金属离子导向合成两个金属配位聚合物:晶体结构和荧光性质
马德运 李湘 郭海福 马燕华 林婉纯 温美玲 蔡立文 朱立烽
(肇庆学院化学化工学院,肇庆526061)
金属离子导向合成两个金属配位聚合物:晶体结构和荧光性质
马德运*李湘 郭海福 马燕华 林婉纯 温美玲 蔡立文 朱立烽
(肇庆学院化学化工学院,肇庆526061)
以2,5-呋喃二羧酸和六水硝酸锌/四水硝酸镉为原料,在溶剂热条件下,合成出2个金属有机配位聚合物,[Zn(FDC)(DMF)2]n(1)和{(Me2NH2)2[Cd2(FDC)3(H2O)2]·4H2O}n(2)(H2FDC=2,5-呋喃二甲酸,DMF=N,N-二甲基甲酰胺)。通过元素分析、红外光谱、差热分析、X射线粉末衍射和X射线单晶衍射等手段对配合物进行了结构表征。结果显示,化合物1为一维链结构,通过分子间氢键作用构筑成二维结构;而2为二维(4,4)网络结构。热稳定性表明化合物1脱去DMF配体后稳定到300℃;而2脱去配体水、阴离子和溶剂分子后结构立即发生坍塌。常温固态下,激发波长分别为303和350 nm时考察了配合物1和2的荧光性质,结果显示2个化合物均发射蓝色荧光(λmax=406,470 nm),荧光寿命分别为76.2和138.1 ns。
配位聚合物;晶体结构;2,5-呋喃二羧酸;金属离子效应;荧光性质
0 Introduction
Metal-organic coordination polymers(CPs)have received widespread attention over the past decade due to their structural diversity and fascinating topology,as well as their excellent properties with promising applications such as gas storage and separation,nonlinear optics,catalysis,magnetism, luminescence,drug delivery,sensing,and detection[1-8]. During the attainment of CPs,many factors can influence the construction progress,e.g.,metal ions, organic ligands,solvents,pH values,reaction temperatures,and so on[9-10].Amongmany on-going efforts to develop CPs materials,solvent effect is one of the most significant factors that affect the structures and properties of final products[11].Moreover,most of the studies about the effects of solvents on the resultant structures are obtained by changing the types of the solvents[11-13].We are interested in the coordination chemistry of 2,5-furandicarboxylic acid(H2FDC),a ligand with versatile binding and coordination modes, which has been proven to be useful in the construction of coordination polymers[14].As part of an on-going study related to functional CPs,H2FDC and Zn(NO3)·6H2O were chosen to afford two zinc/ cadmium-based coordination polymers,[Zn(FDC) (DMF)2]n(1)and{(Me2NH2)2[Cd2(FDC)3(H2O)2]·4H2O}n(2),which are characterized by elemental analyses,IR spectrum,thermogravimetric analyses,powder X-ray diffraction and single-crystal X-ray crystallography. Their structural diversities reveal that the metal ions play important role in the self-assembly processes. Furthermore,their luminescent properties were also investigated.
1 Experimental
1.1 M aterials and measurements
All chemicals purchased were of reagent grade and used without further purification.All syntheses were carried out in 23 mL Teflon-lined autoclaves under autogenous pressure.Elemental analyses(C,H and N)were performed on a Perkin-Elmer 240 CHN elemental analyzer.Infrared spectra were taken on a Shimadzu IR-440 spectrometer with a KBr disk in the 4 000~400 cm-1region.Thermogravimetric analyses (TGA)were carried out on an automatic simultaneous thermal analyzer(DTG-60,Shimadzu)under N2atmosphere at a heating rate of 10℃·min-1within a temperature range of 25~800℃.Powder XRD investigations were carried out on a Bruker AXSD8-Advanced diffractometer at 40 kV and 40 mA with Cu Kα(λ= 0.154 06 nm)radiation.Luminescence spectra and lifetimes for crystal solid samples were recorded at room temperature on an Edinburgh FLS920 phosphorimeter.
1.2 Synthesis of 1
A mixture of Zn(NO3)2·6H2O(0.15 g,0.5 mmol) and H2FDC(0.078 g,0.5mmol)in 12mL DMF.The resulting solution was sealed in a 23 mL Teflon-lined stainless steel autoclave and heated at 130℃for 3 daysunderautogenouspressure.Colorlesssinglecrystal was obtained(Yield:68%,based on FDC)upon cooling the solution to room temperature at rate of 5℃·h-1.Anal.Calcd.for C12H16N2O7Zn(%):C 39.4,H 4.4,N 7.7;Found(%):C 39.7,H 4.0,N 7.3.IR(KBr, cm-1):1 625(s),1 534(m),1 461(w),1 407(s),1 357(s), 1 248(m),1 162(w),1 044(w),881(w),826(m),781(m), 590(s)(Fig.S1).
1.3 Synthesis of 2
Complex 2 was prepared by the same procedure as 1 except that Zn(NO3)2·6H2O were replaced by Cd(NO3)2·4H2O(0.154 g,0.5 mmol).Colorless single crystal was obtained(Yield:62%,based on FDC) upon cooling the solution to room temperature at 5℃·h-1.Anal.Calcd.for C22H34O21N2Cd2(%):C 29.8,H 3.8,N 3.2;Found(%):C 28.7,H 3.5,N 3.6.IR(KBr, cm-1):3 378(vs),3 132(m),1 573(vs),1 471(w),1 425 (w),1 402(w),1 374(vs),1 225(w),1 154(w),1 085 (w),1 017(m),998(w),967(m),794(s),702(m),622(w), 520(w)(Fig.S1).
1.4 Crystal structure analysis
Single crystal X-ray diffraction analyses of complexes 1 and 2 were performed on a Bruker Smart Apex Duo CCD diffractometer operating at 50 kV and 30 mA using Mo Kαradiation(λ=0.071 073 nm). Data collection and reduction were performed usingthe APEXⅡsoftware[15].Multi-scan absorption corrections were applied for all the data sets using SADABS,as included in the APEXⅡprogram[15]. The structures were solved by direct methods and refined by least squares on F2using the SHELXTL program package[16].All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms attached to carbon and oxygen were placed in geometrically idealized positions and refined using a riding model.The routine SQUEEZE (PLATON)[17]was applied to remove diffuse electron density caused by badly disordered[Me2NH2]+cations and water molecules of 2.The formula unit of 2 was achieved through combination of elemental analyses, IR spectra and thermogravimetric characterization. Themore detailed information is listed in the CIF file. Crystallographic data and structural refinement detail of complexes 1 and 2 can be found in Table 1. Selected bond lengths and bond angles are given in Table 2.
CCDC:1489352,1;1527106,2.
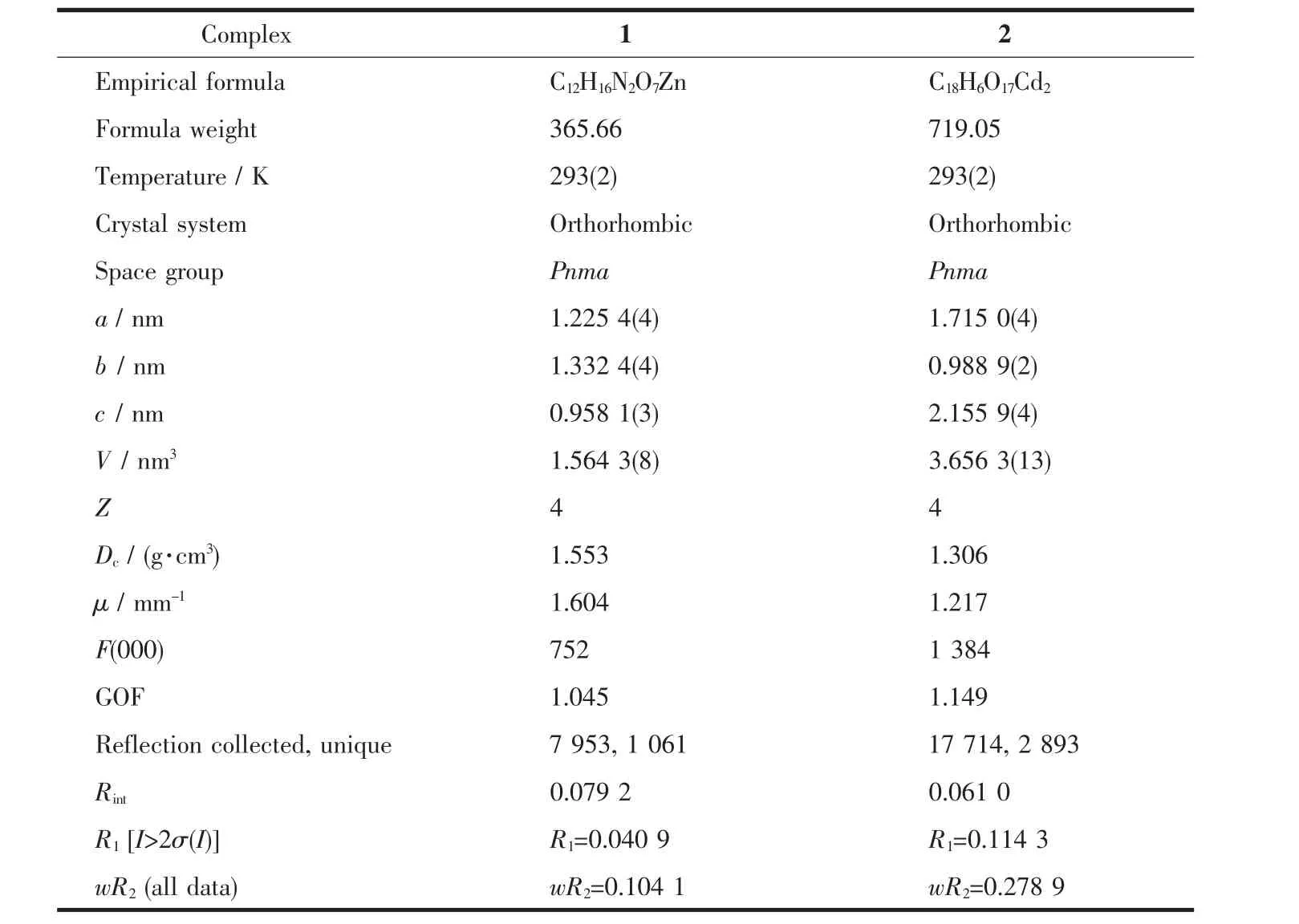
Table 1 Crystal data and structure refinement information for com plexes 1 and 2
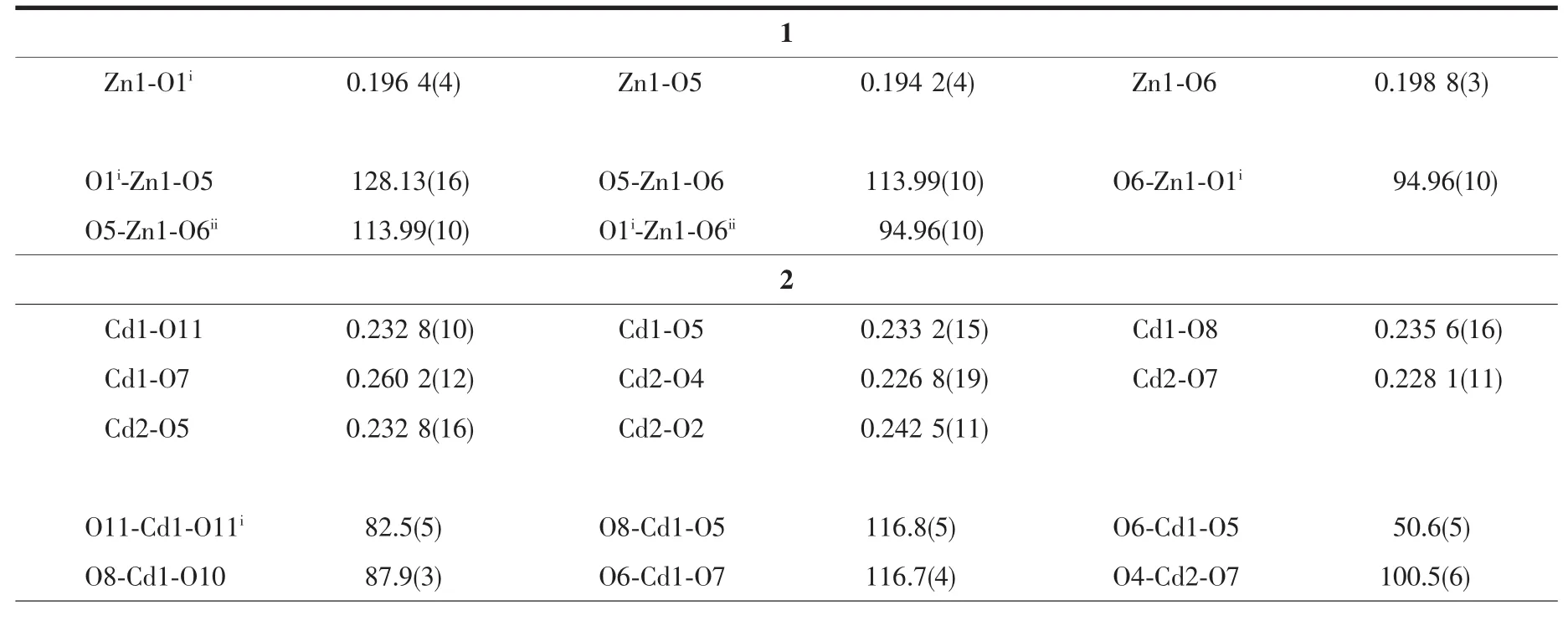
Table 2 Selected bond distances(nm)and angles(°)of 1 and 2

Continued Table 2
2 Results and discussion
2.1 Structure description
Single-crystal X-ray diffraction analysis reveals that complex 1 has 1D wave-like chain structure and crystallizes in orthorhombic Pnma space group. Coordination environment of Znatom is shown in Fig.1a.In the asymmetric unit of 1,there is one Zncation,a FDC anion and two DMF ligands.The Zncenter is four-coordinated by two oxygen atoms from two FDC ligands and two DMF ligands,adopting a distorted tetrahedron geometry.The Zn-O bond lengthsand O-Zn-O bond angles range from 0.194 2(4) to 0.198 8(3)nm and 94.96(10)°to 128.13(16)°, respectively,which is within the reasonable range of observed values for other four-coordinated Zncomplexes with oxygen donating ligands[18-19].In the crystal structure of 1,the FDC ligand adopt one bridging mode to link two Znions(modeⅠin Scheme 1).In this manner,the zinc ions are linked into a wave-like chain through FDC anions with the separation of0.958 1 nm between two neighboring zinc centers(Fig.1b).Finally,the intra/intermolecular C-H…O hydrogen bonds rings(Fig.1c)connect the chains to form a two-dimensional network structure(Fig.1d).
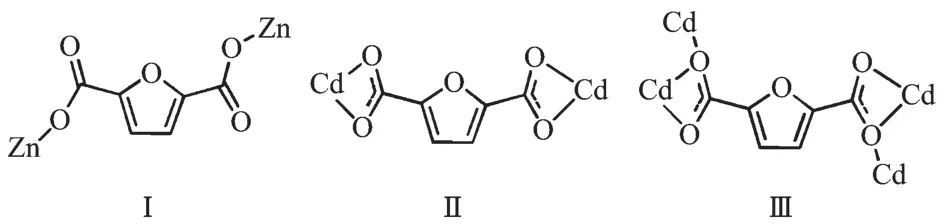
Scheme 1 Coordinationmodes of FDC ligand in the structures of 1 and 2
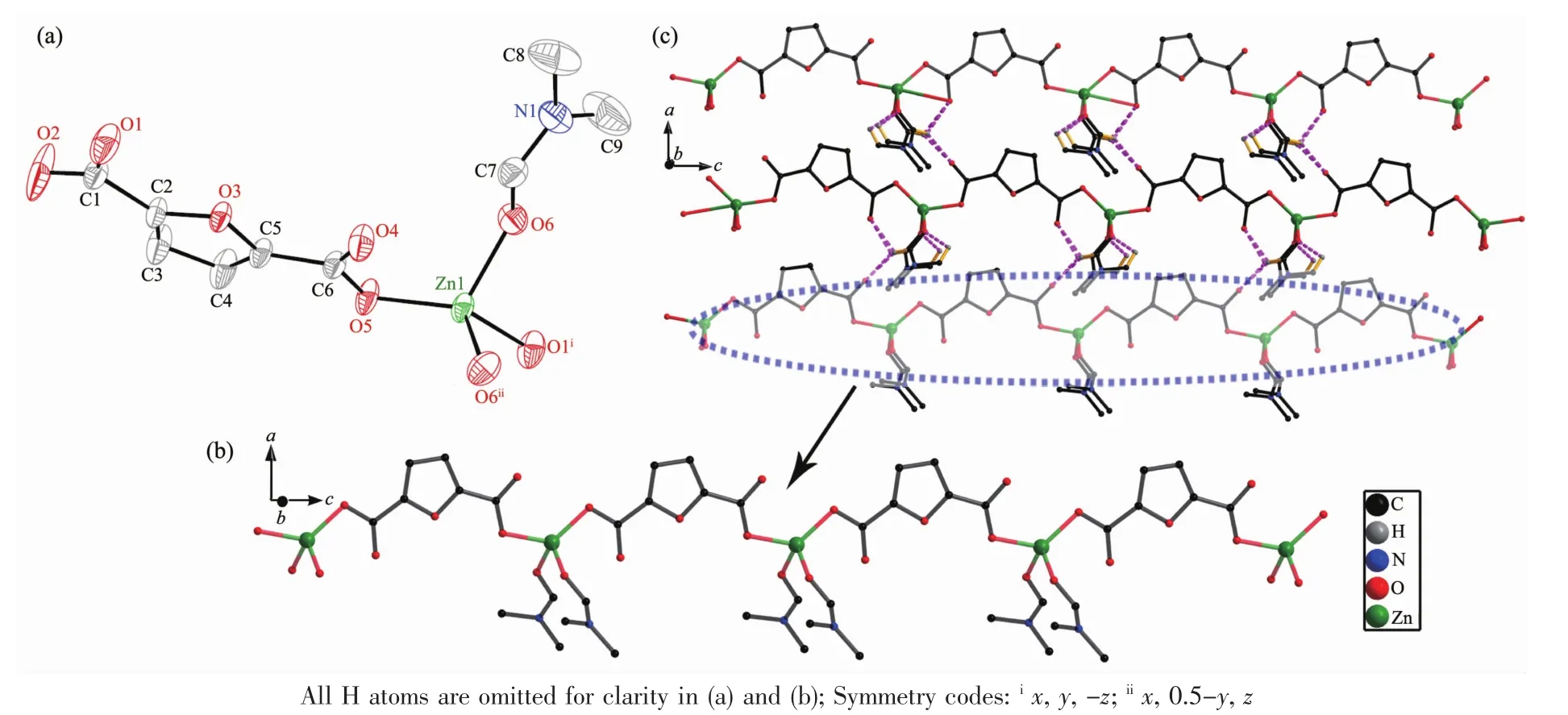
Fig.1(a)Thermal ellipsoid plotof asymmetric unitof 1 showing 30%probability displacement ellipsoids; (b)View of a wave-like chain of 1;(c)View of the 2D layer structure of 1
Complex 2 crystallizes in the monoclinic space group Pnma,with two Cd cations,three FDC anions, two charge-balancing[Me2NH2]+cations,two aqua ligands and four free water molecules in the asymmetric unit.Cd1 is eight-coordinated by eight carboxylate oxygen atoms from four different FDC ligands, adopting a dodecahedron geometry;Cd2 is sevencoordinated by six carboxylate atoms from three FDC ligands and an aqua ligand,displaying a pentagonal bipyramid geometry.The Cd-O distances and O-Cd-O bond angles range from 0.232 8(10)nm to 0.276 0(15)nm and 50.6(5)°to 173.0(6)°,respectively,allofwithin the range of those found in other Cdcomplexes with oxygen donating ligands[20-21].In the polymeric structure of 2,the FDC ligands act as two coordination fashions:one uses two chelating modes to link two Cdions;the other uses the chelating-bridging modes to connect four Cdions(modeⅡandⅢin Scheme 1).Based on the above connections,a ladder chain is formed through linking the dinuclear cadmium cores byμ2-FDC ligands with a Cd…Cd separation of 0.389 1 nm.The adjacent neutral chains are bridged byμ4-FDC ligands to form a 2D framework.The 2D framework can be topologically recognized as a(4,4) net where the dinuclear cadmium core is regarded as quadruply-connected node.
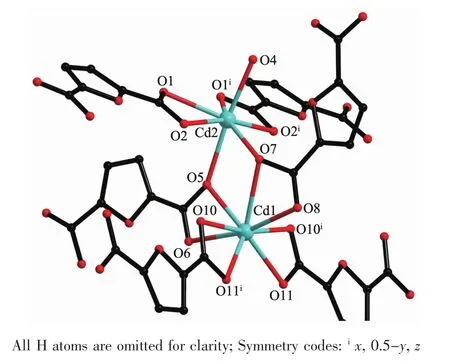
Fig.2 Coordination environmentof Cdin 2
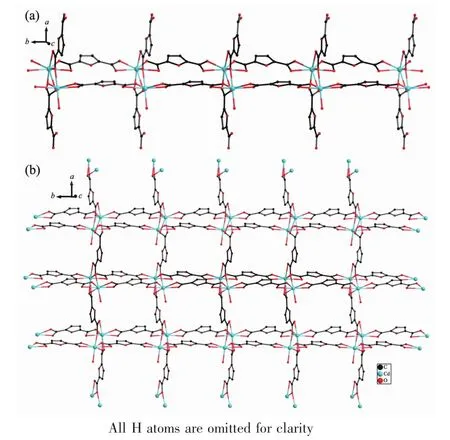
Fig.3(a)Ladder chain in 2 generated through linking the dinuclear cadmium cores;(b)View of the (4,4)layer in 2
2.2 Different geometries of 1 and 2
By changing the metal salts,two new H2FDC-based CPs are synthesized based on the 2,5-furandicarboxylic acid under the same reaction conditions(130℃).From the structures descriptions above,we can see that the metal ions are crucial in determining the structures of the resultant CPs.
When the Zn(NO3)2·6H2O was used,complex 1 is obtained and features a 1D chain structure,where the chains are further linked into a layer structure though hydrogen bonds.During the synthesis of 2,the Zn(NO3)2·6H2O is changed to Cd(NO3)2·4H2O.Complex 2 shows a 2D(4,4)network structure.
To summarize,the metal ions may plays a dominating and steering role in increasing the binding sitesof the ligand and further framework dimensionality of two coordination polymers.Further studies are ongoing with differentmetal ions(e.g.Cu2+,Mg2+)to get an insight how the metal salts affect the final structures and to enrich such inorganic-organic hybrid materials with interesting network topologies and properties.
2.3 IR spectra and TGA
The IR spectra shows broad band in the 3 378 cm-1for 2,which may be assigned to theν(O-H) stretching vibrations of the water molecules(Fig.S1). The features at1 625 and 1 357 cm-1for 1,1 573 and 1 374 cm-1for 2,are associated with the asymmetric (COO)and symmetric(COO)stretching vibrations.
Thermal gravimetric analyses(TGA)were carried out to examine the thermal stability of 1 and 2.The samples were heated up in flowing N2with a heating rate of10℃·min-1.The TG curve for 1 shows aweight loss in the temperature range of 150~220℃corresponds to the removal of two DMF ligands(Calcd. 39.97%,Obsd.40.27%).Upon further heating,the framework was stable up to 320℃and then a sharp weight loss was observed above 320℃due to the collapse of the framework(Fig.4).The TGA curve for 2 shows a weight loss between 50~260℃,which
corresponds to the loss of two[Me2NH2]+cations,two aqua ligands and four free water molecules(Calcd. 22.55%,Obsd.22.81%).Upon further heating,a sharp weight loss was observed above 260℃due to the collapse of the framework.
2.4 Powder X-ray diffraction analysis
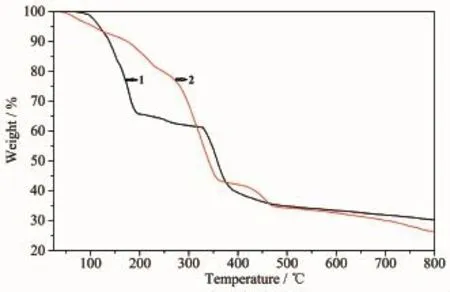
Fig.4 TGA curves of complexes 1 and 2
In order to check the purity of complexes 1 and 2,bulk samples were measured by X-ray powder diffraction at room temperature with the 2θrange from 5°to 40°.As shown in Fig.5,the peak positions of the experimental patterns are in good agreement with the simulated patterns,which clearly indicates the good purity of the complexes.
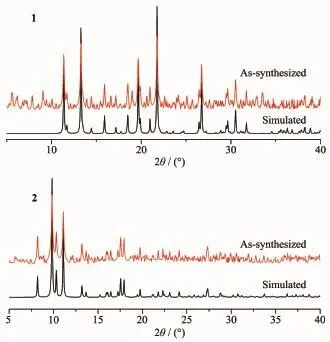
Fig.5 PXRD patterns of 1 and 2
2.5 Lum inescent properties
Luminescent properties of coordination polymers with zinc/cadmium centers have attracted intense interest due to their potential applications[22-25].Herein, we examined the luminescent properties of the two CPs in the solid state at room temperature under the excitations of 303 and 350 nm,respectively.The photoluminescenct spectrum of H2FDC ligand shows emission maxima at 410 nm[26].The emission band of the free ligand is probably attributable to theπ*-π transition.Upon complexation of the ligandswith Znions,the emission peak occur at 406 nm(λex=303 nm) for 1 and 470 nm(λex=350 nm)for 2,respectively (Fig.6).The short wavelength band of 1 is almost same as that of the H2FDC ligand,which indicatesthat the emission of 1 probably origins from the H2FDC ligand,whereas the emission maximum of 2 is clearly red-shifted compared with the H2FDC ligand.Complex 2 represents an obvious qualitative change of luminescent property resulted from the interaction between metal ion and ligand.The emission of 2 probably origins from ligand-to-metal charge transfer excited state[27].The luminescent lifetimes of solid 1 and 2 using an Edinburgh FLS920 phosphorimeter with 450 W xenon lamp as excitation source show lifetimes of 76.2 and 138.1 ns,respectively(Fig.7).
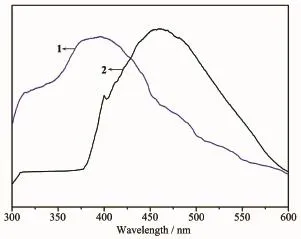
Fig.6 Fluorescence spectra of 1 and 2 at room temperature
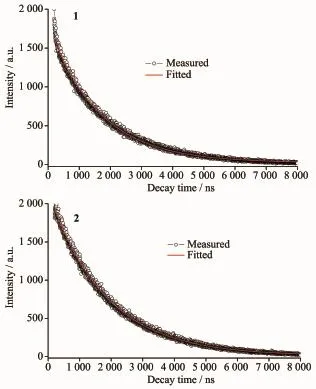
Fig.7 Luminescent lifetimes of 1 and 2 in solid state at room temperature
3 Conclusions
In summary,by changing themetal ions,two CPs have been prepared based on the 2,5-furandicarboxylic acid under the same reaction conditions(130℃). Complex 1 performs a 1D chain structure,where the chains are further connected into 2D layer structure by hydrogen bonds,whereas2 shows2D(4,4)network. Moreover,two complexes emit bright blue fluorescence(406 nm,λex=303 nm for 1;470 nm,λex=350 nm for 2)with the lifetimes of 76.2 and 138.1 ns, respectively.
Supporting information isavailable athttp://www.wjhxxb.cn
[1]Wu H,ThibaultCG,Wang H,etal.MicroporousMesoporous Mater.,2016,219:186-189
[2]Song T,Yu J,Cui Y,et al.Dalton Trans.,2016,45:4218-4223
[3]ZhangW,Xiong R G.Chem.Rev.,2012,112:1163-1195
[4]Wu Y,Wang J,Zou L,et al.Inorg.Chem.Commun.,2016, 69:13-15
[5]Chowdhuri A R,Bhattacharya D,Sahu S K.Dalton Trans., 2016,45:2963-2973
[6]Ma D Y,Li Z,Xiao JX,et al.Inorg.Chem.,2015,54:6719-6726
[7]Hu Y,Liu Z,Xu J,et al.J.Am.Chem.Soc.,2013,135:9287 -9290
[8]Nazari M,Rubio-Martinez M,Tobias G,et al.Adv.Funct. Mater.,2016,26:3244-3249
[9]Rao A S,Pal A,Ghosh R,et al.Inorg.Chem.,2009,48:1802 -1804
[10]Liu L,Huang S P,Yang G D,et al.Cryst.Growth Des., 2010,10:930-936
[11]LiC P,Du M.Chem.Commun.,2011,47:5958-5972
[12]Singh M K,Banerjee A.Cryst.Growth Des.,2013,13:2413-2425
[13]Zlatongrorsky S,Ingleson M J.Dalton Trans.,2012,41:2685-2693
[14]Bu F,Lin Q,ZhaiQ,et al.Angew.Chem.Int.Ed.,2012,51: 8538-8541
[15]Bruker.APEXⅡSoftware,Version 6.3.1,Bruker AXS Inc, Madison,Wisconsin,USA,2004.
[16]Sheldrick GM.Acta Crystallogr.,Sect.A,2008,A64:112-122 [17]Spek A L.PLATON,a Multipurpose Crystallographic Tool, Utrecht University,Utrecht,The Netherlands,2005.
[18]Liang L C,Chou K W,Su W J,et al.Inorg.Chem.,2015, 54:11526-11534
[19]Dar A A,Sen S,Gupta S K,et al.Inorg.Chem.,2015,54: 9458-9469
[20]Vaidhyanathan R,Natarajan S,Rao C N R.J.Solid State Chem.,2001,162:150-157
[21]Chen H X,Ma Y,Zhou F,etal.J.Solid State Chem.,2013, 203:1-7
[22]Mautner FA,Berger C,Fischer R C,etal.Polyhedron,2016, 111:86-93
[23]Qiu Y,Deng H,Mou J,et al.Chem.Commun.,2009:5415-5417
[24]LIU Ji-Wei(刘继伟).Chinese J.Inorg.Chem.(无机化学学报),2017,33(4):705-712
[25]HU Sheng(胡升),ZHOU Chang-Xia(周常侠).Chinese J. Inorg.Chem.(无机化学学报),2016,32(6):1111-1119
[26]Sen R,Mal D,Brandao P,et al.Cryst.Growth Des.,2013, 13:5272-5281
[27]Qiu Y,Liu B,Peng G,et al.Inorg.Chem.Commun.,2010, 13:749-752
M etal Ions Controlled Assembly of Two Coordination Polymers: Structures and Lum inescence Properties
MA De-Yun*LIXiang GUO Hai-Fu MA Yan-Hua
LINWan-Chun WENMei-Ling CAILi-Wen ZHU Li-Feng
(School of Chemistry and Chemical Engineering,Zhaoqing University,Zhaoqing,Guangdong 526061,China)
Two zinc/cadmium-based coordination polymers(CPs),[Zn(FDC)(DMF)2]n(1)and{(Me2NH2)2[Cd2(FDC)3(H2O)2]·4H2O}n(2)(H2FDC=2,5-furandicarboxylic acid,DMF=N,N-dimethyl formamide)have been prepared based on 2,5-furandicarboxylate under the same reaction conditions(130℃and DMF)and were structurally characterized by elemental analysis,IR,TGA,powder X-ray diffraction and single-crystal X-ray diffraction.Complex 1 performs a 1D chain structure,where the chains are further connected into 2D layer structure by hydrogen bonds,whereas 2 shows 2D(4,4)network and is unstable in air.The results of thermal analysis indicate that 1 is stable up to 300℃after the DMF molecules are removed,but 2 is unstable after the aqua ligands,cations and solvent molecules are removed.Moreover,the luminescent properties of 1 and 2 were detected at room temperature under the excitations of 303 nm and 350 nm,respectively.Both of 1 and 2 emit the intensely blue characteristic luminescence at room temperature(λmax=406 and 470 nm),with lifetimes of up to 76.2 and 138.1 ns,respectively. CCDC:1489352,1;1527106,2.
coordination polymers;crystal structure;2,5-furandicarboxylate;metal ions effect;luminescent
O614.24+1;O614.24+2
A
1001-4861(2017)07-1266-07
10.11862/CJIC.2017.162
2017-01-18。收修改稿日期:2017-05-15。
大学生创新训练计划项目(No.201610580008)、广东省自然科学基金(No.2015A030310407)、广东省普通高校特色创新项目(No.2015KTSCX156)、2017年大学生科技创新培育专项资金(“攀登”计划专项资金)(No.pdjh2017b0551)和广东高校优秀青年教师培训计划项目(No.YQ2015168)资助。
*通信联系人。E-mail:mady@zqu.edu.cn

