The kinetic analysis of optimization and selective transportation of Cu(II)ions with TNOA as carrier by MDLM system☆
R.Donat*,Ö.Durmaz ,H.Cetişli
1 Department of Chemistry,Faculty of Sciences Arts,Pamukkale University,20070 Kınıklı,Denizli,Turkey
2 Department of Chemistry,Faculty of Sciences Arts,Afyon Kocatepe University,03200 Afyon,Turkey
1.Introduction
The last ten years have witnessed a series of milestone contributions which inspired researchers to discover new research areas.Along with the developments in especially industry,the amount of wastes dropped into the environment has increased apparently.Among them,Cu(II)ion containing industrial wastes caused by mining,electroplating,printed circuit board manufacturing,metal processing,hydrometallurgical,and electric industries threatens the environment because of their toxicity[1,2].
By forming complexes with proteins,copper is a fundamental element for biochemical reactions of proteins,enzymes and coenzymesviasubstituting metal ions in several catalytic or structural processes[3,4].Copper deficiency can lead to many diseases in humans,such as hematological deficiencies,anemia,neutropenia,thrombocytopenia,and skin depigmentation.On the other hand,high level of copper deposits or consumption can cause intestinal distress,kidney damage,and anemia.Additionally,ingestion of large amount of copper sulfate can result in even death[4].The most dangerous form of Cu(II)to living organisms and the most abundant copper species is cupric ion[5].
Nowadays,due to some advantages of traditional separation and solid membrane techniques such as the lower capital and operating costs,low energy consumption,high concentration factors,high fluxes,etc.,for the extraction and recovery of heavy metals from aqueous solutions,liquid membrane systems are being used by researchers[5,6].
There are different methods to extract and retrieve Cu(II)ions from aqueous solutions like chemical precipitation,reverse osmosis,ion exchange,solvent extraction,membrane filtration and adsorption[5].But these techniques have some problems such as less effectiveness,delicate running conditions,resulting secondary sludge,high fundamental and running costs,and additional high-cost ejection[5,7].
In liquid membrane systems,kerosene,hexane,chloroformbased,benzene,dinitrile and chlorinated organic solvents are used as organic solvents[5,6,8–14].Different carriers have been conducted for the transportation of Cu(II)ionsvialiquid membrane systems such as DEHPA,erythromycin ethyl succinate,D2EHPA,CYANEX 272,LIX 984 N,pyridine-2-acetaldyde benzoylhydrazone(2-APBH),2,2′-bis(p-octyloxybenzyl)diethylenetriamine(bis-pODET),Acorga M5640(salicylaldoxime derivative),LIX 54,and TNOA[2,3,8,15–20].
Selective extraction of Cu(II)ions has been carried out in different studies such as separation of Cu(II),Co(II),and Ni(II)ions with TOA and TlOA as carrier[21],simultaneous extraction of Cu(II),Zn(II),Fe(III),and Ni(II)ions with Cyanex 272 as carrier[22],selective transport of copper ions with 8-hydroxy quinoline as carrier[23],and the removal of Cd(II)and Cu(II)ions with Aliquat 336 as a carrier[24].
In the previous work[25],transportation of Mo(VI)ions achieved successfullyviamulti-dropped liquid membrane(MDLM)system used tri-n-octylamine(TNOA)as the carrier with the help ofNa2CO3as acceptor phase.
The intention of this article is not only to high light the transportation of Cu(II)ions attractively but also to universalize our newly designed MDLM system and expose pathways to be followed by scientists.Firstly,the batch transportation study was first performed to figure out the optimumcircumstances through the MDLM technique.Secondly,selective transportation of Cu(II)ions with alkaline,alkaline earth,and different heavy metal ions at optimum conditions of single Cu(II)extraction was conducted.MDLM system was handled to examine the particular parameters involving the transportation rate of copper by TNOA in kerosene.TNOA is selected as carrier since it is water insoluble and can form oil soluble salts of anionic species at low pH[8].
2.Materials and Methods
2.1.Chemicals and solutions
Refined kerosene was provided by TUPRAŞ A.Ş (Turkish Petroleum Refineries Company),copper(II)chloride,ammonium molybdate,iron(III)nitrate,zinc nitrate,sodium chloride,potassium chloride,calcium chloride,bariumchloride,sulphuric acid,tri-n-octylamine(TNOA),thiosemicarbazide and all other chemicals were acquired by Merck.
Stock solutions of 250 mg·L−1Cu(II),Fe(III),Mo(VI),and Zn(II)were prepared by CuCl2·2H2O,Fe(NO3)3·9H2O,(NH4)6Mo7O24·4H2O,and Zn(NO3)2·6H20,respectively and other concentrations of these solutions were made up by serial dilution of the stock solution.
Stock solution of 100 mg·L−1Na+,K+,Ca2+,and Ba2+was prepared by NaCl,KCl,CaCl2and BaCl2,respectively.All solutions were prepared in double distilled water,and the solution pH was adjusted by adding either 0.10 mol·L-1HCl or NH4OH solution as required.
2.2.Experimental setup
Batch experiments were accomplished by MDLM technique.More detailed information about how MDLM system work can be found in the previous studies[20,25].During the analysis of Cu(II)ions,samples were drawn from the first(donor phase)and second reactor(acceptor phase)in 10 minute time interval and analyzed by UV vis spectrophotometer(thiosemicarbazide method λ=357 nm)[26].
2.3.Analytical instruments
All absorbance measurements of Cu(II)ions in solutions were performed by Shimazdu UV-1201 V Model spectrophotometer with 1.00 cm quartz cells.In the batch experiments;to fix the temperature,Circu-WCR-P8 Wise model creosote device;to fix upon the concentration of Cu(II)ions in the solution,Shimazdu UV-1201 V model spectrophotometer;in order to transport the organic phase from one reactor to the other and supply compressive force,BT30-2 J(YZ1515X titled)peristaltic pump device and finally;for the determination of pH changes of the solution,WTW model Microprocessor pH meter is applied[20].Flame spectrophotometer is applied for the determination of alkaline and alkaline earth metalions,and ICP-OES is used for the determination of Cu(II),Zn(II),Fe(II),and Mo(VI)ions in the selective extraction.
2.4.Data analysis
Determination of Cu(II)ion concentration in the donor,organic and acceptor phase is explained in details in the previous studies[20,25].Summary of some formulas used for the calculations are given below.

whereRd,Rm,andRastand for the dimensionless reduced concentrations of Cu(II)in the donor,organic,and acceptor phases,respectively;k1andk2are pseudo- first-order apparent rate constants of the extraction and stripping,respectively;andJrepresents the flux rate.
The kinetics of the transportation process through MDLM were described as a first-order reaction in metal ion concentration:

whereCeis the metal ion concentration at a given time in the feed phase,Cois the initial concentration of metal ion in the feed phase,kis the rate constant(min−1),andtis the time(min).Thekvalues were determined by the plots of ln(Co/Ce)vs.time.
The percentage of metal ions transported was determined according to Eq.(6):

where Cu(II)strip(t)is the metal concentration in the stripping phase at elapsed time and Cu(II)(o)is the initial concentration of metal ions.
3.Results and Discussions
3.1.Single continuous extraction of Cu(II)ions
3.1.1.Effect of pH of donor phase
In this subsection,experiments were conducted to find the effect of pH of donor phase on the transportation of Cu(II)ions from donor phaseviaTNOA as organic carrier to acceptor phase in order to evaluate the performance of the MDLM system and obtain the best conditions for maximum extraction.Three sets of experiments were carried out in the pH range of donor phase from 8 to 10.pH of the donor phase is set by diluted ammonium solutions and the initial concentration of 100 ml of Cu(II)ions 250 mg·L−1as well as the temperature(298.15 K)and flow rates of both donor and acceptor phase were kept constant as 50 ml·min−1.100 ml 0.10 mol·L−1H2SO4is used as acceptor phase.The liquid membrane phase is composed of 100 ml 5.00 × 10−3mol·L−1TNOA as carrier in kerosene.
Kinetic pro files of each phase(donor,membrane and acceptor)at different pH of the donor phase are presented in Fig.1.
Cu(II)extraction and stripping processes obey consecutive first order reaction kinetics and reaction rate constants assume different values at different pH of donor phase.Hence,the plots of ln(Co/Ce)vs.time graph is given in Fig.2.
Accordingly,when TNOA is used as carrier,in conjunction with Cu(II)ions in the membrane phase,more than 99%of Cu(II)ions transported from donor phase to acceptor phase.Transportation duration for pH of 8,9,and 10 is 140,120,130 min,respectively.
Alagurajet al.removed Cu(II)ions from feed solution using emulsified liquid membrane,obtained best results at pH of feed solution range 8 to 10 H2SO4are used as acceptor phase and extraction of copper ions increased by decrease in the acidity of the feed solution[8].Sadeghiet al.transported Cu(II)ions selectively through the bulk liquid membrane with 93.50%transport efficiency mediated by erythromycin ethyl succinate(EES).Maximum extraction occurred by maintaining the pH of the feed solution at6.00 and the pH of the stripping phase that is L-histidine at 7.00[3].
The molecular structure and the chemistry of organic carrier in the complexation and transport processes are one of the most important factors ruling the selectivity of the membrane.And also,the carrier molecular structures can be adjusted to manage as particular selectivity[27].
Summary of the mechanism of Cu(II)extractionviaMDL Msystem is illustrated in Fig.3.The extraction rate of Cu(II)was assumed to be influenced by the rate of the chemical reactions(ion-exchange)occurring at the interface and by diffusion of the TNOA/Cu(II)species within the membrane).This is the extraction process of which the rate controlling step is the diffusion ofCu(II),NH4OH−and/or NR3molecules in donor and organic phases.Next,the(R3NH)2CuCl24−formed diffuses across the organic phase to the O/A interface.Mass transport within the membrane was the result of Fickian diffusion only.Fast mass transport occurred in the bulk of the aqueous phase as the aqueous solution was stirred so that the diffusion factor can be ignored.At the O/A interface,Cu(II)is released from(R3NH)2CuCl24−and diffuses into the bulk of acceptor phase in exchange of two moles of H+derived from H2SO4.During this process,one mole of NR3is regenerated and diffuse back to the bulk of organic phase due to their concentration gradient.This is the stripping process of which the rate-controlling step is the decomplexation reaction between(R3NH)2CuCl42−and H+at the O/A interface.The next separation cycle is started when two moles of NR3bind with Cu(II)that comes by to form another mole of(R3NH)2CuCl42−at the D/O interface.This is a facilitated counter-coupled transport mechanism since Cu(II)and H+travel in the opposite direction across the organic phase.

Fig.1.Concentrations of Cu(II)ions versus time graphs for the single continuous extraction studies through the kerosene based MDLM of(a)donor,(b)organic,and(c)acceptor phase at different pH of donor phase(8–10).

Fig.2.ln(C o/C e)versus time graphs for the single continuous extraction studies for the transportation of Cu(II)ions through the kerosene based MDLM at different pH of donor phase(8–10).
In the result of investigation,the following mechanism of reactions with chloride complexes of Cu(II)and TNOA was proposed:


Fig.3.Scheme of the single transportation of Cu(II)ions through MDLM system.
3.1.2.Effect of temperature
The experiments were performed using aqueous solutions of Cu(II)ions at pH value of 9 for donor phase,a membrane phase containing 5.00 × 10−3mol·L−1TNOA as carrier in kerosene,and 1.00 mol·L−1H2SO4as acceptor phase.The volume of donor,membrane,and acceptor phase is 100 ml.Temperature is one of the most important factors that determine the transport efficiency and mechanism of metal ions through MDLM system.Six sets of experiments were conducted and kinetic pro files of each phase(donor,organic,and acceptor)at different temperatures are presented in Fig.4.
The change of ln(Co/Ce)vs.time graphs are plotted in Fig.5.All the data were fitted adequately(R2≥0.99),accuracy and precision of the fitted parameters were verified.As it is seen in Fig.5,extraction and stripping of Cu(II)processes obey consecutive first order reaction kinetics at different temperatures.

Fig.4.Concentrations ofCu(II)ions versus time graphs forthe single continuous extraction studies through the kerosene based liquid membrane of(a)donor,(b)organic,and(c)acceptor phase at different temperatures(283.15–308.15 K).

Fig.5.ln(C o/C e)versus time graphs for the single continuous extraction studies for the transportation of Cu(II)ions through the kerosene based liquid membrane at different temperatures(283.15–308.15 K).
Transportation efficiency of Cu(II)ions at different temperatures(283.15–308.15 K)from donor phase to acceptor phase is 99.89%,99.98%,99.53%,99.90%,99.89%,and 99.48%respectively.Extraction time for different temperatures(283.15–308.15 K)is 170,150,130,120,130,and 150 min,respectively.
Transportation efficiency is not enough to determine the optimum temperature for the extraction of Cu(II)ions.Hence,it is better to compare the results in Table 1.
As shown in Table 1,in a continuous system with TNOA,reaction rate constants for the transportation from donor phase to membrane phase(k1)varying temperatures(283.15–308.15 K)are 2.91×10−2,2.75× 10−2,3.06× 10−2,3.42× 10−2,2.54× 10−2,and 2.65×10−2min−1,respectively,but those(k2)for the transportation from organic phase to acceptor phase are 1.79 × 10−2,2.39 × 10−2,3.10×10−2,3.66×10−2,2.65×10−2,and 2.77×10−2min−1,respectively.The smallest increment ink1than ink2justifies thatk1has minor andk2has a major effect on the transportation of Cu(II)ions with temperature changes.In low temperatures(283.15 K and 288.15 K)k1is greater thank2values and in higher temperatures(293.15 K and 298.15 K)k1is smaller thank2.These findings show that MDLM is governed by donor phase at the low temperatures and by acceptor phase at high temperatures.Additionally,Rmmaxandtmaxvalues decrease while flux rates(JdmaxandJamax)increase considerably with increasing temperature.Accordingly,the optimum temperature for Cu(II)extraction is 298.15 K since reducing the cost of the extraction and the best results obtained in this temperature.
Gathered values by batch experiments show that temperature is an important parameter in order to determine the mechanism of the transportation of Cu(II)ions.Cu(II)ion's transportation is consecutive first order irreversible reaction and the calculation of the activation energy of the process with the help of the input and output transport rate constants can be difficult due to instability of the rate constants.As it is mentioned in the literature[20,28,29],activation energy can be calculated with the help of maximum input(Jdmax)and output rates((Jamax))by using the Eq.(4).Maximum output ratesversus1/Tgraph is shown in Fig.6.The activation energy is calculated by using the slope of the line of the graph as 5.22 kcal·mol−1(21.82 kJ·mol−1).

Fig.6.Maximum output rates versus 1/T graph.
Generally,any process is diffusion-controlled if its activation energy(Ea)value 20 kJ·mol−1,intermediated-controlled if it is between 20 and 50 kJ·mol−1,chemical reaction-controlled if it is more than 50 kJ·mol−1[28].In this study,since the activation energy is found as 21.77 kJ·mol−1,this process is intermediated-controlled process.According to these results,transportation of Cu(II)ions achieved successfully by using MDLM system.
3.1.3.Effect of different concentration of acceptor phase
The reaction at the membrane/acceptor interface plays an important role on the transportation of Cu(II)ions through MDLM system.As the reaction given in Section 3.1,if the metal complex is not completely stripped,Cu(II)ions accumulate in the membrane phase and that makes membrane phase saturated with the complex which will gradually lower the permeation rate.The influence of the acceptor phase concentration on the extraction of Cu(II)ions was covered by varying the H2SO4concentration from 0.250 to 1.50 mol·L−1.The other parameters kept constant.Fig.7 reveals the kinetic pro files of each phase(donor,organic and acceptor)at different acceptor phase concentrations.
Fig.8 proves that extraction and stripping of Cu(II)at different acceptor phase concentration processes obey consecutive first order reaction kinetics.Because ln(Co/Ce)vs.time is directly proportional.All the data were fitted adequately(R2≥0.99),accuracy and precision of the fitted parameters were verified.

Table 1Comparison of kinetic parameters for the transportation of Cu(II)ions through the kerosene-based MDLM at different temperature
Transportation efficiency of Cu(II)ions at different acceptor phase concentration(0.25,0.50,0.75,1.00,1.25,and 1.50 mol·L−1)from donor phase to acceptor phase is 99.75%,99.97%,99.69%,99.90%,99.97%,and 99.97%,respectively.Extraction time for different acceptor phase concentration(0.25,0.50,0.75,1.00,1.25,and 1.50 mol·L−1)is 170,160,150,120,140,and 150 min,respectively.This data reveals that with the increase of H2SO4concentration from 0.25 to 1.00 mol·L−1in the acceptor phase,extraction of Cu(II)ions increases marginally with further increase in concentration.At a higher concentration(1.00 to 1.50 mol·L−1)ofH2SO4,more number of H+ions are added to acceptor phase and itmay cause a traffic on the interface of the membrane and acceptor phase.Additionally,extraction duration may shorten.Best results are obtained at 1.00 mol·L−1H2SO4in the acceptor phase.
Alagurajet al.transported Cu(II)ions from feed solution using emulsified liquid membrane,best results obtained at pH of acceptor phase range 1.00 to 2.00 and H2SO4used as acceptor phase[8].
Alguacilet al.removed 88%of Cu(II)ions from acidic waste waters using 2-hydroxy-5-nonylbenzaldehyde oxime as ionophore in pseudo-emulsion membrane with strip dispersion technology by using 180 g·L−1H2SO4as stripping phase[2].Changet al.investigated the simultaneous extraction and stripping of Cu(II)from aqueous solutions through a soybean oil-based bulk liquid membrane(BLM)viadi-2-ethylhexyl phosphoric(D2EHPA)acid as carrier by using sulphuric acid(H2SO4)at various concentrations and best results(86%stripped)obtained at 3.0 mol·L−1H2SO4[5].
In another study of Changet al.extracted Cu(II)ions from aqueous solutions using di-2-ethylhexylphosphoric acid(D2EHPA)and tributylphosphate(TBP)diluted in soybean oil.In the stripping studies,transportation efficiency of Cu(II)attained by various dilute(<2.00 mol·L−1)mineral acids(H2SO4,HCl,HNO3)increased with their concentrations.The order of decreasing transportation yields for various types of acids was found to be H2SO4>HCl>HNO3and highest yield obtained at 1.50 mol·L−1H2SO4[30].

Fig.7.Concentrations of Cu(II)ions versus time graphs for the continuous extraction studies through the kerosene based MDLMof(a)donor,(b)organic,and(c)acceptorphase at different acceptor phase concentrations(0.25,0.50,0.75,1.00,1.25,and 1.50 mol·L−1).

Fig.8.ln(C o/C e)versus time graphs for the single continuous extraction studies for the transportation of Cu(II)ions through the kerosene based liquid membrane at different acceptor phase concentrations(0.25,0.50,0.75,1.00,1.25,and 1.50 mol·L−1).
3.1.4.Effect of different extractant concentration on the transportation efficiency
The concentration of extractant in the organic phase has an important effect on the transportation of Cu(II)ions through liquid membrane systems especially on the transportation duration and flux rates.The effect of concentration of TNOA on the transportation of Cu(II)ions was studied by varying it in the range of 0.001–0.0075 mol·L−1.The results by means of the kinetic pro files of each phase(donor,membrane,and acceptor phases)at different organic phase concentrations are presented in Fig.9.
According to Fig.10,extraction and stripping of Cu(II)at different acceptor phase concentration processes obey consecutive first order reaction kinetics.Because ln(Co/Ce)vs.time is directly proportional.
Transportation efficiency of Cu(II)ions at different extractant concentration(0.0010,0.0025,0.0050,and 0.0075 mol·L−1)from donor phase to acceptor phase is 99.95%,99.97%,99.90%,and 99.84%,respectively.Extraction time for different organic phase concentration(0.0010,0.0025,0.0050,and 0.0075 mol·L−1)is 170,160,110,and 110 min,respectively.Transportation rate of Cu(II)ions rises with increasing carrier concentration.Further increase in carrier concentration decline the trend of transportation.Strong binding of Cu(II)ions to the extractant may obstruct delivering Cu(II)ions to the acceptor phase or increasing the concentration may raise the viscosity of the membrane phase.This can slow down the flux rate of the solution from donor phase to acceptor phase[3].Thus,0.005 mol·L−1TNOA was selected for the next steps as optimum carrier concentration.

Fig.10.ln(C o/C e)versus time graphs for the continuous extraction studies for the single transportation of Cu(II)ions through the kerosene based liquid membrane at different carrier concentrations(0.0010,0.0025,0.0050,and 0.0075 mol·L−1).

Fig.9.Concentrations of Cu(II)ions versus time graphs for the single continuous extraction studies through the kerosene based MDLM of(a)donor,(b)organic,and(c)acceptor phase at different carrier concentrations(0.0010,0.0025,0.0050,and 0.0075 mol·L−1).
Sadeghiet al.transported Cu(II)ions through bulk liquid membrane with 93.5%transportation efficiency by 5.0 × 10−3mol·L−1erythromycin ethylsuccinate[3].Alagurajet al.transported Cu(II)ionsthrough the emulsion liquid membrane system with 4%TNOA by using 5%SPAN80 as surfactant for stable emulsion formation[8].Lazarovaet al.extracted Cu(II)ions through a Hollow- fiber membrane from aqueous nitrate media by LIX reagents and best results obtained with 2 wt%LIX®860 N–I and 5 wt%LIX®984 N[31].
3.1.5.Effect offlux rate
The effect of flux rate on the transportation of Cu(II)ions was studied varying the flux rate by peristaltic pump in the range of 25 ml·min−1to 75 ml·min−1.The results by means of the kinetic profiles of each phase(donor,membrane,and acceptor phases)at different flux rates are presented in Fig.11.
According to Fig.12,extraction and stripping of Cu(II)at different acceptor phase concentration processes obey consecutive first order reaction kinetics.Because ln(Co/Ce)vs.time is directly proportional.
Transportation efficiency of Cu(II)ions at different flux rates(25,50,and 75 ml·min−1)from donor phase to acceptor phase is 99.96%,99.90%,and 99.92%,respectively.Extraction time for different organic phase concentration(25,50,and 75 ml·min−1)is,170,120,and 100 min,respectively.Transportation rate of Cu(II)ions rises with increasing flux rates.During the kinetic studies,the flux rates shouldn't be so fast,especially for the MDLM system since it causes foaming in the reactors.Thus,50 ml·min−1was selected as optimum flux rate.

Fig.12.ln(C o/C e)versus time graphs for the single continuous extraction studies for the transportation of Cu(II)ions through the kerosene based liquid membrane at different flux rates(25,50,and 75 ml·min−1).

Fig.11.Concentrations ofCu(II)ions versus time graphs for the single continuous extraction studies through the kerosene based MDLM of(a)donor,(b)organic,and(c)acceptor phase at different flux rates(25,50,and 75 ml·min−1).
3.2.Selective transportation of Cu(II)ions
3.2.1.Selective transportation of Cu(II)ions with alkaline and alkaline earth metals
The competitive transport of Cu(II),alkali and alkaline earth metal ions through MDLM with TNOA as the carrier has been investigated by using 100 mg·L−1Cu(II)and 40 mg·L−1NaCl,KCl,CaCl2,BaCl2,simultaneously.The comparative kinetics of transportation is given in Fig.13.Batch experiments are held at optimum conditions of transportation of Cu(II)ions(volume of donor,organic and acceptor phase 100 ml,pH of donor phase 9.00,temperature 298.15 K,concentration of H2SO4in acceptor phase 1.00 mol·L−1,concentration of TNOA in organic phase 5.00 × 10−3mol·L−1and rate of peristaltic pump 50 ml·min−1).ln(Co/Ce)versustime plots for the continuous extraction studies for the transportation of Cu(II)ions without/with alkaline and alkaline earth metal ions through the kerosene-based MDLM is also illustrated in Fig.14.
The effect of alkaline and alkaline earth metal ions on the simultaneous competitive transportation of Cu(II)ions is available in Table 2.According to the results,Na+,K+,and Ba2+ions are not detected in the acceptor phase,but 12.00%of Ca2+ions is transported from donor phase to acceptor phase with Cu(II)ions.
It is found that the extraction percentage of Cu(II)with/without alkaline and alkaline earth metal ions does not show great diversity(99.90%,99.31%),whereas in competitive transportation,extraction duration increases from 120 to 140 min.Besides,k1of selective transportation decreases from 0.0342 to 0.0213 min−1,butk2for that arises from 0.0366 to 0.0447 min−1.In addition, flux rates of donor and acceptor phases fall to 0.0108 from 0.0130 min.Hence,selective transportation of Cu(II)ions with alkaline and alkaline earth metal ions has been conducted successfully through the kerosene-based MDLM.
3.2.2.Selective transportation of Cu(II)ions with heavy metal ions
Simultaneous batch experiments have been carried out by using 50 mg·L−1Fe(III),50 mg·L−1Zn(II),and 50 mg·L−1Mo(VI)with 100 mg·L−1Cu(II)ions in the donor phase by MDLM system at optimum conditions given above(see Section 3.2.1).Kinetic pro files of transportation of Cu(II)ions with/without heavy metals are presented in Fig.15.ln(Co/Ce)versustime plots for the continuous extraction studies for the transportation of Cu(II)ions without/with heavy metal ions through the kerosene-based MDLM is also shown in Fig.16.

Fig.13.Kinetics of Cu(II)transportation(a)without alkali and alkaline earth metal ions and(b)with alkali and alkaline earth metal ions.

Fig.14.ln(C o/C e)versus time graphs for the continuous extraction studies for the transportation of Cu(II)ions(a)without alkaline and alkaline earth metal ions and(b)with alkaline and alkaline earth metal ions through the kerosene-based MDLM.
When the effect of Mo(VI),Fe(III),and Zn(II)metal ions on the extraction ofCu(II)transportation using TNOA as carrier through kerosene based MDLM technique is investigated,interesting results have been obtained.Extraction yield has not changed a lot(99.90%and 99.53%),whereas extraction time rises to 150 min from 120 min.The effect of Mo(VI),Fe(III),and Zn(II)metal ions on the selective transportation of Cu(II)ions is given in Table 3.
Under the selective experimental condition,k1andk2values are dropped to 0.0308 and 0.0208 min−1from 0.0342 and 0.0366 min−1,respectively.As well as flux rates of Cu(II)ions of donor and acceptor phase decreases from 0.0130 to 0.0092 min.
Bariet al.studied the simultaneous extraction and separation of Cu(II),Zn(II),Fe(III),and Ni(II)by polystyrene microcapsules coated with Cyanex 272.Almost 100%of Cu(II),Zn(II),Fe(III),and Ni(II)ions from the loaded MC-Xs has been stripped by 0.10 and 0.50 mol·L−1H2SO4solution for Cu(II),0.10 mol·L−1H2SO4solution for Zn(II),and 0.50 mol·L−1of H2SO4solution for Fe(III)[22].
Pospiechet al.separated Cu(II),Co(II),and Ni(II)from chloride solution by polymer inclusion membranes containing cellulose triacetate(CTA)as the support.Cu(II)and Co(II)ions were extracted by TOA and TlOA as the ionic carriers to 0.10 mol·L−1NaOH solution as the receiving phase.But Ni(II)ions weren't detected in the acceptor phase[21].

Table 2Comparison of kinetic parameters for the selective transportation of Cu(II)ions with/without alkaline and alkaline earth metal ions through the kerosene-based MDLM
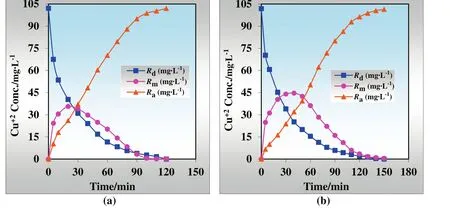
Fig.15.Kinetics of Cu(II)transportation(a)without heavy metal ions and(b)with heavy metal ions.

Fig.16.ln(C o/C e)versus time graphs for the extraction studies for the transportation of Cu(II)ions(a)without heavy metalions and(b)with alkaline and alkaline earth metalions through the kerosene-based MDLM.

Table 3Comparison of kinetic parameters for the transportation of Cu(II)ions with/without heavy metal ions through the kerosene-based MDLM
The percentage extraction of heavy metal ions is given in Table 4.

Table 4The percentage extraction of Zn(II),Fe(III),and Mo(VI)ions from donor phase to acceptor phase
At the end of the simultaneous extraction of Zn(II),Fe(III),and Mo(VI)with Cu(II)ions,2.20%of Mo(VI),0.80%of Fe(III),and 3.60%of Zn is detected in the acceptor phase.So,simultaneous batch extraction of Cu(II),Zn(II),Fe(III),and Mo(VI)shows the possible separation of these metal ions.
4.Conclusions
MDLM syste musing TNOA dissolving in kerosene as the extractantis applied successfully for the transportation of Cu(II)ions from donor phase to acceptor phase.The possible mechanism of transport was discussed.The simplicity,low cost,high efficiency and selectivity and a relatively short time period for Cu(II)ion transport obtained by the MDLM system demonstrate its potential applicability to selective removal,concentration or purification of copper from its mixtures.
Cu(II)ion transportation is consecutive first order irreversible reaction.Activation energy is found as 5.22 kcal·mol−1(21.82 kJ·mol−1),this process is called as diffusion controlled system.These observations suggest that the TNOA can be successfully employed as carriers for the removal of toxic Cu(II)ions in suitable wastewater treatment systems.
[1]S.H.Chang,T.T.Teng,N.Ismail,A.F.M.Alkarkhi,Selection of design parameters and optimization of operating parameters of soybean oil-based bulk liquid membrane for Cu(II)removal and recovery from aqueous solutions,J.Hazard.Mater.190(1–3)(2011)197–204.
[2]F.J.Alguacil,F.A.Lopez,I.Garcia-Diaz,Copper removal from acidic wastewaters using 2-hydroxy-5-nonbenzaldehyde oxime as ionophore in pseudo-emulsion membrane with strip dispersion(PEMSD)technology,J.Ind.Eng.Chem.18(1)(2012)255–259.
[3]S.Sadeghi,M.Jahani,E.Ghiamati,Selective transport of Cu2+ions through bulk liquid membrane mediated by erythromycin ethyl succinate,Sep.Sci.Technol.46(2)(2011)215–223.
[4]D.de Agreda,I.Garcia-Diaz,F.A.López,F.J.Alguacil,Supported liquid membranes technologies in metals removal from liquid effluents,Rev.Metal.47(2)(2011)146–168.
[5]S.H.Chang,T.T.Teng,N.Ismail,Soybean oil-based bulk liquid membrane for simultaneous extraction and stripping of Cu(II)from aqueous solutions,Int.J.Environ.Sci.Dev.2(2011)389–393.
[6]Z.Ren,W.Zhang,Y.Liu,Y.Dai,C.Cui,New liquid membrane technology for simultaneous extraction and stripping of copper(II)from wastewater,Chem.Eng.Sci.62(22)(2007)6090–6101.
[7]S.S.Ahluwalia,D.Goyal,Removal of heavy metals by waste tea leaves from aqueous solution,Eng.Life Sci.5(2005)158–162.
[8]M.Alaguraj,K.Palanivelu,M.Velan,Removal of Cu(II)using emulsion liquid membrane,Int.J.Chem.Technol.Res.1(3)(2009)722–726.
[9]A.Mellah,D.Benachour,The solvent extraction of zinc and cadmium from phosphoric acid solution by di-2-ethylhexylphosphoric acid in kerosene diluent,Chem.Eng.Process.Process Intensif.45(8)(2006)684–690.
[10]M.Atanassova,I.L.Dukov,Solvent extraction and separation of lanthanoids with mixtures of chelating extractant and 1-(2-pyridylazo)-2-naphthol,Sep.Purif.Technol.49(1)(2006)101–105.
[11]N.Alizadeh,K.Ashtari,Coalescence extraction of silver(I)using the temperature induced phase separation(TPIS)process,Sep.Purif.Technol.44(1)(2005)79–84.
[12]M.Ak,D.Taban,H.Deligöz,Transition metal cations extraction by ester and ketone derivatives of chromogenic azocalix[4]arenes,J.Hazard.Mater.154(1–3)(2008)51–54.
[13]G.Muthuraman,T.T.Teng,C.P.Leh,I.Norli,Use of bulk liquid membrane for the extraction of chromium(VI)from aqueous acidic solution with tri-n-butyl phosphate as a carrier,Desalination249(2)(2009)884–890.
[14]N.Singh,D.O.Jang,Selective and efficient tripodal receptors for competitive solvent extraction and bulk liquid membrane transport of Hg2+,J.Hazard.Mater.168(2–3)(2009)727–731.
[15]C.Mendiguchia,C.Moreno,M.Garcia-Vargas,Determination of copper in seawater based on a liquid membrane preconcentration system,Anal.Chim.Acta460(1)(2002)35–40.
[16]G.León,M.A.Guzmán,Faciliated transport of copper through bulk membranes containing different carriers:Compared kinetic studies,Desalination223(1)(2008)330–336.
[17]M.D.Granado-Castro,M.D.Galindo-Riaño,F.C.Domínguez-Lledó,C.Díaz-López,M.García-Vargas,Study of the kinetics of the transport of Cu(II),Cd(II)and Ni(II)ions through a liquid membrane,Anal.Bioanal.Chem.391(3)(2008)779–788.
[18]N.Spreti,L.Brinchi,R.Germani,M.V.Mancini,G.Savelli,A new carried for for selective removal of heavy metal ions from aqueous solutions through bulk liquid membranes,Eur.J.Org.Chem.2004(18)(2004)3865–3871.
[19]Y.T.Mohamed,A.H.Ibrahim,Extraction of copper from waste solution using liquid emulsion membrane,J.Environ.Prot.3(2012)129–134.
[20]R.Donat,Ö.Durmaz,H.Cetişli,Transportation and kinetic analysis of Mo(VI)ions through a MDLM system containing TNOA as carrier,J.Hazard.Mater.294(2015)17–26.
[21]B.Pośpiech,W.Walkowiak,Separation of copper(II),cobalt(II)and nickel(II)from chloride solutions by polymer inclusion membranes,Sep.Purif.Technol.57(3)(2007)461–465.
[22]M.F.Bari,M.S.Hossain,I.M.Mujtaba,S.B.Jamaluddin,K.Hussin,Simultaneous extraction and separation of Cu(II),Zn(II),Fe(III)and Ni(II)by polystyrene microcapsules coated with Cyanex 272,Hydrometallurgy95(3–4)(2009)308–315.
[23]T.R.Reddy,J.Ramkumar,S.Chandramouleeswaran,A.V.R.Reddy,Selective transport of copper across a bulk liquid membrane using 8-hydroxy quinoline as carrier,J.Membr.Sci.351(1–2)(2010)11–15.
[24]L.Wang,R.Paimin,R.W.Cattrall,W.Shen,S.D.Kolev,The extraction of cadmium(II)and copper(II)from hydrochloric acid solutions using an Aliquat 336/PVC membrane,J.Membr.Sci.176(1)(2000)105–111.
[25]K.E.Erden,R.Donat,Ş.Aytas,Simultaneous extraction and stripping of uranium ions via multi-dropped liquid membrane system,Russ.J.Appl.Chem.88(11)(2015)1902–1912.
[26]H.Arslan,E.Hasdemir,Direct determination of copper with thiosemicarbazide by UV–Vis spectrophotometric method and effect of presence some other cations,G.U.J.Sci.17(1)(2004)49–58.
[27]L.D.Nghiem,P.Mornane,I.D.Potter,J.M.Perera,R.W.Cattrall,S.D.Kolev,Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes(PIMs),J.Membr.Sci.281(1–2)(2006)7–41.
[28]M.Kobya,N.Topçu,N.Demircioǧlu,Kinetic analysis of coupled transport of thiocyanate ions through liquid membranes at different temperatures,J.Membr.Sci.130(1‐2)(1997)7–15.
[29]Z.Lazarova,L.Boyadzhiev,Kinetic aspects of copper(II)transport across liquid membrane containing LIX-860 as a carrier,J.Membr.Sci.78(3)(1993)239–245.
[30]S.H.Chang,T.T.Teng,I.Norli,Efficiency,stoichiometry and structural studies of Cu(II)removal from aqueous solutions using di–2-ethylhexylphosphoric acid and tributylphosphate diluted in soybean oil,Chem.Eng.J.16(2011)249–255.
[31]Z.Lazarova,M.Lazarova,Hollow- fiber membrane extraction of copper from aqueous nitrate media by LIX reagents:comparison of extraction efficiency and fractional resistances,Solvent Extr.Ion Exc.29(1)(2011)128–145.
 Chinese Journal of Chemical Engineering2017年4期
Chinese Journal of Chemical Engineering2017年4期
- Chinese Journal of Chemical Engineering的其它文章
- Suppressing secondary reactions of coal pyrolysis by reducing pressure and mounting internals in fixed-bed reactor☆
- Transformation mechanism of nutrient elements in the process of biochar preparation for returning biochar to soil☆
- Preparation of cross-linked enzyme aggregates of nitrile hydratase ES-NHT-118 from E.coli by macromolecular cross-linking agent☆
- Development of a new cleaner production process for cassava ethanol☆
- Multi-objective regulation in autohydrolysis process of corn stover by liquid hot water pretreatment
- Modelling of adsorption of textile dyes over multi-walled carbon nanotubes:Equilibrium and kinetic
