Implications from protein uptake kinetics onto dextran-grafted Sepharose FF coupled with ion exchange and affinity ligands☆
Aiying Xue,Linling Yu,Yan Sun*
Department of Biochemical Engineering and Key Laboratory of Systems Bioengineering of the Ministry of Education,School of Chemical Engineering and Technology,Tianjin University,Tianjin 300072,China
1.Introduction
Polymer-grafted adsorbent has been researched extensively in ion exchange chromatography(IEC)in recent years[1-4].For example,as the representative polymer-grafted ion-exchangers,dextran-grafted IEC resins[2,5],such as the commercial SP Sepharose XL,Streamline Q XL[2,6-8],and the customized T40-SP-X-S6B[9]were widely used for antibody purification.Besides,poly(ethylenimine)(PEI),a synthetic polyelectrolyte,was also modified on agarose gels to prepare polymergrafted IEC media[3].Compared with conventional IEC adsorbents,polymer-grafted IEC adsorbents have exhibited significant advantages,especially with respectto the enhanced uptake kinetics[5,10].The faster uptake rate has been explained mainly by the “chain delivery”effect,driven by the chemical potential of the bound proteins as well as the interactions between neighboring flexible chains mediated by the bound proteins[11,12].In dextran-grafted[12]and PEI-modified IEC adsorbents[4,11],the “chain delivery”effect has been clearly identified.
However,protein uptake kinetics in polymer-grafted adsorbentwith affinity ligands has not been studied.It is of significance to elucidate protein uptake behavior in polymer-grafted affinity chromatography(AC)adsorbents,because AC has been playing a vital role in protein purification[13].Our group has developed a biomimetic design strategy for affinity peptide ligands of human immunoglobulin G(hIgG)based on the affinity motif of Protein A,and octapeptide FYCHWQDE was identified as a high-affinity ligand for hIgG[14,15].
In this work,dextran-grafted Sepharose gels were prepared and FYCHWQDE was coupled at three different ligand densities.Adsorption behaviors of hIgG on the three adsorbents were determined and compared with those on the non-grafted FYCHWQDE resins,in order to investigate the effects of ligand density and grafted dextran on the affinity adsorption of hIgG.In concert with experimental evidence obtained in the dextran-grafted and non-grafted IEC adsorbents with diethylaminoethyl(DEAE)ligand,the results are expected to deepen our understanding ofthe roles ofgrafted polymer chains in the transport of bound proteins in ion-exchange and affinity adsorbents.
2.Materials and Methods
2.1.Materials
Sepharose FF was purchased from GE Healthcare(Uppsala,Sweden).Dextran(Mw500000)and human immunoglobulin G(hIgG,purity>90%)were obtained from Sigma-Aldrich(St.Louis,MO,USA).Octapeptide FYCHWQDE was synthesized by the solid-phase synthesis method and provided by GL Biochem Ltd.(Shanghai,China).All other reagents were of analytical grade from local suppliers.
2.2.Preparation of adsorbents
The dextran-grafted adsorbent was prepared with Sepharose FF by the method described previously[10,16].The initial concentration of dextran solution was 200 mg·ml-1.The obtained product was denoted as D-FF.
In the preparation of IEC adsorbents,the functional group DEAE was introduced as described by Shiet al.[17].DEAE was coupled onto D-FF and Sepharose FF at similar ligand densities by adjusting the concentration of diethylaminoethyl chloride(DEAE-Cl)in the reaction systems.The obtained resins were denoted as DEAE-D-FF and DEAE-FF,respectively.
The dextran-grafted and FYCHWQDE-modified affinity gels were prepared by coupling FYCHWQDE onto D-FFviathe thiol group on the cysteine residue in the affinity ligand as described below.First,a resin named 2-pyridyl-disul fide-D-FF was prepared by introducing the functional group 2-pyridyl disul fide to D-FF by the method described by Ferrazet al.[18].Then,FYCHWQDE was coupled onto 2-pyridyldisul fide-D-FF by the method reported previously[14].Dextrangrafted affinity adsorbents with three different ligand densities(31.1,45.3 and 59.4 μmol·ml-1)were synthesized,named W-D-31,W-D-45 and W-D-59,respectively.Here,W-D is used to denote the dextran-grafted FYCHWQDE adsorbents.Besides,the ligand FYCHWQDE was also coupled directly onto Sepharose FF following the above-mentioned method.For comparisons,non-grafted affinity resins at the three similar ligand densities were prepared and denoted as W-31,W-45 and W-59,respectively.
2.3.Measurement of dextran content and ligand density
The quantitative determination of dextran content was carried out based on gravimetric determination as described in the literature[5,19].The results indicated a dextran content of 35 mg·ml-1.Ionexchange capacities of the DEAE-D-FF and DEAE-FF were determined by the silver chloride precipitation titration reported previously[20].The peptide ligand densities of the affinity resins were determined by the method described by Xueet al.[15].
2.4.Adsorption isotherms and uptake kinetics
Static adsorption experiments of hIgG on IEC and AC adsorbents were carried out in 20 mmol·L-1Tris-HCl(pH 8.0)and 20 mmol·L-1phosphate buffer(pH 6.0),respectively,as described earlier[15,21].Initial protein concentration was in the range of 0.2-4.0 mg·ml-1and the residual concentration was detected after adsorption for 24 h,which was con firmed to have achieved the adsorption equilibrium.The adsorption density of protein(q)was calculated by mass balance with the free protein concentration in equilibrium(c),and the Langmuir model was used to describe the adsorption isotherms,

whereqmis the adsorption capacity andKdis the dissociation constant.
Dynamic uptake experiments for hIgG were conducted in a 100-ml three-neck round-bottom flask at 25°C as described by Zhaoet al.[22].The buffer systems used in the dynamic experiments were the same as those in static adsorption experiments.Initial protein concentration was set at 1 mg·ml-1.The effective pore diffusivity of the protein(De)was calculated by fitting the experimental uptake data to the pore diffusion modeldescribed previously[19].De,a lumped kinetic parameter describing the overall uptake rate of protein adsorption,was normalized with the free solution diffusivity of hIgG(D0)to compare the intensification of intraparticle mass transfer[10,23].TheD0value of hIgG is 4.0 × 10-11m2·s-1at 25 °C[24].In the kinetic studies,each experiment was conducted in triplicate and the average value was reported with its standard deviation.
3.Results and Discussion
3.1.Protein uptake to ion exchangers
The adsorption isotherms of hIgG on the two IEC adsorbents are shown in Fig.1(a).It can be seen that all the isotherms could be well fitted by the Langmuir equation.Moreover,neither of the isotherms reached a plateau atthe concentration range tested herein,so the values of fittedqmwere not suitable to describe the adsorption abilities.However,the fittedKdwas little affected,because it is determined by the equilibriumdata atlowerconcentrations(around theKdvalue).Besides,initial hIgG concentration selected for investigating the uptake kinetics was 1 mg·ml-1,and the experimental concentration was 0-1 mg·ml-1,within concentration range tested in the adsorption isotherms.Thus,adsorption isotherms in Fig.1(a)covered the concentration range used in the uptake experiment and met the requirement of the present research.
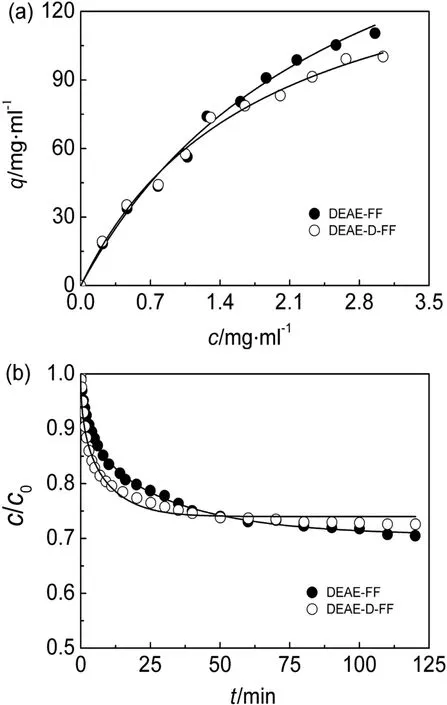
To have a clear view of the adsorption ability of hIgG to the IEC adsorbents and make a legible comparison,the adsorbed protein density(qc)at an equilibrium liquid-phase concentration(c)of 2 mg·ml-1,calculated from Eq.(1)using the Langmuir parameters,was used as a substitute of protein adsorption capacity[11].The calculatedqcand LangmuirparameterKdare listed in Table 1.Itis found thatthe adsorption capacities of hIgG were comparative on the DEAE-D-FF and DEAE-FF resins with similar ion-exchange capacities.Similar phenomenon was observed in dextran-grafted hydrophobic charge-induction chromatography resins with ligand densities of 60-90 μmol·g-1reported by Liuet al.[25].For DEAE-D-FF,the grafted dextran chains partially occupied the pore volume,which might lead to a decrease in the accessible pore space[26]and was then negative on hIgG adsorption.However,the grafted dextran in the resin could provide a 3-D binding volume with better accessibility for protein binding,which was bene ficial in hIgG adsorption[12,19].Interplay of the two effects would make the adsorption capacities of DEAE-FF and DEAE-D-FF comparative.
Fig.1(b)shows the uptake curves ofhIgG on the two IEC adsorbents.The pore diffusion model provided good simulations of the uptake kinetics and the correspondingDe/D0values are given in Table 1.It can be seen that the uptake curve of DEAE-D-FF changed more quickly with the uptake time than that of DEAE-FF.The results in Table 1 show that theDe/D0value of DEAE-D-FF was 2.7 times higher than that for DEAE-FF,indicating that the uptake rate of hIgG on DEAE-D-FF was accelerated remarkably by the dextran grafting,which was coincident with the results of dextran-grafted IEC adsorbents reported previously[5,10,12,19,23].Namely,the flexible dextran layer[19]offered a“chain delivery”effect to the bound protein[3,12,27],which facilitated hIgG uptake onto DEAE-D-FF.
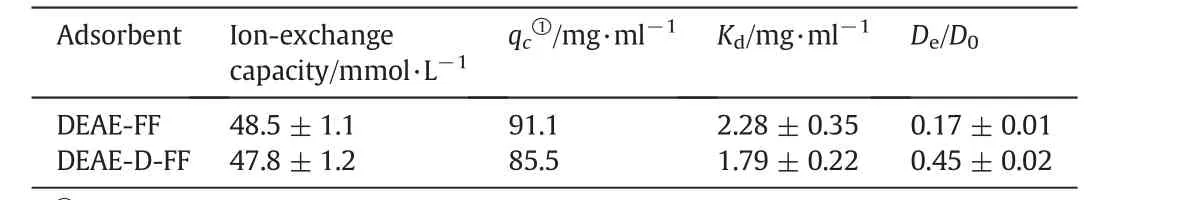
Table 1Langmuir and uptake parameters of hIgG adsorption onto ion exchangers
3.2.Af finity adsorption to FYCHWQDE-Sepharose
The adsorption isotherms of hIgG on the dextran-grafted and non-grafted FYCHWQDE adsorbents are shown in Figs.2(a)and 3(a),respectively.Similar with the above ion exchange,qc(c=2 mg·ml-1)was used to approximate hIgG adsorption capacity and the calculated values are listed in Table 2 together with theKdvalues for comparison of the three pairs of dextran-grafted and non-grafted FYCHWQDESepharose resins.
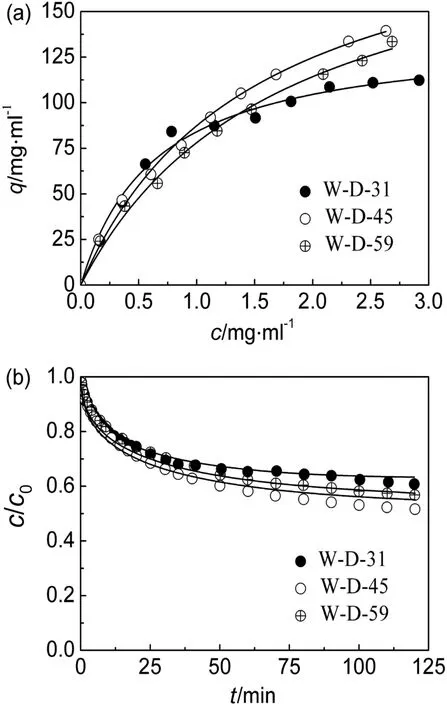
Fig.2.Adsorption isotherms(a)and uptake curves(b)ofhIgGin 20 mmol·L-1 phosphate buffer(pH 6.0)on dextran-grafted FYCHWQDE adsorbents.

Fig.3.Adsorption isotherms(a)and uptake curves(b)ofhIgGin 20 mmol·L-1 phosphate buffer(pH 6.0)on non-grafted FYCHWQDE adsorbents.
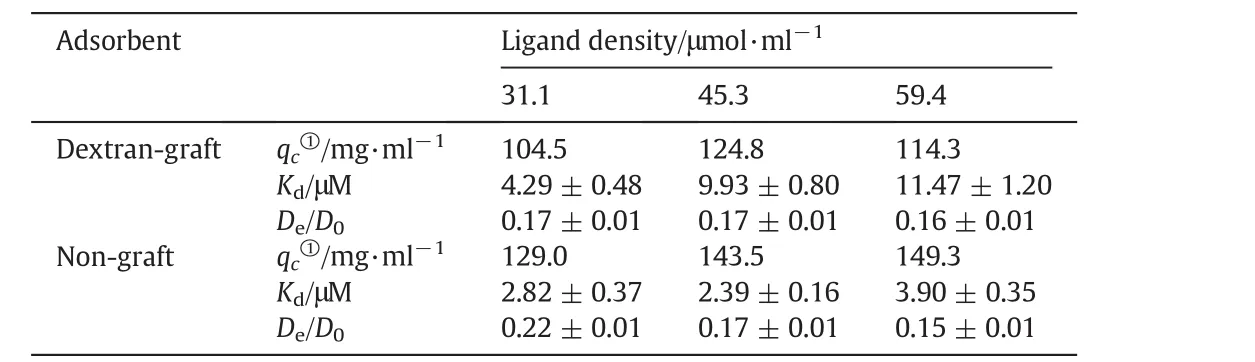
Table 2Langmuir and uptake parameters of hIgG adsorption onto FYCHWQDE-Sepharose resins
It is found from Table 2 that,for the three pairs of FYCHWQDE adsorbents,the adsorption capacities of hIgG on the W-D resins decreased by the dextran grafting,distinctly different from the ion-exchange adsorption with dextran-grafted IEC adsorbents reported in literatures[5,10,23].However,it is similar to the dextran-grafted mixed-mode chromatography(MMC)adsorption reported by Yuet al.[19],in which the decrease of adsorption capacity was attributed to the collapse of dextran layer caused by hydrophobic interaction between the ligands(4-(1H-imidazol-1-yl)aniline).Besides,it was reported that hydrophobic interaction,generated between the hydrophobic ligands(benzoyl groups or butyl groups)immobilized on PEI chains,also led to the collapse of PEI chains grafted in MMC adsorbents at high NaCl concentrations[27,28].In this research,there are three hydrophobic residues(phenylalanine,tyrosine and tryptophane)in FYCHWQDE.Therefore,hydrophobic interactionsviathe FYCHWQDE ligands coupled on the dextran chainsmightlead to partialorcomplete collapse of the dextran layer.Thus,the grafted dextran on the W-D resins could not provide a 3-D binding volume,but the chain collapse caused a decrease in the accessible pore space,resulting in the decrease of the adsorption capacities of the W-D resins.This speculation was supported by the increase inKdvalues with dextran grafting,as given in Table 2.The dextran layer collapse in the W-D resins made the binding sites for hIgG less than those in the corresponding non-grafted resins of the same ligand densities[27].Less binding sites for hIgG weakened the binding strength for hIgG[4],resulting in the increase ofKd,the apparent value of dissociation constant.
Only to the W-D resins,the value ofqcpresented an inverted V-shape as ligand density increased.The available binding sites for hIgG increased with ligand density.However,the severer dextran layer collapse with increasing hydrophobic ligand density[28]reduced the available binding sites.By the interplay of the two opposite factors,the adsorption capacity reached the maximum on W-D-45.Moreover,the two opposite effects on the available binding sites for hIgG on the W-D resins also led to weakened affinities for hIgG,namely an increase inKdvalue as the ligand density increased,as shown in Table 2.
Figs.2(b)and 3(b)show the uptake curves of hIgG on the dextrangrafted and non-grafted FYCHWQDE adsorbents,respectively,and calculated valuesofDe/D0are summarized in Table 2.Itcould be observed that the uptake curves of the three W-D resins were similar,in accordance with the values of fittedDe/D0listed in Table 2,implying that the effective pore diffusivity of hIgG on the dextran-grafted AC resin was not influenced obviously by ligand density.From Table 2,it can also be seen that theDe/D0value for W-D-31 was obviously smaller than that for W-31.Namely,there was slower protein uptake to the dextran-grafted affinity resin.This indicates that the grafted dextran layer reduced the pore size[19]and then increased the hindrance for protein pore diffusion[3,20].More importantly,it is found that theDe/D0values for the W-D resins were quite similar with those for the non-grafted ion exchangers listed in Table 1.This implies the lack of chain delivery effect on the affinity adsorbent,even though the presence of polymer chains.
Thus,it can be concluded that different from ion-exchange adsorption,chain delivery does not happen for affinity adsorption to polymer-grafted porous media.This is considered due to the specific binding of the affinity ligand to the protein.Because there is only one single binding site(the consensus inter-CH2-CH3 interaction site)on the Fc fragment of hIgG[15],it is obvious that the bound protein was not able to interact with another ligand on a neighboring polymer chain.That is,the basis for chain delivery does not exist for the affinity adsorption.In other words,chain delivery only happens for multivalent proteins(proteins with many binding sites like charged groups)bound on flexible chains of enough length.
3.3.Further discussion on chain delivery
From the above results,itwas clear thatthe uptake rate ofhIgGcould be enhanced by chain delivery effectin dextran-grafted IEC resin,similar with many other polymer-grafted IEC adsorbents[3,5,10-12],as illustrated in Fig.4(a).The transfer of the bound protein on a polymer chain was facilitated through the electrostatic interaction between the protein and the neighboring chain by the flexible chain swings.
However,chain delivery effect did not happen in the AC adsorbent,as illustrated in Fig.4(b).For the protein molecule with only a single binding site,the bound protein by affinity interaction cannot interact with other affinity ligands on neighboring chains because of the lack ofextra binding sites.So the chain delivery did nothappen in the affinity adsorption.
Besides,chain delivery effect was not yet observed in the dextrangrafted MMC adsorbent with 4-(1H-imidazol-1-yl)aniline as a hydrophobic ligand[19].The main reason was attributed to the collapse of dextran layer due to hydrophobic interaction between the MMC ligand.In PEI-modified MMC adsorbent,a collapse of PEI layer happened as well on account of hydrophobic interaction generated by the immobilized hydrophobic ligands on PEI layer,leading to that the grafted PEI chains could not play a role in accelerating mass transfer of protein,unlike in PEI-modified IEC adsorbents[19,28].
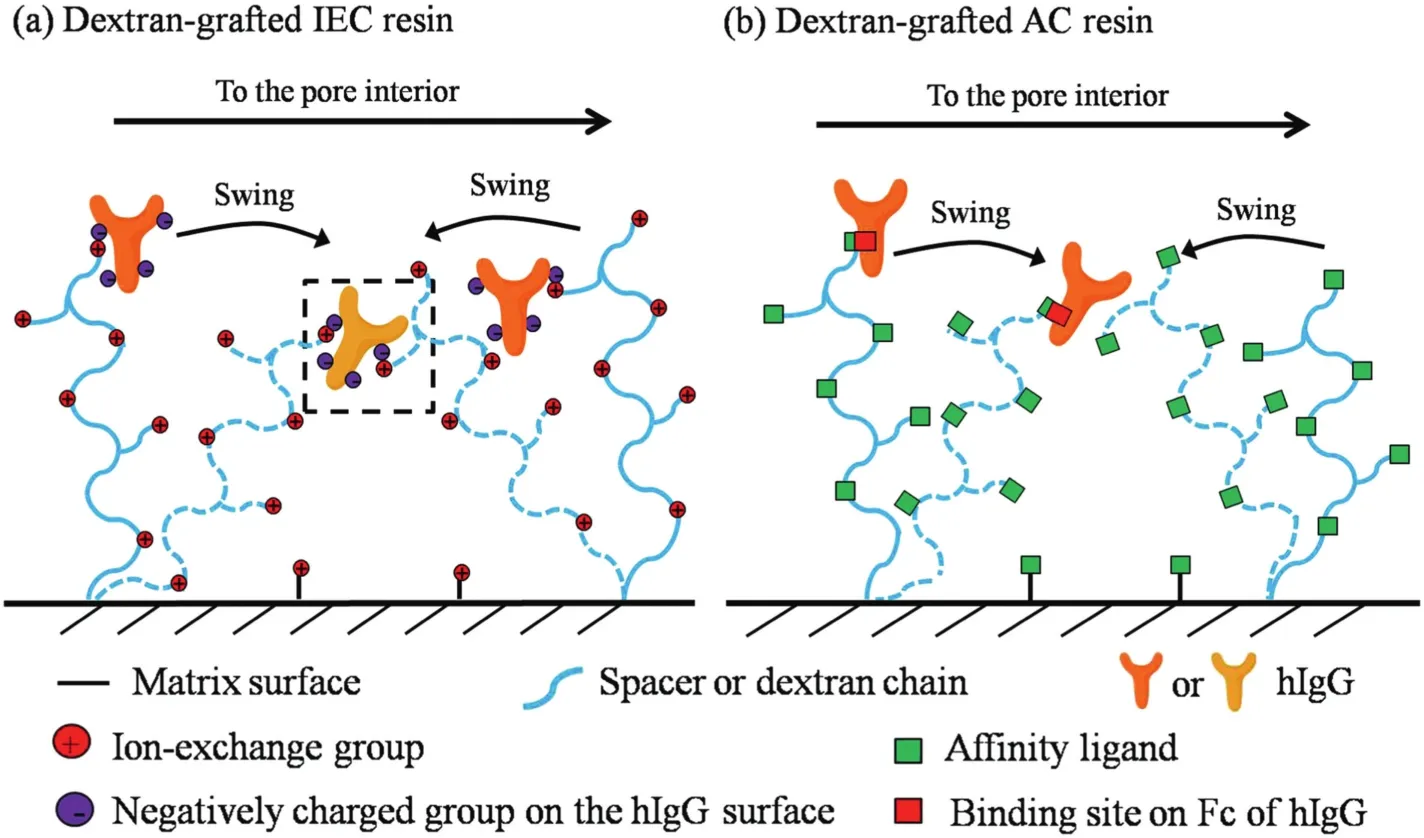
Fig.4.Schematic illustrations of mass transfer in(a)dextran-grafted IEC pore surface and(b)dextran-grafted affinity resin pore surface.The dashed blue curves represent the grafted polymer chains with adsorbed proteins after swing,and the dashed black box with a lightened Y-type pattern in(a)highlights the sites for the delivery of bound protein(hIgG herein).The quantities of the dextran chains and the ligands in this figure do not represent their real densities on the pore surface.The quantity of binding sites on hIgG in(a)does not represent its real density while in(b)indeed does(single binding site).In(a)it shows chain delivery occurs for the bound hIgG via dextran chain swings while in(b)chain delivery does not occur due to the lack of extra affinity binding sites on the protein.
In comparison of the kinetic results in IEC[5,10-12],AC and MMC[19,27,28],three necessary elements for chain delivery in polymergrafted adsorbents have been revealed.First,the polymer should be long enough and of a certain flexibility to guarantee that adsorbed protein on a polymer chain can be transferred to the adjacent polymer chains by the swing of the long and flexible chains.Second,the coupled ligands should be of low hydrophobicity,which does not compromise the chain delivery by causing chain collapses by hydrophobic interactions.Third,the bound protein should be multivalent,making extra binding sites available for interacting with neighboring chains once it is bound to a chain.Thus,only protein adsorption onto flexible polymer-grafted ion exchangers meets all the three requirements.By contract,affinity or hydrophobic adsorption does not due to the lack of binding sites on target proteins or chain collapses caused by hydrophobic interactions.
4.Conclusions
In this work,dextran-grafted Sepharose gel with a dextran grafting density of35 mg·ml-1was fabricated and then ion-exchange and affinity ligands were separately immobilized on its surface to investigate the roles of grafted dextran in different adsorbents.Results of hIgG adsorption on IEC adsorbents showed that the grafted dextran could markedly enhance mass transfer of hIgG by“chain delivery”effect.For affinity adsorption,however,the adsorption capacity of the affinity resins decreased and the dissociation constant of hIgG increased by grafting dextran.Moreover,the uptake rate of hIgG onto the affinity resins was not enhanced by the grafted dextran chains,indicating that“chain delivery”effect did not happen in the dextran-grafted affinity adsorbents.Different roles of grafted dextran in IEC,MMC and AC resins for protein adsorption revealed three necessary elements for the happening of chain delivery in polymer-grafted adsorbents,namely long and flexible polymer chains for ligand coupling,low hydrophobic ligands and multivalent proteins with more than one binding sites for the ligands.
Nomenclature
cfree protein concentration in equilibrium,mg·ml-1
Deeffective pore diffusivity of the protein,m2·s-1
D0free solution diffusivity of hIgG(4.0 × 10-11m2·s-1at25 °C)
Kddissociation constant,mg·ml-1(or μmol·L-1)
qadsorption density of protein,mg·ml-1
qcadsorbed protein density at an equilibrium liquid-phase concentration,mg·ml-1
qmadsorption capacity of protein,mg·ml-1
[1]E.J.Suda,K.E.Thomas,T.M.Pabst,P.Mensah,N.Ramasubramanyan,M.E.Gustafson,A.K.Hunter,Comparison of agarose and dextran-grafted agarose strong ion exchangers for the separation of protein aggregates,J.Chromatogr.A1216(2009)5256-5264.
[2]Y.Tao,G.Carta,G.Ferreira,D.Robbins,Adsorption of deamidated antibody variants on macroporous and dextran-grafted cation exchangers:I.Adsorption equilibrium,J.Chromatogr.A1218(2011)1519-1529.
[3]L.L.Yu,S.P.Tao,X.Y.Dong,Y.Sun,Protein adsorption to poly(ethylenimine)-modified Sepharose FF:I.A critical ionic capacity for drastically enhanced capacity and uptake kinetics,J.Chromatogr.A1305(2013)76-84.
[4]Y.Zhao,X.Dong,L.Yu,Y.Sun,Protein adsorption to poly(ethylenimine)-modified Sepharose FF:VI.Partial charge neutralization drastically increases uptake rate,J.Chromatogr.A1427(2015)102-110.
[5]M.C.Stone,G.Carta,Protein adsorption and transport in agarose and dextrangrafted agarose media for ion exchange chromatography,J.Chromatogr.A1146(2007)202-215.
[6]A.Ljunglöf,K.M.Lacki,J.Mueller,C.Harinarayan,R.van Reis,R.Fahrner,J.M.van Alstine,Ion exchange chromatography of antibody fragments,Biotechnol.Bioeng.96(3)(2007)515-524.
[7]A.Ljunglöf,J.Thömmes,Visualising intraparticle protein transport in porous adsorbents by confocal microscopy,J.Chromatogr.A813(2)(1998)387-395.
[8]R.Wongchuphan,B.T.Tey,W.S.Tan,S.K.Subramanian,F.S.Taip,T.C.Ling,Purification of rabbit polyclonal immunoglobulin G using anion exchangers,Process Biochem.46(1)(2011)101-107.
[9]Y.Tao,G.Carta,Rapid monoclonal antibody adsorption on dextran-grafted agarose media for ion-exchange chromatography,J.Chromatogr.A1211(1)(2008)70-79.
[10]Q.H.Shi,G.D.Jia,Y.Sun,Dextran-grafted cation exchanger based on superporous agarose gel:Adsorption isotherms,uptake kinetics and dynamic protein adsorption performance,J.Chromatogr.A1217(2010)5084-5091.
[11]Y.Hong,N.Liu,W.Wei,L.L.Yu,G.Ma,Y.Sun,Protein adsorption to poly(ethylenimine)-modified Sepharose FF:III.Comparison between different proteins,J.Chromatogr.A1342(2014)30-36.
[12]L.Yu,L.Gong,S.Bai,Y.Sun,Surface DEAE groups facilitate protein transport on polymer chains in DEAE-modified-and-DEAE-dextran-grafted resins,AIChE J62(10)(2016)3812-3819.
[13]Y.D.Clonis,Affinity chromatography matures as bioinformatic and combinatorial tools develop,J.Chromatogr.A1101(2006)1-24.
[14]W.W.Zhao,F.F.Liu,Q.H.Shi,X.Y.Dong,Y.Sun,Biomimetic design of affinity peptide ligands for human IgG based on protein A-IgG complex,Biochem.Eng.J.88(2014)1-11.
[15]A.Xue,W.W.Zhao,X.Liu,Y.Sun,Affinity chromatography of human IgG with octapeptide ligands identified from eleven peptide-ligand candidates,Biochem.Eng.J.107(2016)18-25.
[16]J.Zhao,S.Yao,D.Lin,Adsorbents for expanded bed adsorption:Preparation and functionalization,Chin.J.Chem.Eng.17(2009)678-687.
[17]Q.H.Shi,X.Zhou,Y.Sun,A novel superporous agarose medium for high-speed protein chromatography,Biotechnol.Bioeng.92(2006)643-651.
[18]N.Ferraz,J.Leverrier,F.Batista-Viera,C.Manta,Thiopropyl-agarose as a solid phase reducing agent for chemical modification of IgG and F(ab′)2,Biotechnol.Prog.24(2008)1154-1159.
[19]L.L.Yu,Q.H.Shi,Y.Sun,Effect of dextran layer on protein uptake to dextran-grafted adsorbents for ion-exchange and mixed-mode chromatography,J.Sep.Sci.34(2011)2950-2959.
[20]L.L.Yu,Y.Sun,Protein adsorption to poly(ethylenimine)-modified Sepharose FF:II.Effect of ionic strength,J.Chromatogr.A1305(2013)85-93.
[21]D.Xiang,Y.Wang,J.Zhao,S.Zhu,Effect of pore-size of mesoporous SBA-15 on adsorption of bovine serum albumin and lysozyme protein,Chin.J.Chem.Eng.18(2010)493-499.
[22]W.W.Zhao,Q.H.Shi,Y.Sun,FYWHCLDE-based affinity chromatography of IgG:Effect of ligand density and purifications of human IgG and monoclonal antibody,J.Chromatogr.A1355(2014)107-114.
[23]M.C.Stone,Y.Tao,G.Carta,Protein adsorption and transport in agarose and dextran-grafted agarose media for ion exchange chromatography:Effect of ionic strength and protein characteristics,J.Chromatogr.A1216(2009)4465-4474.
[24]A.R.Özdural,A.Alkan,P.J.A.M.Kerkhof,Modeling chromatographic columns:Non-equilibrium packed-bed adsorption with non-linear adsorption isotherms,J.Chromatogr.A1041(2004)77-85.
[25]T.Liu,D.Q.Lin,H.L.Lu,S.J.Yao,Preparation and evaluation ofdextran-grafted agarose resin for hydrophobic charge-induction chromatography,J.Chromatogr.A1369(2014)116-124.
[26]A.R.Ubiera,G.Carta,Radiotracer measurements of protein mass transfer:Kinetics in ion exchange media,Biotechnol.J.1(2006)665-674.
[27]N.Liu,Z.Wang,X.Liu,L.Yu,Y.Sun,Characterization of novel mixed-mode protein adsorbents fabricated from benzoyl-modified polyethylenimine-grafted Sepharose,J.Chromatogr.A1372(2014)157-165.
[28]L.Yu,N.Liu,Y.Hong,Y.Sun,Protein adsorption and chromatography on novel mixed-mode resins fabricated from butyl-modified poly(ethylenimine)-grafted Sepharose,Chem.Eng.Sci.135(2015)223-231.
 Chinese Journal of Chemical Engineering2017年7期
Chinese Journal of Chemical Engineering2017年7期
- Chinese Journal of Chemical Engineering的其它文章
- Elevating the flexibility and operability of dividing-wall distillation columns via feed thermal condition adjustment☆
- Hydrodynamic dispersion ofreactive solute in a Hagen-Poiseuille flow of a layered liquid
- Interpenetrating polymers supported on microporous polypropylene membranes for the transport of chromium ions☆
- Suppression of gold nanoparticle agglomeration and its separation via nylon membranes☆
- Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions
- Horizontal gas mixing in rectangular fluidized bed:A novel method for gas dispersion coefficients in various conditions and distributor designs
