化疗联合参麦注射液对晚期非小细胞肺癌患者细胞免疫及肿瘤恶性程度的影响
罗丽华
(河北省秦皇岛市第三医院肿瘤科,秦皇岛,066000)
化疗联合参麦注射液对晚期非小细胞肺癌患者细胞免疫及肿瘤恶性程度的影响
罗丽华
(河北省秦皇岛市第三医院肿瘤科,秦皇岛,066000)
目的:探讨化疗联合参麦注射液治疗晚期非小细胞肺癌(NSCLC)的临床疗效及对患者细胞免疫、肿瘤恶性程度的影响。方法:选取2011年5月至2016年2月秦皇岛市第三医院收治的NSCLC患者162例,随机分为观察组和对照组,每组81例。对照组患者给予单纯化疗治疗,观察组在对照组的基础上联合参麦注射液治疗。治疗6个月后统计2组临床疗效;检测并比较治疗前后2组患者血清炎性细胞因子水平及肿瘤标志分子水平;检测并比较治疗前后2组患者全血中T淋巴细胞亚群比例的变化;统计治疗期间2组不良反应。结果:治疗6个月后观察组缓解率为79.01%,显著高于对照组的50.62%(P<0.01);与治疗前比较,治疗后观察组患者血清IFN-γ、IL-2、TNF-α水平均明显升高,且显著高于对照组(P<0.05或P<0.01);观察组患者血清IL-6及IL-10水平均明显降低,且显著低于对照组(P<0.01);与治疗前比较,治疗后对照组CD4+比例及CD4+/CD8+均明显降低(P<0.01),而观察组CD8+比例明显降低,CD4+比例、NK细胞比例及CD4+/CD8+均明显升高(P<0.05或P<0.01),且2组间差异有统计学意义(P<0.05或P<0.01);与治疗前比较,治疗后2组血清CEA、CA125、CYFRA21-1、NSE及SCC-Ag水平均明显降低,且观察组显著低于对照组(P<0.01);与对照组比较,观察组患者恶心、呕吐,白细胞减少及血小板下降发生率明显降低(P<0.05或P<0.01)。结论:化疗联合参麦注射液可明显调节晚期NSCLC患者血清细胞因子水平,提高患者细胞免疫功能,并有效降低血清肿瘤标志物水平,降低肿瘤恶性程度,疗效显著优于化疗单用,同时其也可有效降低患者不良反应发生率,具有较高的安全性。
非小细胞肺癌;化疗;参麦注射液;细胞免疫;肿瘤标志物;疗效
非小细胞肺癌(Non-small Cell Lungcar Cinoma,NSCLC)属于肺癌中最为常见的类型,且发病率和致死率均比较高,约占肺癌总量的80%[1-5]。中晚期NSCLC患者主要临床症状表现为咳嗽、胸部胀痛、气促、咳痰带血、低热等[6-8]。局部晚期NSCLC患者往往无法接受手术治疗,单纯化疗患者肿瘤负荷较重,疗效并不理想,同时化疗具有严重的不良反应,影响患者免疫功能,还易引发骨髓造血功能抑制、血细胞减少等严重并发症[9-10]。中医药也是治疗NSCLC常用的方法之一,为有效减轻化疗的不良反应,可将化疗与中医药有机结合,提高中晚期NSCLC的综合治疗效果[11]。据报道[12-13],参麦注射液可显著抑制NSCLC患者肿瘤血管的生长,调节患者免疫功能,起到明显的增效减毒作用,但有关其与化疗联合应用治疗NSCLC的研究尚不充分,因此本研究采用参麦注射液联合化疗治疗晚期NSCLC,旨在探讨参麦注射液联合化疗对改善NSCLC患者细胞免疫及肿瘤恶性程度效果,现将结果报道如下。
1 资料与方法
1.1 一般资料 选取2011年5月至2016年2月本院收治的NSCLC患者162例,将所有患者随机分为对照组(n=81)及观察组(n=81),对照组男47例,女34例;年龄31~67岁,平均年龄(50.68±10.24)岁;病理类型:鳞癌27例,腺癌39例,其他15例;临床分期:ⅢA期43例,ⅢB期38例。观察组男45例,女36例;年龄32~69岁,平均年龄(49.93±10.14)岁;病理类型:鳞癌26例,腺癌37例,腺鳞癌18例;临床分期:ⅢA期42例,ⅢB期39例。2组患者性别、年龄、病理类型及临床分期等方面一般资料比较均差异无统计学意义(P>0.05),具有可比性。本研究经本院医学伦理委员会讨论决定且所有患者及家属均签署知情同意书。
1.2 诊断标准 所有患者均经病理学或细胞学证实且均符合《中国常见恶性肿瘤诊治规范》中的相关诊断标准[14]。
1.2 纳入标准 所有患者均为原发且初次治疗,无明显手术指征;预计生存期>6个月;据国际抗癌联盟(UICC)分期标准,均为ⅢA或ⅢB期;据东部肿瘤协作组(ECOG)PS评分为0~2分。
1.3 排除标准 肿瘤分期达中晚期及以上者;对本研究所用药物过敏者;依从性较差者;合并严重心、脑等功能障碍及免疫系统疾病者;肿瘤细胞发生转移者;主支气管受侵犯者;治疗前接受过放/化疗治疗者。
1.4 治疗方法 对照组患者进行单纯化疗治疗:鳞癌患者予以多西他赛37.5 mg/m2加至250 mL生理盐水中,d 1及d 8静脉滴注,同时联合顺铂75 mg/m2分3 d给予,即d 1至d 3静脉滴注化疗,21 d为1个疗程;腺癌及其他类型患者予以培美曲塞500 mg/m2d 1静脉滴注或紫杉醇75 mg/m2d 1静脉滴注,同时联合顺铂75 mg/m2d 1静脉滴注化疗,21 d为1个疗程。观察组患者进行化疗联合参麦注射液(四川升和药业股份有限公司生产,国药准字Z20043478,规格:20 mL/支)治疗,40 mL/次,1次/d,每个疗程连续静脉滴注15 d;化疗方案同对照组。2组均至少连续治疗2个疗程。
1.5 观察指标 分别于治疗前后采集患者空腹静脉血5 mL,经离心分离血清,以ELISA法检测2组血清IFN-γ、IL-2、TNF-α、IL-6及IL-10等细胞因子水平;另以流式细胞仪检测2组全血中NK细胞比例及T淋巴细胞比例(CD4+及CD8+),计算CD4+/CD8+;以自动电化学发光免疫分析仪检测2组患者血清癌胚抗原(CEA)、糖类抗原125(CA125)、肿瘤细胞角蛋白19片段(CYFRA21-1)、神经元特异性烯醇化酶(NSE)、鳞状细胞癌相关抗原(SCC-Ag)等肿瘤标志物水平;统计2组治疗期间不良反应发生情况。
1.6 疗效判定标准 分别于治疗前及治疗6个月后对2组患者进行胸部CT扫描,复查,参照实体瘤疗效评价标准(RECIST)[15]评价2组近期临床疗效:完全缓解(CR):肿瘤病灶消失并维持4周;部分缓解(PR):肿瘤组织缩小30%或以上并维持4周;疾病稳定(SD):肿瘤病灶体积缩小<30%或增大<20%;疾病进展(PD):肿瘤病灶体积增大>20%或有新病灶。缓解率(RR)=(CR+PR)例数/总例数×100%。

2 结果
2.1 2组患者临床疗效比较 治疗6个月后对照组患者PD 13例,SD 27例,PR 18例,CR 23例,RR为50.62%(41/81);观察组PD 7例,SD 10例,PR 35例,CR 29例,RR为79.01%(64/81);观察组RR显著高于对照组(P<0.01)。
2.2 治疗前后2组细胞免疫功能比较
2.2.1 治疗前后2组血清细胞因子水平比较 治疗前2组血清细胞因子水平差异无统计学意义(P>0.05);与治疗前比较,治疗后观察组患者血清IFN-γ、IL-2、TNF-α水平均明显升高,且显著高于对照组(P<0.05或P<0.01);观察组患者血清IL-6及IL-10水平均明显降低,且显著低于对照组(P<0.01)。见表1。
2.2.2 治疗前后2组T淋巴细胞亚群及NK细胞比例变化 治疗前2组T淋巴细胞亚群及NK细胞比例差异无统计学意义(P>0.05);与治疗前比较,治疗后对照组CD4+比例及CD4+/CD8+均明显降低(P<0.01),而观察组CD8+比例明显降低,CD4+比例、NK细胞比例及CD4+/CD8+均明显升高(P<0.05或P<0.01),且2组间差异有统计学意义(P<0.05或P<0.01)。见表2。
2.3 治疗前后2组血清肿瘤标志物水平比较 治疗前2组血清肿瘤标志物水平差异均无统计学意义(P>0.05);与治疗前比较,治疗后2组血清CEA、CA125、CYFRA21-1、NSE及SCC-Ag水平均明显降低,且观察组显著低于对照组(P<0.01)。见表3。
2.4 不良反应 与对照组比较,观察组患者恶心、呕吐,白细胞减少及血小板下降发生率明显降低(P<0.05或P<0.01)。见表4。

表1治疗前后2组血清细胞因子水平的比较
注:与治疗前比较,*P<0.05,**P<0.01;与对照组比较,△△P<0.01。
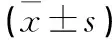
表2 治疗前后2组T淋巴细胞亚群及NK细胞比例变化的比较
注:与治疗前比较,*P<0.05,**P<0.01;与对照组比较,△P<0.05,△△P<0.01。
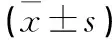
表3 治疗前后2组血清肿瘤标志水平比较
注:与治疗前比较,**P<0.01;与对照组比较,△△P<0.01。
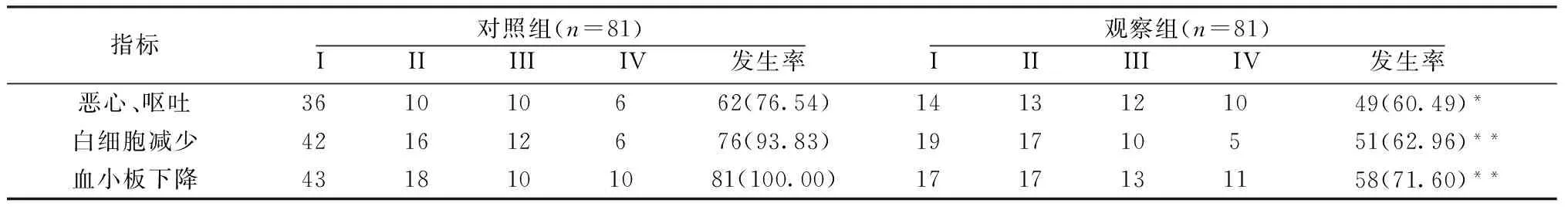
表4 2组不良反应比较[n(%)]
注:与对照组比较,*P<0.05,**P<0.01。
3 讨论
化疗在NSCLC的治疗中具有及其重要的作用,是中晚期NSCLC患者的常规治疗方式,但化疗药物在抑制、杀灭肿瘤细胞的同时,也会严重影响人体正常细胞,造成一系列化疗不良反应,进一步损害机体自身免疫功能[16-19]。加之,晚期肺癌患者体质虚弱,受化疗不良反应影响机体免疫功能进一步受损,细胞因子水平紊乱,大多数患者因不能耐受而影响治疗,因此单纯化疗并不能起到预期的效果,甚至反而会因免疫功能进一步降低而威胁生命安全[20-24]。中药联合化疗治疗中晚期非小细胞肺癌已在临床广泛应用,在稳定瘤体、降低肿瘤标志物水平等方面取得了较好疗效,且中药与化疗有协同作用,可明显改善生活质量并延长患者生存期,同时还可在一定程度上减轻化疗的不良反应[25-29]。
中医学认为肺癌属“咳嗽、痰饮、肺积、肺壅”等范畴,其是因虚而致实、得病,是正虚为本,虚中夹实的疾病。其主要由邪毒乘虚入肺,导致肺脏阴阳失调,宣降失司,气机不利,血行受阻,致痰凝气滞,瘀阻络脉,邪气瘀毒胶结,日久形成肺部积块。故应以益气养阴为主要治疗原则[30]。参麦注射液主要由红参、麦冬组成,麦冬甘寒,可养阴润肺、益胃生津;红参甘温,可补气滋阴、益血生津;共奏益气固脱、养阴生津、运脾化湿之功效[31]。据报道,参麦注射液联合化疗治疗恶性肿瘤具有一定的增效减毒作用。本研究结果显示,观察组RR为79.01%,显著高于对照组的50.62%;且观察组患者恶心、呕吐,白细胞减少及血小板下降发生率较对照组明显降低,与相关研究[32]结果相吻合,提示参麦注射液可明显提高NSCLC患者的临床疗效,同时可明显降低化疗的毒副租用,增效减毒作用显著。
NSCLC患者普遍处于免疫抑制状态,免疫功能障碍也是导致肿瘤细胞持续增殖、侵袭的重要原因[33]。现代药理研究证实,参麦注射液的有效成分主要有人参皂苷、人参多糖、麦冬皂苷、麦冬多糖及麦冬黄酮等,具有广泛的免疫药理活性,可通过抑制巨噬细胞释放炎性反应递质胺减轻机体损伤,有效调节机体免疫功能,明显增加肿瘤患者的抗肿瘤能力,其中人参皂苷具有双相免疫调节作用,可调节机体非特异性及特异性免疫功能;麦冬多糖可诱导机体免疫系统产生多种细胞因子,增强机体体液免疫及细胞免疫应答[34]。T淋巴细胞是恶性肿瘤免疫监视系统中起关键作用的功能细胞,NSCLC患者细胞免疫功能紊乱,表现为CD4+比例及CD4+/CD8+比值下降,且细胞因子水平失衡,其中IL-6及IL-10等Th2型细胞因子占优势地位,而IFN-γ、TNF-α及IL-2等Th1型细胞因子水平较低,T淋巴细胞亚群比例失衡或Th1/Th2偏移越严重,机体的免疫抑制反应也会随之加重,且以上变化随着肿瘤病情加重而进一步发展[35]。本研究结果显示,治疗后观察组患者血清IFN-γ、IL-2、TNF-α水平均明显升高,而IL-6及IL-10水平均明显降低,且2组间差异有统计学意义;此外,治疗后对照组CD4+比例及CD4+/CD8+均较治疗前明显降低,而观察组CD8+比例较治疗前明显降低,CD4+比例、NK细胞比例及CD4+/CD8+均较治疗前明显升高,且2组间差异有统计学意义,与王蓉等[36]研究结果相似。肿瘤标志物水平在肿瘤疗效评价方面意义重大,其水平升高与肿瘤负荷密切相关,是判断肿瘤恶性程度的首要指标。CEA主要存在于胚胎性肿瘤组织中的糖蛋白抗原,是动态监测反映肺癌治疗效果的公认指标;CA125是一种似黏液糖蛋白复合物,可普遍用于肺癌、胃癌等的检出及疗效判断;CYFRA21-1主要存在于肺组织尤其是肺肿瘤上皮细胞的胞质中,SCC-Ag属于鳞状上皮细胞癌相关抗原,NSE是肺癌的特异性辅助诊断指标,3者均会随着患者病情变化而出现明显波动[37-39]。本研究中治疗后2组血清CEA、CA125、CYFRA21-1、NSE及SCC-Ag水平均较治疗前明显降低,且观察组显著低于对照组,提示参麦注射液可明显调节NSCLC患者细胞因子水平,增强其细胞免疫功能,有效抑制肿瘤进展,降低机体肿瘤标志物水平,减轻患者恶性程度。
综上所述,参麦注射液联合化疗可明显调节晚期NSCLC患者血清细胞因子水平,提高患者细胞免疫功能,增强机体抵抗肿瘤的能力,有效降低患者血清肿瘤标志物水平,抑制肿瘤恶性发展,同时患者不良反应明显减少,提示参麦注射液是一种安全、有效的NSCLC化疗辅助治疗药物,可起到明显的增效减毒作用,值得临床上推广使用。
[1]李艳,郭其森.晚期非小细胞肺癌维持治疗进展[J].中华肿瘤防治杂志,2014,21(10):800-804.
[2]D′Addario G,Früh M,Reck M,et al.Metastatic non-small-cell lung cancer:ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up[J].Ann Oncol,2010,21 Suppl 5:v116-119.
[3]Velcheti V,Schalper K A,Carvajal D E,et al.Programmed death ligand-1 expression in non-small cell lung cancer[J].Laboratory investigation; a journal of technical methods and pathology,2014,94(1):107-116.
[4]Peters S,Adjei AA,Gridelli C,et al.Metastatic non-small-cell lung cancer (NSCLC):ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up[J].Ann Oncol,2012,23 Suppl 7:vii56-64.
[5]Shaw A T,Kim D W,Mehra R,et al.Ceritinib in ALK-rearranged non-small-cell lung cancer[J].New England Journal of Medicine,2014,370(13):1189.
[6]Lynch TJ,Bell DW,Sordella R,et al.Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib[J].N Engl J Med,2004,350(21):2129-39.
[7]Schiller JH,Harrington D,Belani CP,et al.Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer[J].N Engl J Med,2002,346(2):92-98.
[8]Inoue A,Suzuki T,Fukuhara T,et al.Prospective phase II study of gefitinib for chemotherapy-naive patients with advanced non-small-cell lung cancer with epidermal growth factor receptor gene mutations[J].J Clin Oncol,2006,24(21):3340-3346.
[9]胡毅,冯奉仪.晚期非小细胞肺癌化疗研究现状及展望[J].国外医学:肿瘤学分册,2002,29(3):197-201.
[10]Dimitroulis J,Stathopoulos GP.Evolution of non-small cell lung cancer chemotherapy (Review)[J].Oncol Rep,2005,13(5):923-930.
[11]Cao Y,Li P,Tan KJ.[Clinical observation on shenmai injection in preventing and treating adverse reaction of chemotherapy on advanced non-small cell lung cancer][J].Zhongguo Zhong Xi Yi Jie He Za Zhi,2006,26(6):550-552.
[12]Yang,Yuzhen,Peng,et al.Clinical observation of Shenmai injection in the treatment for adverse reactions of chemotherapy on advanced non-small cell lung cancer[J].The Chinese-German Journal of Clinical Oncology,2008,7(2):81-83.
[13]W.Leszczyński,L.Hawrylewicz,A.Namys-Kaletka,et al.Description of non-complanar conformal radiotherapy technique used in stomach cancer patients[J].Onkologia I Radioterapia,2014,26(1):30-35.
[14]全国肿瘤防治办公室.中国常见恶性肿瘤诊治规范[S].北京:北京医科大学,中国协和医科大学联合出版社,1990.
[15]杨学宁,吴一龙.实体瘤治疗疗效评价标准-RECIST[J].循证医学,2004,4(2):85-90,111.
[16]Danesi R,Pasqualetti G,Giovannetti E,et al.Pharmacogenomics in non-small-cell lung cancer chemotherapy[J].Adv Drug Deliv Rev,2009,61(5):408-417.
[17]Scagliotti GV,Lodico D,Gozzelino F,et al.Unresectable non-small cell lung cancer chemotherapy with high-dose cisplatin and etoposide[J].Oncology,1985,42(4):224-228.
[18]Ginopoulos P,Spyropoulos K,Kardamakis D,et al.Advanced non-small cell lung cancer chemotherapy:a randomized trial of two active regimens (MVP and PE)[J].Cancer Lett,1997,119(2):241-247.
[19]Wang S,Liu F,Zhu J,et al.DNA Repair Genes ERCC1 and BRCA1 Expression in Non-Small Cell Lung Cancer Chemotherapy Drug Resistance[J].Med Sci Monit,2016,22:1999-2005.
[20]Srdic D,Plestina S,Sverko-Peternac A,et al.Cancer cachexia,sarcopenia and biochemical markers in patients with advanced non-small cell lung cancer-chemotherapy toxicity and prognostic value[J].Support Care Cancer,2016,24(11):4495-4502.
[21]Vieira G B B,Gonçalves R B,Milan G S,et al.The comparison of quality of life with matching paired method in non-small cell lung cancer chemotherapy-an interim report[J].Revista Gestão Industrial,2013,9(3):88.
[22]Tilden D,Aristides M,Kielhorn A,et al.Major determinants of cost in advanced non-small-cell lung cancer chemotherapy regimes in France[J].Value in Health,2002,5(6):538-539.
[23]Liu CH,Peng YJ,Wang HH,et al.Heterogeneous prognosis and adjuvant chemotherapy in pathological stage I non-small cell lung cancer patients[J].Thorac Cancer,2015,6(5):620-628.
[24]Lin S Y,Liu L M,Wu L C.Effects of Shenmai injection on immune function in stomach cancer patients after chemotherapy[J].Chinese Journal of Integrated Traditional & Western Medicine,1995,15(8):451-453.
[25]Lin Y S,Liu L M,Wu L C,et al.Effect of Shenmai Injection on lmmunologic Function in Stomach Cancer Patients after Chemotherapy[J].Chinese Journal of Integrative Medicine,1996,4(3):195-197.
[26]Zhu W R,Zheng L,Guo Y B,et al.Clinical research of intraperitoneal chemotherapy plus Shenmai Injection in treating advanced colorectal cancer[J].Journal of Chinese Integrative Medicine,2005,3(4):266-269.
[27]Wang L,Huang XE,Cao J.Clinical study on safety of cantharidin sodium and shenmai injection combined with chemotherapy in treating patients with breast cancer postoperatively[J].Asian Pac J Cancer Prev,2014,15(14):5597-5600.
[28]Lin S Y,Liu L M,Wu L C,et al.Effect of Shenmai injection,on immunologic function in stomach cancer patients after chemotherapy) on immunologic function in stomach cancer patients after chemotherapy[J].Chinese Journal of Integrative Medicine,1996,2(3):195-197.
[29]Zhou Y F,Min-Hua W U,Chen X F,et al.Effection of Shenmai Injection for chemotherapy patients with cancer on blood tests and CD4/CD8 ratio[J].Chinese Journal of Clinical Pharmacology & Therapeutics,2011,16(12):1410-1413.
[30]李莉.参麦注射液在肺癌综合治疗中的临床价值分析[J].临床医药文献电子杂志,2016,3(23):4680-4680,4681.
[31]汤建军.参麦注射液联合吉西他滨对老年非小细胞肺癌患者生活质量及免疫功能的影响[J].河南中医,2014,34(2):349-351.
[32]Wang X,Lin H,Liyuan LV,et al.A meta-analysis of Kang`ai injection combined with chemotherapy in the treatment of advanced non-small cell lung cancer[J].J Cancer Res Ther,2015,11(3):558-564.
[33]Remark R,Becker C,Gomez JE,et al.The non-small cell lung cancer immune contexture.A major determinant of tumor characteristics and patient outcome[J].Am J Respir Crit Care Med,2015,191(4):377-390.
[34]刘怀民,杨峰,王怀璋.参麦注射液对非小细胞肺癌化疗增效减毒作用的临床观察[J].四川中医,2007,25(8):72.
[35]Roepman P,Jassem J,Smit EF,et al.An immune response enriched 72-gene prognostic profile for early-stage non-small-cell lung cancer[J].Clin Cancer Res,2009,15(1):284-290.
[36]王蓉,李大鹏,冯军.参麦注射液联合化疗对晚期非小细胞肺癌患者T细胞亚群及细胞因子的影响[J].南京中医药大学学报,2016,32(2):125-128.
[37]Molina R,Filella X,Augé JM,et al.Tumor markers (CEA,CA 125,CYFRA 21-1,SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis.Comparison with the main clinical and pathological prognostic factors[J].Tumour Biol,2003,24(4):209-218.
[38]李前进,潘杰.支气管动脉化疗栓塞联合放射性粒子植入治疗IIIa-IIIb期非小细胞肺癌后的肿瘤恶性程度评估[J].海南医学院学报,2016,22(15):1718-1721.
[39]Cedrés S,Nuez I,Longo M,et al.Serum tumor markers CEA,CYFRA21-1,and CA-125 are associated with worse prognosis in advanced non-small-cell lung cancer (NSCLC)[J].Clin Lung Cancer,2011,12(3):172-179.
(2017-03-24收稿 责任编辑:王明)
Effect of Chemotherapy Combined with Shenmai Injection on Cellular Immunity and Tumor Malignance of Advanced Non-small Cell Lung Cancer Patients
Luo Lihua
(DepartmentofOncology,TheThirdHospitalofQinhuangdao,Qinhuangdao066000,China)
Objective: To investigate the clinical efficacy of chemotherapy combined with Shenmai injection in the treatment of advanced non-small cell lung cancer (NSCLC) and its effect on cellular immunity and tumor malignant degree of patients. Methods: A total number of 162 patients with NSCLC admitted in the hospital from May 2011 to February 2016, and were randomly divided into control group and observation group, with 81 cases in each group. The patients in the control group were treated with chemotherapy, while patients in the observation group were additionally treated with Shenmai Injection. The clinical efficacy of the two groups was evaluated after 6-month treatment. The levels of serum inflammatory cytokines and tumor markers were measured and compared between the two groups before and after the treatment. Changes of T lymphocyte subsets in the whole blood of the two groups were detected and compared before and after treatment. Adverse reactions during the treatment were observed. Results: After 6-month treatment, the remission rate was 79.01% in the observation group, which was significantly higher than that of 50.62% in the control group (P<0.01). The levels of serum IFN-γ, IL-2 and TNF-α in the observation group were significantly higher than those in the control group (P<0.05 orP<0.01). The levels of IL-6 and IL-10 in the observation group were significantly lower than those in the control group (P<0.05 orP<0.01). The proportion of CD4+and CD4+/CD8+in the control group were significantly lower (P<0.01), while the proportion of CD8+in the observation group significantly decreased.CD4+ratio, NK cell ratio and CD4+/CD8+significantly increased (P<0.05 orP<0.01), and there was significant difference between the two groups after the treatment (P<0.05 orP<0.01).The levels of serum CEA, CA125, CYFRA21-1, NSE and SCC-Ag in the two groups were significantly lower and the observation group was significantly lower than the control group after the treatment (P<0.01). The incidence of nausea, vomiting, leukopenia and thrombocytopenia was significantly lower, compared with the control group (P<0.05;P<0.01). Conclusion: Chemotherapy combined with Shenmai injection may significantly regulate the serum cytokine levels in patients with advanced NSCLC, improve the cellular immune function, effectively reduce serum tumor marker level and reduce tumor malignancy. The effect is significantly better than chemotherapy and it may effectively reduce the incidence of adverse reactions with high safety.
Non-small cell lung cancer; Chemotherapy; Shenmai Injection; Cellular immunity; Tumor markers; Efficacy
罗丽华(1978.09—),女,本科,主治医师,研究方向:肿瘤内科学,E-mail:13933521310@163.com
R273
A
10.3969/j.issn.1673-7202.2017.05.034

