions and suture along the incision sites are considered as a difficult and time-consuming procedure. The mean operation time with the NEWS technique was 153 min in porcine models, whereas it was more than 3 h in patients with small subepithelial tumors (31). Moreover, one in six patients experienced conversion to EFTR with subsequent laparoscopic suture closure because of poor recognition of the tumor margin. Therefore, further efforts to overcome the technical problems of NEWS are required.

Figure 2Images of the endolaparoscopic full-thickness with simple suturing procedures. (A) Endoscopic circumferential incision of the mucosal layer; (B) Laparoscopic seromuscular suturing which results in inversion of the stomach wall; (C) Endoscopic full-thickness resection; (D) Endoscopic mucosal suturing by placement of endoloops and clips.
Recently, non-exposure endolaparoscopic full-thickness resection with a simple suturing technique was introduced in a porcine model (Figure 2) (33). In this procedure, laparoscopic seromuscular suturing is done without seromuscular dissection after both mucosal and serosal markings (Table 1). Then, EFTR of the inverted stomach wall is performed with a conventional needle knife, and finally endoscopic mucosal suturing is performed with endoloops and clips. The operation time was shorter in this procedure compared with the NEWS procedure, and thus seems to be more practical. Now, a prospective feasibility study for this procedure is ongoing in patients with subepithelial tumors, and the next step is expected to expand this method to early gastric cancer. This procedure could be a promising non-exposure approach for primary tumors in SN navigation surgery.
Application of SN navigation surgery after non-curative endoscopic resection
According to the Japanese Gastric Cancer Treatment Guidelines 2010 (ver. 3), additional surgery is recommended for patients who underwent non-curative endoscopic resection for gastric cancer (34). Previous studies reported 3%–18% of lymph node metastases in patients with tumors out of indication, and standard gastrectomy with D1+ lymph node dissection is recommended for these patients (35-40). However, the majority of these patients have no lymph node metastasis and additional surgery is considered as overtreatment. Therefore, SN navigation surgery could have a critical role in reducing unnecessary treatment.
To date, few studies have evaluated the role of SN mapping after non-curative endoscopic resection. Arigamiet al. examined SN mapping using a single tracer (99mTctin colloid) in patients who underwent non-curative endoscopic resection (41). A total of 16 patients were included in this study, and the detection rate and falsenegative rate were 100% and 0%, respectively. In a larger study by Mayanagiet al., forty patients underwent sentinel mapping using a dual tracer (99mTc-tin colloid plus blue dye), and similar results were demonstrated; a 100%detection rate (40/40) and 0% false-negative rate. These studies suggested that the SN is not significantly affected by endoscopic resection and that sentinel concepts could also be applied to lesions following endoscopic resection (42). Sentinel concepts may be beneficial to patients who underwent non-curative endoscopic resection, because additional gastrectomy can be omitted if sentinel lymph nodes are negative. Therefore, further prospective studies and clinical trials are essential to confirm the feasibility and safety of SN navigation surgery after non-curative endoscopic resection.

Table 1 Comparison between NEWS and simple suturing method in porcine models
Conclusions
Although many feasibility studies and some multicenter phase II clinical trials have been reported, there are still unclear issues regarding SN navigation surgery. The phase III randomized controlled trial (SENORITA trial) is ongoing, and long-term outcomes can help to elucidate these issues. Recently, image-guided technologies, such as infrared ray and fluorescence, have emerged as promising SN mapping methods, and further studies are required prior to clinical application. The approach for primary lesions after SN mapping is also an important issue. The non-exposure endolaparoscopic full-thickness technique can be an alternative that avoids peritoneal contamination and tumor seeding. Moreover, sentinel concepts are tried to apply to lesions following non-curative endoscopic resection. In these cases, SN navigation surgery can lead to organ-preserving surgery and play a key role in improving the quality of life of patients with early gastric cancer.
Acknowledgements
This work was supported by a grant of the National Cancer Center (No. NCC-1710160-1).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 2006;355:1307-17.
2.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 2003;349:546-53.
3.Ryu KW, Eom BW, Nam BH, et al. Is the sentinel node biopsy clinically applicable for limited lymphadenectomy and modified gastric resection in gastric cancer? A meta-analysis of feasibility studies J Surg Oncol 2011;104:578-84.
4.Wang Z, Dong ZY, Chen JQ, et al. Diagnostic value of sentinel lymph node biopsy in gastric cancer: a meta-analysis. Ann Surg Oncol 2012;19:1541-50.
5.Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer 2016;16:131-40.
6.Nashimoto A, Akazawa K, Isobe Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 2013;16:1-27.
7.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer 2000;3:219-25.
8.Park JY, Ryu KW, Eom BW, et al. Proposal of the surgical options for primary tumor control during sentinel node navigation surgery based on the discrepancy between preoperative and postoperative early gastric cancer diagnoses. Ann Surg Oncol 2014;21:1123-9.
9.Kitagawa Y, Fujii H, Mukai M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am 2000;80:1799-809.
10.Ryu KW. The future of sentinel node oriented tailored approach in patients with early gastric cancer. J Gastric Cancer 2012;12:1-2.
11.Kitagawa Y, Takeuchi H, Takagi Y, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol 2013;31:3704-10.
12.Miyashiro I, Hiratsuka M, Sasako M, et al. High falsenegative proportion of intraoperative histological examination as a serious problem for clinical application of sentinel node biopsy for early gastric cancer: final results of the Japan Clinical Oncology Group multicenter trial JCOG0302. Gastric Cancer 2014;17:316-23.
13.Miyashiro I. What is the problem in clinical application of sentinel node concept to gastric cancer surgery? J Gastric Cancer 2012;12:7-12.
14.Park DJ, Park YS, Lee HS, et al. Phase II, prospective, single-arm, single-institutional, openlabel clinical trial on laparoscopic sentinel node navigation surgery in early gastric cancer. J Clin Oncol 2017;35(suppl 4S):abstract 90.
15.Park JY, Kim YW, Ryu KW, et al. Assessment of laparoscopic stomach preserving surgery with sentinel basin dissection versus standard gastrectomy with lymphadenectomy in early gastric cancer-A multicenter randomized phase III clinical trial (SENORITA trial) protocol. BMC Cancer 2016; 16:340.
17.Park JY, Kook MC, Eom BW, et al. Practical intraoperative pathologic evaluation of sentinel lymph nodes during sentinel node navigation surgery in gastric cancer patients — Proposal of the pathologic protocol for the upcoming SENORITA trial. Surg Oncol 2016;25:139-46.
18.Lee YJ, Jeong SH, Hur H, et al. Prospective multicenter feasibility study of laparoscopic sentinel basin dissection for organ preserving surgery in gastric cancer: quality control study for surgical standardization prior to phase III trial. Medicine (Baltimore) 2015;94:e1894.
19.Nimura H, Narimiya N, Mitsumori N, et al. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg 2004;91:575-9.
20.Ohdaira H, Nimura H, Mitsumori N, et al. Validity of modified gastrectomy combined with sentinel node navigation surgery for early gastric cancer. Gastric Cancer 2007;10:117-22.
21.Kelder W, Nimura H, Takahashi N, et al. Sentinel node mapping with indocyanine green (ICG) and infrared ray detection in early gastric cancer: an accurate method that enables a limited lymphadenectomy. Eur J Surg Oncol 2010;36:552-8.
22.Tajima Y, Yamazaki K, Masuda Y, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg 2009;249:58-62.
23.Tajima Y, Murakami M, Yamazaki K, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol 2010;17:1787-93.
24.Mitsumori N, Nimura H, Takahashi N, et al. Sentinel lymph node navigation surgery for early stage gastric cancer. World J Gastroenterol 2014;20:5685-93.
25.Takahashi N, Nimura H, Fujita T, et al. Laparoscopic sentinel node navigation surgery for early gastric cancer: a prospective multicenter trial. Langenbecks Arch Surg 2017;402:27-32.
花期授粉:猕猴桃的花期一般为7~10天,“贵长”“红阳”等品种必须借助人工授粉或蜜蜂授粉可以弥补自然授粉不足。
26.Takeuchi H, Kitagawa Y. New sentinel node mapping technologies for early gastric cancer. Ann Surg Oncol 2013;20:522-32.
27.Kinami S, Oonishi T, Fujita J, et al. Optimal settings and accuracy of indocyanine green fluorescence imaging for sentinel node biopsy in early gastric cancer. Oncol Lett 2016;11:4055-62.
28.Hur H, Lim SG, Byun C, et al. Laparoscopy-assisted endoscopic full-thickness resection with basin lymphadenectomy based on sentinel lymph nodes for early gastric cancer. J Am Coll Surg 2014;219:e29-37.
29.Maehata T, Goto O, Takeuchi H, et al. Cutting edge of endoscopic full-thickness resection for gastric tumor. World J Gastrointest Endosc 2015;7:1208-15.
30.Goto O, Mitsui T, Fujishiro M, et al. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer 2011;14:183-7.
31.Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17:594-9.
32.Mitsui T, Goto O, Shimizu N, et al. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech 2013;23:e217-21.
33.Kim CG, Yoon HM, Lee JY, et al. Nonexposure endolaparoscopic full-thickness resection with simple suturing technique. Endoscopy 2015;47:1171-4.
34.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011;14:113-23.
35.Oda I, Gotoda T, Sasako M, et al. Treatment strategy after non-curative endoscopic resection of earlygastric cancer. Br J Surg 2008;95:1495-500.
36.Choi JY, Jeon SW, Cho KB, et al. Non-curative endoscopic resection does not always lead to grave outcomes in submucosal invasive early gastric cancer. Surg Endosc 2015;29:1842-9.
37.Jung H, Bae JM, Choi MG, et al. Surgical outcome after incomplete endoscopic submucosal dissection of gastric cancer. Br J Surg 2011;98:73-8.
38.Son SY, Park JY, Ryu KW, et al. The risk factors for lymph node metastasis in early gastric cancer patients who underwent endoscopic resection: is the minimal lymph node dissection applicable? A retrospective study Surg Endosc 2013;27:3247-53.
39.Eom BW, Yu JS, Ryu KW, et al. Optimal submucosal invasion of early gastric cancer for endoscopic resection. Ann Surg Oncol 2015;22:1806-12.
40.Eom BW, Kim YI, Kim KH, et al. Survival benefit of additional surgery after noncurative endoscopic resection in patients with early gastric cancer. Gastrointest Endosc 2017;85:155-163.e3.
41.Arigami T, Uenosono Y, Yanagita S, et al. Feasibility of sentinel node navigation surgery after noncurative endoscopic resection for early gastric cancer. J Gastroenterol Hepatol 2013;28:1343-7.
42.Mayanagi S, Takeuchi H, Kamiya S, et al. Suitability of sentinel node mapping as an index of metastasis in early gastric cancer following endoscopic resection. Ann Surg Oncol 2014;21:2987-93.
Cite this article as:Eom BW, Kim YI, Yoon HM, Cho SJ, Lee JY, Kim CG, Kim SJ, Rho JY, Kim SK, Kook MC, Kim YW, Ryu KW. Current status and challenges in sentinel node navigation surgery for early gastric cancer. Chin J Cancer Res 2017;29(2):93-99. doi: 10.21147/j.issn.1000-9604.2017.02.01
10.21147/j.issn.1000-9604.2017.02.01
Keun-Won Ryu, MD, PhD. Center for Gastric Cancer, Research Institute &Hospital, National Cancer Center, 323 Ilsan-ro, Ilsandong-gu, Goyang-si 410-769, Republic of Korea. Email: docryu@ncc.re.kr.
Submitted Mar 31, 2017. Accepted for publication Apr 17, 2017.
 Chinese Journal of Cancer Research2017年2期
Chinese Journal of Cancer Research2017年2期
- Chinese Journal of Cancer Research的其它文章
- Satisfactory surgical outcome of T2 gastric cancer after modified D2 lymphadenectomy
- Postoperative chemotherapy with S-1 plus oxaliplatin versus S-1 alone in locally advanced gastric cancer (RESCUE-GC study): a protocol for a phase III randomized controlled trial
- Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis
- Apparent diffusion coefficient by diffusion-weighted magnetic resonance imaging as a sole biomarker for staging and prognosis of gastric cancer
- Helicobacter pylori antibody responses in association with eradication outcome and recurrence: a population-based intervention trial with 7.3-year follow-up in China
- Adjuvant chemotherapy with paclitaxel and cisplatin in lymph node-positive thoracic esophageal squamous cell carcinoma
