Modelling of adsorption of textile dyes over multi-walled carbon nanotubes:Equilibrium and kinetic
Danilo Vuono ,Enrico Catizzone ,Alfredo Aloise ,Alfonso Policicchio ,Raffaele G.Agostino ,Massimo Migliori,*,Girolamo Giordano
1 University of Calabria,Department of Environmental and Chemical Engineering,via P.Bucci,I-87036 Rende,CS,Italy
1.Introduction
Because of its extensive usage in agricultural(70%),industrial(22%)and civil(8%)applications,the water demand is tremendously increased in the last years[1].This situation leads to a huge amount of wastewater to be treated,removing any pollutant compound,before recycling or discharging[2–4].Among the several inorganic and organic pollutants potentially present in wastewater[5],because of their high solubility dyes are difficult to remove.In addition,due to their not easy biodegradability,dyes dissolved in water represent highly toxic and carcinogenic compounds,determining dangerous effect on the environment[6]with serious problems to animals,plants and human health[7,8].On the other hand,textile,paper printing,paint,leather,food and cosmetic factories are the most users of dyes with an estimate production of dyes of 1 million ton per year.Many dyes are visible in water at concentration lower than 1 mg·L-1,nevertheless the dyes concentration in textile industrial wastewater is in the range of 10–200 mg·L-1and their treatment is still an open issue[9].
The removal process is even more complicated as the complexity and variety of dyes are considered,usually mixed with other reactants in the waste stream[10–12].For these reasons,it is difficult to assess a general water treatment process for textile waste waters and several methods(chemical,biological and physical)can be adopted in order to remove dyes.Chemical methods decompose the molecules of dyes by oxidative processes by using hydrogen peroxide(H2O2)or ozone(O3)or by photochemical reactions under UV treatment in the presence of H2O2[13,14].Biological methods require the use of particular microbial culture(e.g.white-rot fungi)for the decolourization of textile dyes in both aerobic and anaerobic processes[15].Physical methods(involving mainly adsorption processes)are the most used methods for the textile wastewater treatments because of their versatility(both inorganic and organic contaminants can be removed)and lower process costs compared to the other depuration methods[16].Furthermore,this treatment permits to recover and to reuse the adsorbent without producing additional materials to be further treated.The choose of adsorbent material is based on the type of pollutant to be removed:silica gel is an effective material for removing basic dyes,peat can be used to remove polar organic molecules and transition metals,whilst activated carbons efficiently remove acid dyes,pigment and reactive dyes[17–19].
In this concern,due to their unique chemical–physical properties and low cost synthesis procedures,carbon nanotubes have been extensively studied for adsorption application.Physical properties such as well-sized cylindrical pores,high surface-to-volume ratio,hydrophobic walls and easily functionalizable surface have addressed a large number of investigations towards the use of carbon nanomaterials as potential adsorbents for water purification apart from several other applications[20–23].Carbon nanotubes can be classified in single-walled(SWCNT)[24,25]and multi-wall(MWCNTs)[26],both considered as 1D systems consisting of one or more graphene sheets,respectively,rolled up in a concentric form.This configuration is a unique nanostructure with interesting electronic and mechanical properties that depend to its chirality and diameter[27].Because of these particular properties,carbon nanotubes are attractive for several practical applications,such as,devices for energy storage[28]and adsorption,with high sensitivity,selectivity,and efficiency[29].
Three methods were developed for the synthesis of these reliable materials:electric arc discharge,laser evaporation/ablation and chemical vapour deposition(CVD).Among these methods,the last one is the most diffused technique in the large scale production because of its high performances in terms of efficiency of both carbon deposit and production costs[30–32].In this concern,the formation of carbon filaments from the catalytic decomposition of carbon-containing gas over metal surfaces has been known for a long time[30,33–38].
Referring to adsorption processes,MWCNTs exhibits higher performances than SWCNTs due to their structural configuration[30,39].In MWCNTs the porous nature can be classified as inner hollow cavities of small diameter and aggregated pores,formed by interaction between the single CNTs as a result of van der Waals forces[40–42].The adsorption efficiency offered by MWCNTs can be also attributed to the high amount of structural defects that act as potential active sites for adsorption.In particular,the aggregated pores play a role in the adsorption of a large number biological contaminants[41].In general,the post synthesis treatments(e.g.purification or functionalization by oxidation)change the properties of CNTs,such as specific surface area and total volume pore,as well as resulting in the modification of the chemical surface of these nanomaterials.Kayiranet al.suggest that the oxygen content(OH,COOH,C=O)on CNTs influences the maximum adsorption capacity and can depend on the synthesis and purification steps or intentionally generated by oxidation with acids,plasma,ozone or removed by thermal treatment[43,44].For these reasons CNTs,can be considered as suitable option in the wastewater treatment via adsorption processes[45–47].In particular,potential applications as powdered or supported adsorbent materials of textiles dyes and other kinds of pollutants have been recently investigated[48–51].Their use could be considered as a valid alternative to granular activated carbon and synthetic resins[52–61].
Wu[51]investigated the equilibrium and the dynamic adsorption of Procion Red MX-5B using CNTs at different pH and temperature values,showing that the dye adsorption phenomena is endothermic with a spontaneous physi-sorption mechanism.Kuo and coworkers[53]examined the adsorption performances of CNTs on two kinds of dyes,defining a pseudo-second order equation as kinetic model also showing that Freundlich equation can be adopted as equilibrium adsorption model.Mishraet al.[59]studied the removal performances of functionalised MWCNTs towards the azo-dyes.In this research the authors hypothesis was the involvement of electrostatic interaction between oxidised MWCNTs surface and anionic azo-dyes along with van der Waals interaction,justifying the good adsorption behaviour of this materials.The equilibrium evaluations pointed out a good fitting of experimental data with either Langmuir or Freundlich models.Moreover,the adsorption capacities as a function of treatment time found a satisfactory fitting with the pseudo-second order kinetic model.Yaoet al.[62]investigated the adsorption behaviour of methylene blue using CNTs as adsorbent at different temperatures observing a positive value of adsorption value but too low to support the evidence of the adsorption as a chemical phenomenon.The studies of Wanget al.can be considered very interesting.The authors defined the adsorption of a binary dye system in aqueous solution,highlighting a synergistic effect of dyes during the adsorption treatment on MWCNTs.Kinetic studies showed that adsorption processes followed also in this case a pseudo second order kinetic in both single or binary dye solutions[63]con firmed by other authors[64,65].In this work the MWCNTs were used to treat dye-contaminated solutionviaadsorption process,evaluating the effect of dye concentration,nanotubes amount and treatment time on the adsorption.From experimental data analysis a semiempirical kinetic model has been developed,able to describe the effect influence of operating conditions on the adsorption process efficiency.Also equilibrium data were used to compare different adsorption isotherm models.
2.Experimental
2.1.Materials and characterisation
The MWCNTs(CNTsUNP)used in this study are synthesised by Chemical Catalytic Vapour Deposition(CCVD)[64].The synthesis temperature is 700°C and the Co-Fe based catalyst is supported on a natural zeolite.In order to check the carbon deposition efficiency,the Carbon Deposit(Cd)parameter,was calculated as it follows:

wheremcarbandmcatare the mass of synthesis product and dried catalyst,respectively.The supported catalyst is removed in a purification step by 5 days acidic treatment,using 1 g of CNTs in 200 ml HF(40%,Sigma-Aldrich)followed by several of washing with distilled water,obtaining a purified sample of MWCNTs(CNTsPUR)[64].This as-made CNTs can be further chemically treated to insert surface functionalization to be tested in the adsorption test.The oxidation step,carried out with purified nanotubes,was performed in order to distribute on the caps and the external walls of nanotubes--OH groups(CNTsOH).A hydroxylation step was performed by using 1 g of CNTsPURin a 200 ml of a H2SO4/HNO3mixture homogenised at 250 rpm and room temperature with a volumetric ratio value of 0.6(H2SO498%,HNO365%Sigma-Aldrich),with a contact time of 24 h and washed with distilled water[66].This treatment is similar to other oxidation procedures carried out in recent studies[45].
Structural analysis of synthesised MWCNTs was performed using Raman spectroscopy and TEM.Raman spectroscopy measurements were carried out by using a Thermo Fisher DXR Raman microscope,equipped with OMNICxi Raman imaging software 1.0.The low resolution transmission electron microscopy(TEM)pictures were taken on a Philips CM10 transmission electron microscope using 100 V accelerating voltage.Sample preparation relied on the classical method.About 10 mg of CNT was suspended in 3 ml of ethanol and the suspension was then deposited on a carbonated Cu–Rh grid.
The specific surface area was measured by performing a BET and t-plot analysis of porosimetry data(ASAP 2020 Micromeritics)under nitrogen adsorption at 77 K,after a pre-treatment in vacuum at 200°C for 12 h.
2.2.Adsorption study
In order to perform adsorption test,commercial reactive dyes were purchased from local market(Coloreria It aliana,Italy).Three different colours(reactive Blue 116,reactive Red 159 and reactive Yellow 81)have been selected because of their different structural,physical and chemical properties in terms of molecular weight and chemical element composition(Table S1 of Supporting Information).
The benchmark of the CNTs adsorption performances was established as activated carbons(AC)available in powder form(Sigma-Aldrich purity 99.9%;particle size of 44-74μm),using a 100 mg·L−1initial solution of dye in water.All adsorption tests were carried in batch conditions,at room temperature,by adding different amounts of adsorbent in 20 ml of a dye solution and stirring the suspension at 500 r·min−1.At the end of the test,the suspension is filtrated to separate the adsorbent solid from the treated liquid.The solid phase is then dried at 120°C for 18 h,whilst the liquid phase directly undergoes through further characterisations.Dye concentration in any solution was detectedviaUV–VIS analysis(Evolution 300,Thermo Scientific).Any test was conducted at room temperature and for three independent replications;data shown in the test are the average of the tests always with a standard deviation below 5%.
In order to assess the adsorption performances of CNTs with respect to the benchmark,0.06 g of adsorbent(CNTsUNP,CNTsPUR,CNTsOHor AC)is added in the dye solution(100 mg dye·L−1),for a contact time in the range 0–240 min.The adsorption capacityq(t)(mg dye·(g CNTs)−1)of nanotubes is calculated by the following equation:

wherec0is the initial concentration of the dye solution,c(t)is the current dye concentration both in mg·L−1,tis the contact time(min).Vis the solution volume(L)and m is the CNTs or AC mass(g).When this parameter is calculated in the steady state conditions,it represents the plateau adsorption capabilityqp,in principle depending upon the process conditions and residual dye solution concentration.
In order to study the effects on kinetic of adsorption process of different process parameters such as:CNTs amount,contact time and dye concentration,experimental tests are carried out by varying the initialdye solution concentration in the range of50–250 mg·L−1as wellas the mass of carbon nanotubes between 0.03 and 0.15 g for a contact time up to 240 min.
Adsorption isotherms were derived by mixing 0.03 g of CNTsUNPin dye solutions with concentrations between 20 and 300 mg·L−1at room temperature and monitoring either solid adsorption and residual dye concentration in the solution for240 min.The maximum investigated contact time(240 min)was always sufficient to reach constant dye concentration in the liquid phase.
2.3.Adsorption isotherms models
Data for adsorption isotherm are collected at constant temperature andquasi-equilibrium conditions(after 240 min)by measuring either the solute concentration in the final solutionC(mg dye·(L solution)−1),as well as the concentration of the adsorbed dye on solid particles ω (mg dye·(g CNTs)−1)assumed as the equilibrium concentration of the solute on the solid surface.
Different models have been developed and deeply investigated[67,68]to fit adsorption isotherms and in this work,Langmuir,Freundlich and Temkin models are adopted in order to fit the experimental result and to estimate the model parameters.
The Langmuir isotherm is based on the well-known assumptions of the Langmuir theory:the adsorbate forms a monolayer on the adsorbent surface[54],all adsorption sites are energetically identical,each sites can retain only one adsorbate molecule and each site is energetically and sterically independent from the other.Langmuir adsorption isotherm model can be mathematically described by the following equation[67]:

where ωMax(mg dye·(g CNTs)−1)is the maximum adsorption capacity of the adsorbent andKL(L solution·(mg dye)−1)is the equilibrium constant defined as:

Conversely to the Langmuir model,Freundlich model considers the surface heterogeneity,also taking into account the mutual interaction between adsorbed molecules[54].Freundlich model can be described by the following equation[68]:

whereKF((L solution)n·(mg dye)1−n·(g CNTs)−1)is the Freundlich equilibrium parameter andnis a fitting parameter related to adsorption intensity[54].According to the mathematical form,the Freundlich model does not allow to fit a concentration plateau in the adsorbed solute ω when increasing the solute concentrationC.
Temkin model takes explicitly into account the interaction between adsorbent and adsorbate molecules,considering that(1)the adsorption-heat linearly decreases when increasing the surface coverage(2)a uniform distribution of the bonding energies was also assumed[54].
Temkin model can be described by the following equation:

whereAT(L solution·(mg dye)−1)is an equilibrium/ fitting parameter[60,69]andBT(mg dye·(g CNTs)−1)is a constant related to heat of sorption.
Model parameters estimation was carried outby using a commercial software(Curve Expert Professional 2.0)for non-linear regression by adopting 0.95 as confidential interval.
To discriminate among different adsorption isotherm models,a statistical criterion has been adopted,based onF-test.Since the three models have same number of parameters P,the same degree of freedom(DOF)holds DOF=N–P,whereNis the number of experimental observationsyi(i=1…N).For any model,if the predicted isy^icalculated in the experimental conditions of the measuredyi,the sum of the least squaresSScan be calculated as:

and the according to the hypothesis that the population follows the Fisher distribution:

The best- fit model is assumed as the model with the highestFvalue[70].
2.4.Adsorption kinetic models
Together with equilibrium curves as described in the previous paragraph,adsorption kinetic has to be also considered,in order to gain more insight about adsorbent performances and adsorption mechanism.In this paper,three adsorption kinetic models described below have been compared.
The pseudo- first order model(PFOM)is described by the following equation:

is the earliest adsorption kinetic model,widely used to describe the adsorption of dyes(malachite green)from waste water[71].qpandq(mg dye·(g CNTs)−1)are the plateau adsorption capacity and the current-one at any time respectively,k1(min−1)is the pseudo first order rate constant for the kinetic model and the productk1qp(mg dye·(g CNTs)−1·min−1)can be used to estimate the initial adsorption rate.Another kinetic model widely used to describe pollutants adsorption(such as dyes are considered)on solids[72]is the following pseudo-second order model(PSOM):

wherek2(g CNTs·(mg dye)−1·min−1)is the pseudo-second order constant rate and the productk2qp2(mg dye·(g CNTs)−1·min−1)can be assumed as the initial adsorption rate(mg dye·(g CNTs)−1·min−1).In case of chemisorption phenomena,Elovich's equation has been also widely used for pollutants removal from either gas or liquid streams[73]:

where the parameter β (g CNTs·(mg dye)−1)is related to either the extent of covered surface or the activation energy involved in chemisorption and α (mg dye·(g CNTs)−1·min−1)is the initial adsorption rate.As already described at the end the previous paragraph,an F-test was adopted in order to discriminate among the investigated kinetic models.
3.Results and Discussion
3.1.CNTs characterisation
The CNTs synthesis was con firmed as very effective withCdvalue around 1500%.Data of specific surface area are reported in Table 1.The observed increase in both specific surface area and micropore volume after the MWCNTs purification can be attributed to the removal ofCo--Fe based catalyst[74,75].In fact,the different arrangement of the CNTUNPsample grouped in bundles lost after purification can contribute to this small increase in surface area.According to that,when considering CNTOH,the further specific surface area increase could be due to thequasiloss of the bundle organisation as con firmed by comparison of TEM images of Fig.1[66].A qualitative characterisation by FT-IR analysis(not shown)has defined the presence of OH−groups on the external walls of nanotubes with analogous spectra to other studies[44,66].
Fig.2 shows the Raman spectra of unpurified,purified and oxidised samples respectively,revealing three characteristic bands:the first one in the range 1330–1350 cm−1is due to the disorder-induced phonon mode(D-band),the second one in the range 1569–1590 cm−1is due to the high degree of symmetry and order of nanotubes structure(G-band)whilst the third one(between 2668 and 2688 cm−1)identifies the second order2D-band(or G′-band),related to the packing in three-dimensional space.The intensities ratios of D and G peaks(ID/IG)are related to the overall order of all of three type of CNTs and a value of 0.77,0.97 and 0.91 for CNTsUNP,CNTsPURand CNTsOHrespectively,indicate the presence of a degree of order with a certain number of defects in the structure,increasing with the acidic treatment of purification and oxidation steps.The intensities ratios of G′and G peaks(IG′/IG)of 0.53,0.40 and 0.41 for unpurified,purified and oxidised nanotubes respectively,are indicative of the loss of packing degree of the structure due to destruction of the bundles organisation of CNTs during the purification[74].

Table 1Textural properties of multi-walled carbon nanotubes and active carbon
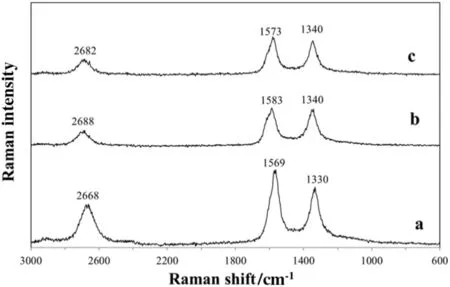
Fig.2.Raman spectra of unpurified(a),purified(b)and oxidised(c)CNTs.
3.2.Adsorption test:effect of CNTs treatment
In order to verify the effect of CNTs treatment over the adsorption performances,the three investigated samples(CNTsUNP,CNTPUR,CNTsOH)were tested in combination with each of the three dyes.Data of Fig.3 reveal a similar behaviour of the three types of CNTs as a function of time,suggesting that both acidic treatments to purify and oxidise the nanotubes do not improve the adsorption performances of the material and the presence of impurities(around 4 wt%of calcined catalyst)in the CNTs sample does not affect the adsorption capability.This behaviour is in agreement with previous studies performed on these materials,as reliable alternative for adsorbing aromatic compounds[76].
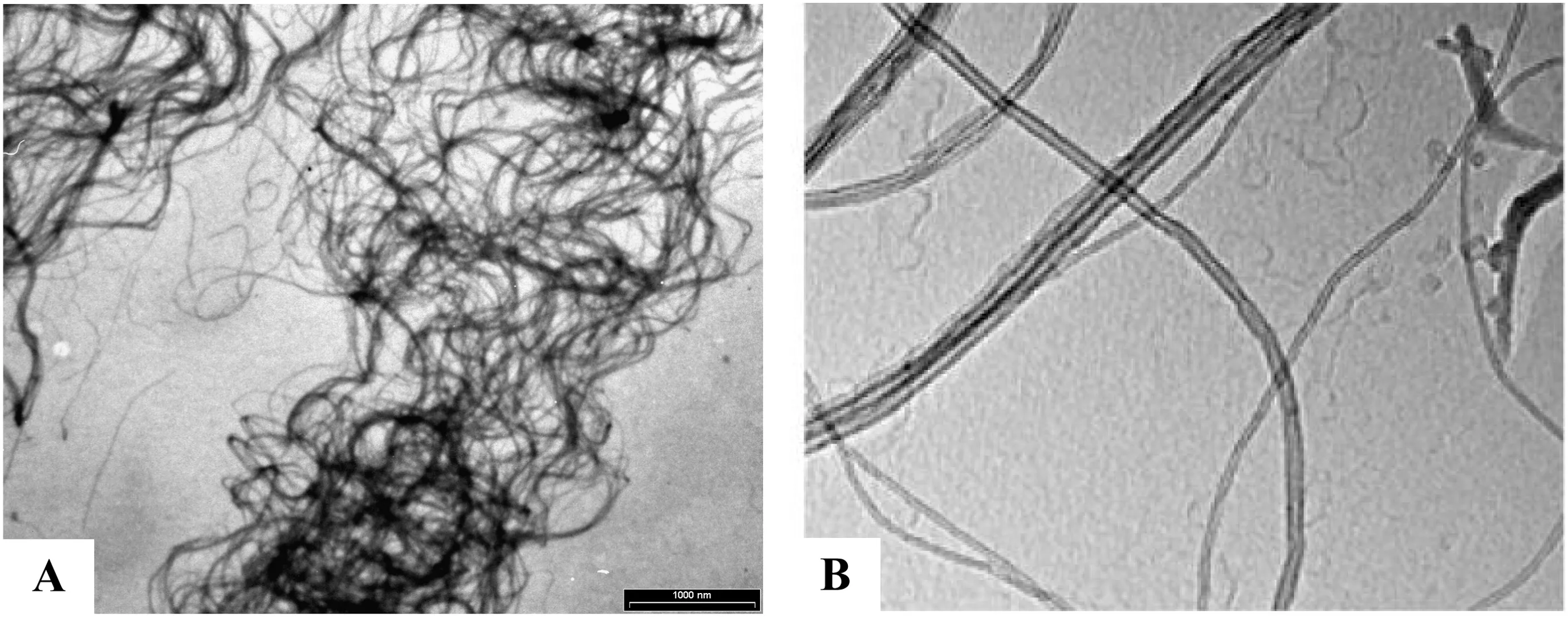
Fig.1.TEM image of unpurified(A)and oxidised(B)MWCNTs.
Therefore,due their lower production costs for both experimental and industrial applications,the CNTsUNPwas selected and used for any further experimental investigation.

Fig.3.CNTsUNP,CNTsPUR and CNTsOH adsorption performances as a function of contact time;(a)Blue 116,(b)Red 159,(c)Yellow 81.
3.3.Adsorption test:AC benchmark
The adsorption performance of CNTsUNPand AC,under the same test conditions described above,is reported in Fig.4.Despite the higher super ficialspecific area(see Table 1),it is noteworthy that active carbon[77],widely recognised as very efficient adsorbent,exhibits an adsorption capability significantly lower than those of nanotubes.In addition,whilst the CNTsUNPadsorption ability does not depend on the dye type,the ACs show a low adsorption performance towards the Yellow 81 dye since theqpvalue is significantly lower than those of Blue 116 and Red 159.This test con firms the CNTs as very good adsorbent materials for removing very soluble compounds,such as dyes,from wastewater.
3.4.Kinetic analysis and pseudo second order model development
In order to obtain adsorption kinetic,test where performed under different operating conditions and data were fitted with the PFOM,PSOM and Elovich kinetic models described in the Section 2.4.A detailed comparison of the model reliability is summarised in Table S2 of Supporting Information,based onF-values.Data analysis reveals that a pseudo-second order model exhibits the best correlation among the investigated kinetic models for a 92%of the reported experimental conditions.Therefore,PSOM was adopted to fit all the data and the research was focused on establish the relationship between the model parametersk2andqpand the most relevant process parameters.In order to verify the effect of the amount of both initial dye and CNTs,experimental tests were carried out by simultaneously varying these parameters in the way to keep the ratio(D/C)to the constant value to 33.3[mg dye·(g CNTs)−1].The value of PSOM kinetic parameters evaluated for this value of D/C are reported in Table 2 for the three investigated dyes and reported data have a quite robust correlation factor(higher than 0.992).It clearly appears that the plateau adsorption capacityqpis not affected by different operation conditions(dyes concentration or amount of carbon nanotubes)as far as the ratio D/C is kept as a constant.On the other hand,the same trend is not observed for the constant rate parameterk2.
The dependence ofk2parameter from initial dye concentration can be associated to the presence of multiple adsorption kinetic mechanisms.Similar behaviour was reported in an investigation performed by Saleh[54],where it was demonstrated that the intra-particle diffusion kinetically might affect the adsorption process.In this concern,the intra-particle diffusion phenomena can be described by Weber and Morris model:


Fig.4.CNTsUNP and AC adsorption performances as a function of contact time;(a)Blue 116,(b)Red 159,(c)Yellow 81.
If the plot ofqpversust1/2generates a straight line intra-particle diffusion is the unique controlling step of sorption mechanism,on the contrary multiple adsorption steps take place if the curve slope changes[54–56].Weber and Morris plot is shown in Fig.5 and it clearly appears that experimental data exhibit non-constant slope,suggesting that the adsorption process could be governed by more than one kinetic mechanism[54].In addition,since the obtained plot does not pass through the origin it can be assumed that intra-particle diffusion is not the limiting step of the investigated adsorption process[54].
After these preliminary tests,it was investigated as the plateau adsorption capacityqpis affected by the process conditions by varying the D/C ratio between 21.6 and 83.3.As shown in Fig.6,a linear correlation can be used to fit the experimental points for the three differentdyes.This is an interesting result since from these data the amount of adsorbed dye can be correlated to the initial one and the slope of interpolation line(dimensional unit are(mg dye)adsorbed·(mg dye)−1initial)can be considered as a recovery factor of dye on the adsorbent material:the more is performing the material,the more the slope value approaches the bisector.Data reported in Fig.6 show that the recovery factors are 95%,98%and 100%for Blue 116,Red 159 and Yellow 81 respectively,again con firming these materials as reliable adsorbents for wastewater treatment.As a final result,also referring to Eq.(2)notation,the following correlation can be assumed to calculate theqpvalue:

Table 2Parameters of pseudo-second order kinetic model at constant D/C=33.3 and 20 ml of treated solution
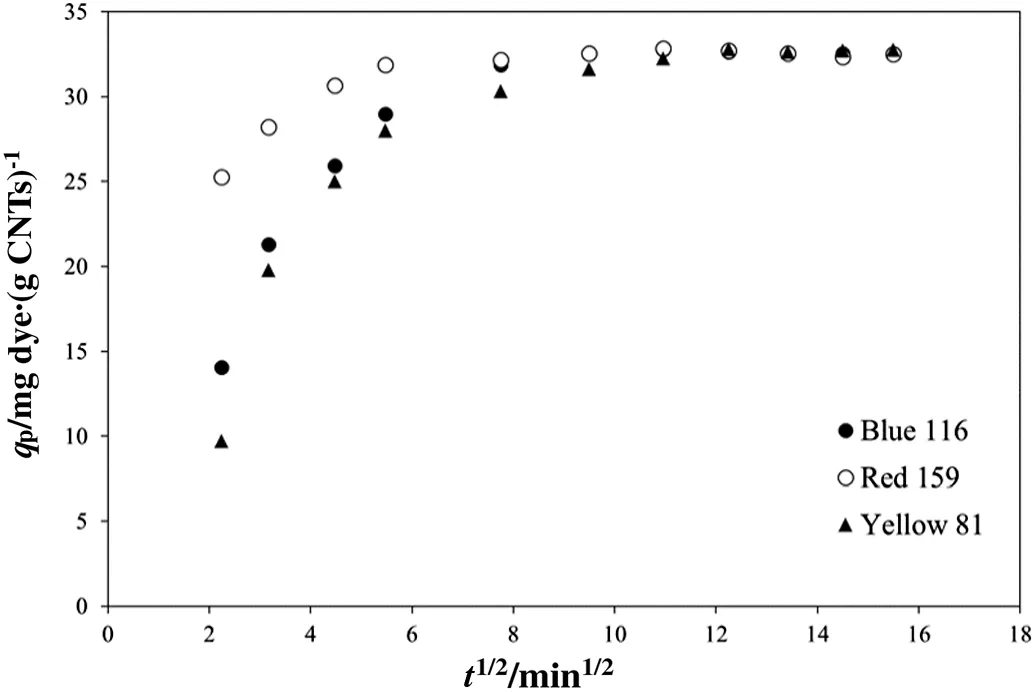
Fig.5.Weber and Morris plot.

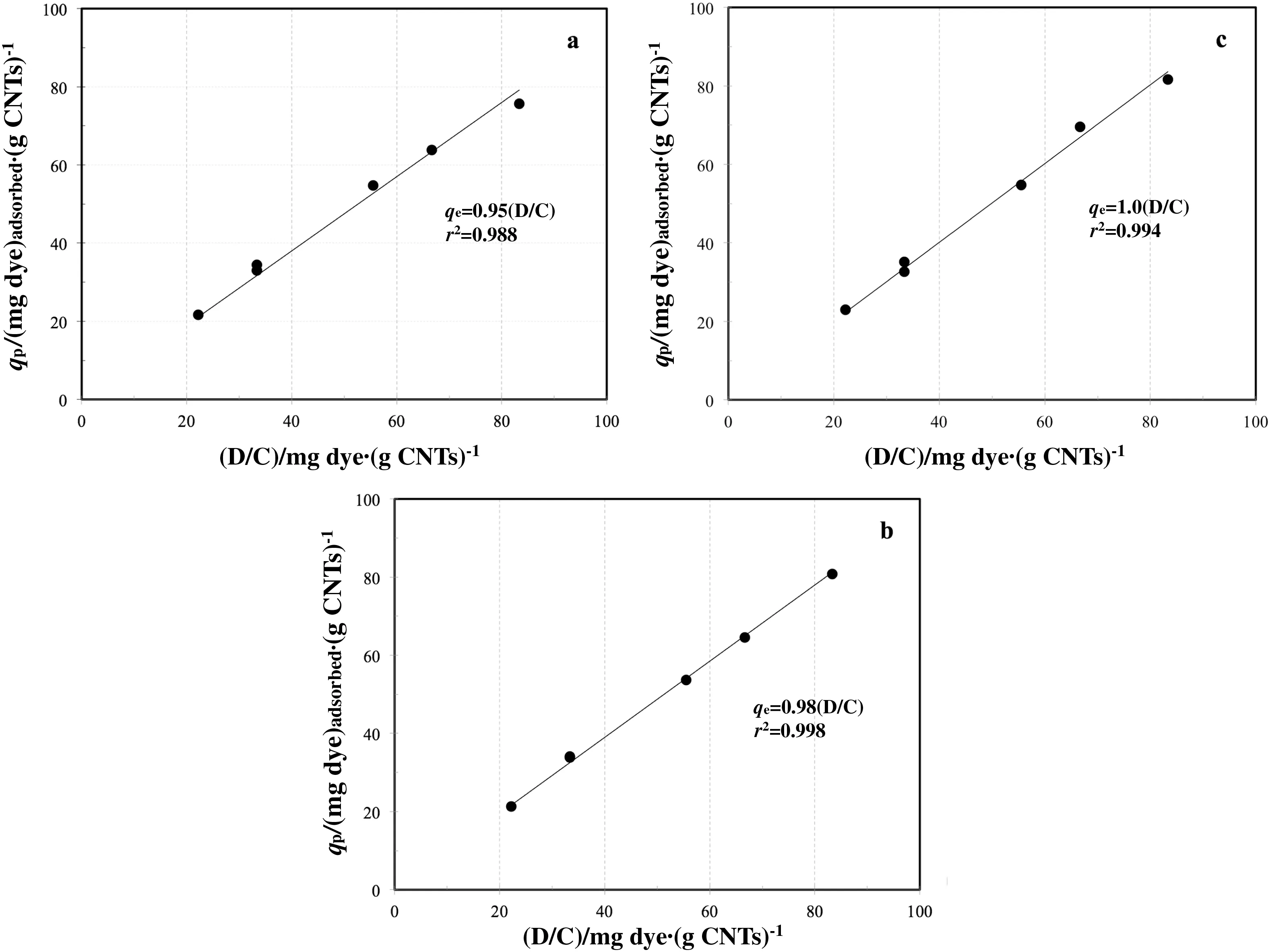
Fig.6.Effect of Dye/CNTs ratio(D/C)on q p;(a)Blue 116,(b)Red 159,(c)Yellow 81.
wherec0(mg·L−1)the initial dye concentration andra fractional recovery factor equal to 0.95,0.98 and 1 for Blue 116,Red 159 and Yellow 81 respectively.
The relationship betweenk2parameter and the adsorption operation conditions was established by independently varying the CNT mass and the initial dye concentration and assuming a factorial dependence of the parameters upon the process variables[78]:

wherefandaare the fitting function and MCNT is the specific mass of carbon nanotubes(as g CNTs·(L solution)−1).The choice of decoupling the functionality allows to separately select thefandaexpression.Data in Fig.7 were derived at different MCNTs and for three values ofcdye.The trend of the experimental points suggests the use of a parabolic equation with zero-origin as:

and data fitting is reported in Table 3 for the three dye types.Data comparison reveals a((L solution)2·(g CNTs)−2·min−1)values higher for Red 159,suggesting that adsorption of this compound is much more faster than adsorption of the other dyes at any investigated dye concentrations.As final step,in order to improve the model generality,the functionality ofawith the initial dye concentrationc0was established by a power law as:

The fitting of experimental points is reported in Fig.8 for the three investigated dyes and the estimated values ofbandnare reported in Table 4.
3.5.Adsorption isotherms modelling
Experimental data of adsorption isotherms tests are reported in Fig.9 whereqpis plotted versus the residual dye concentration after 240 min of contact time.Previous kinetic study revealed that no change of concentration is observed at higher treatment times and an equilibrium condition can be assumed.The observed data were fitted by the three adsorption isotherm models described in the Section 2.3 and the estimated parameter values with correlation coefficientr2are summarised in Table 5.All models seem to be meaningful due to their highr2values(r2>0.9)but,as already done for the kinetic modelling,anF-value for each model was estimated for all investigated dyes and results are showed in Table 6.For Yellow 81 is not possible to discriminate among the adopted models due their similar estimatedF-values.For Blue 116 both Langmuir and Temkin seem to be the more accurate models to describe the adsorption isotherm while for Red 159 an adsorption isotherm model as Temkin type exhibits the best fitting goodness and the fitting of experimental point with the model is shown in Fig.9 also.Despite the fact that an adsorption mechanism cannot be hypothesised with the available data,for the sake of generality,it is suggested to use the Temkin model to describe dye adsorption from wastewater,allowing performance comparison also between different dye types.The estimated BTvalues suggest that adsorption heat of investigated dyes should follows the order Red 159>Yellow 81>Blue 116.
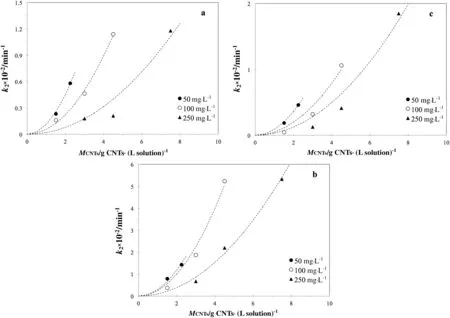
Fig.7.Effect of quantity of CNTs on K2-pseudo-second order parameter for(a)Blue 116,(b)Red 159,(c)Yellow 81.Dashed lines are the trend of the fitting zero-origin parabolic model.
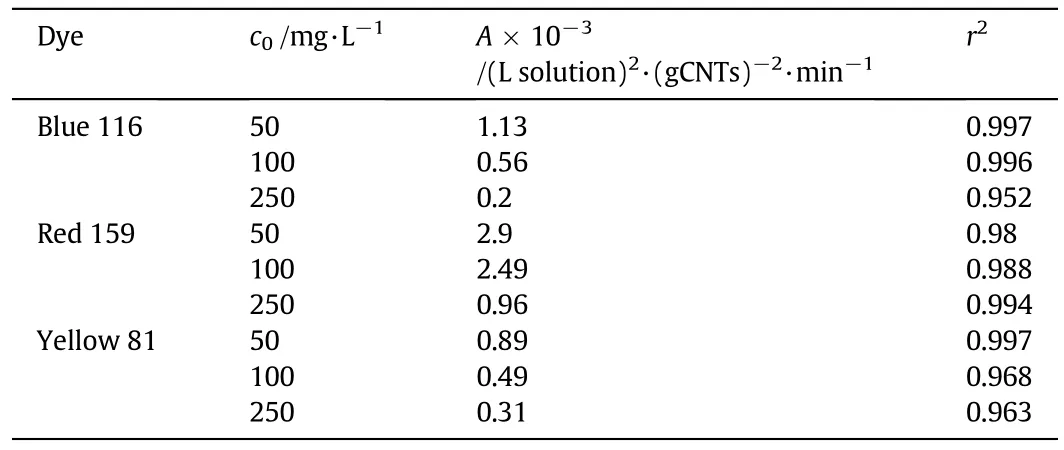
Table 3a value as a function of initial concentration c0 for the investigated dyes

Fig.8.Effect of dye concentration on parameter a of the proposed semi-empirical kinetic model.

Table 4b and n of the proposed semi-empirical kinetic model for the investigated dyes
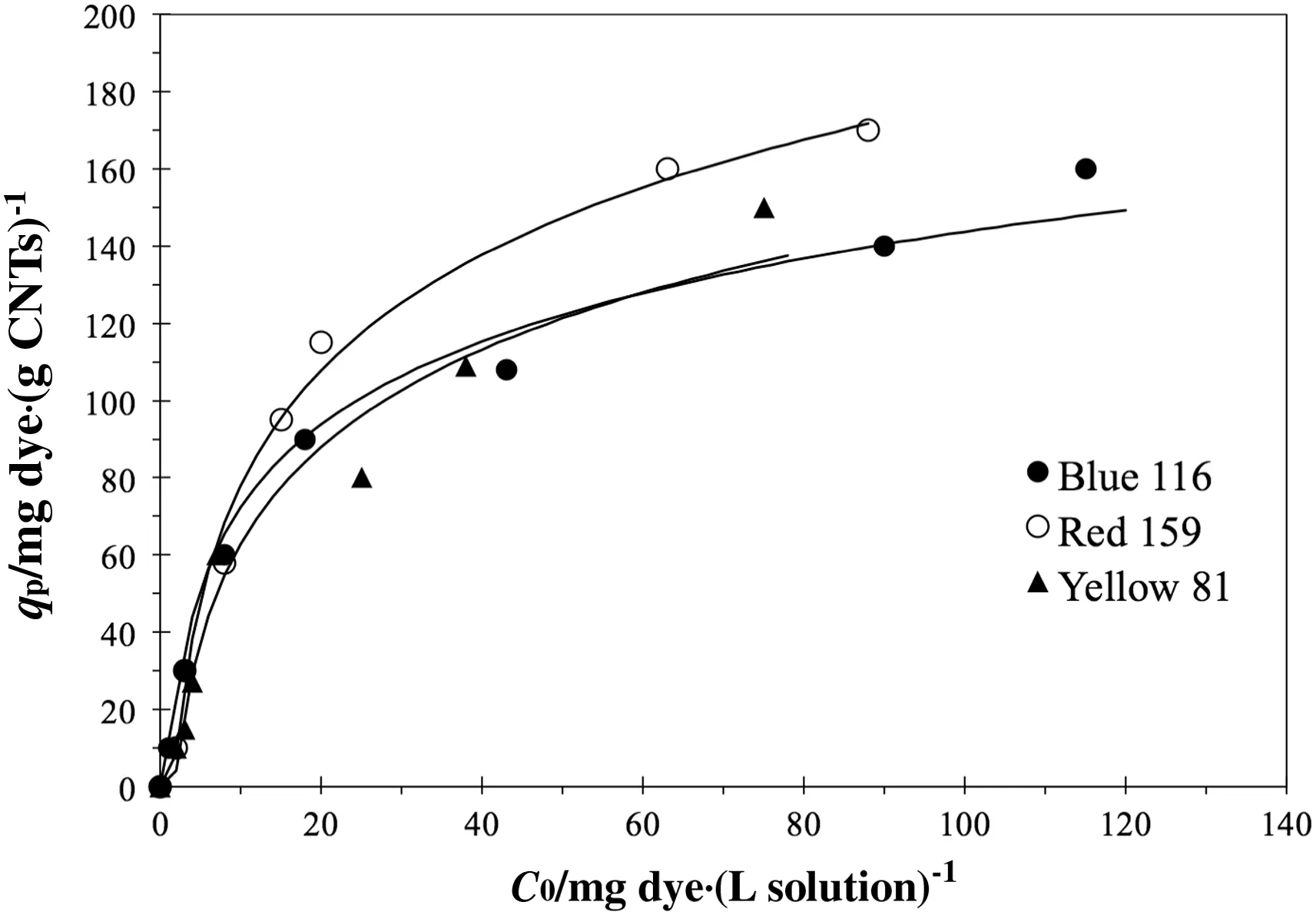
Fig.9.Experimental data and Tamkin model fit.Experimental conditions:0.03 g of CNTsUNP in 20 ml of solution.
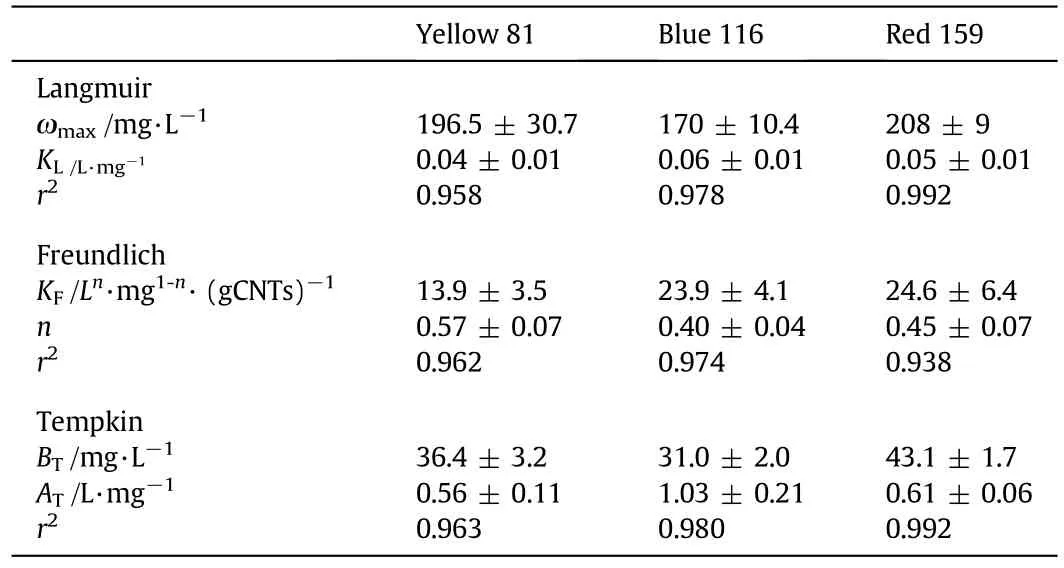
Table 5Parameters of the adsorption isotherm models for the investigated dyes

Table 6F-values of the adopted adsorption isotherm models
4.Conclusions
In this paper the use of multiwall CNTs for the removal of dye from wastewater has been proposed.The unique structural characteristics of CNTs allow this material to be used as effective adsorbent in many applications and also in the case of dyes the adsorption test revealed very good performances with respect to classic Active Carbon.In addition also the effect of surface treatments of CNTs was investigated,revealing how neither the presence of residual catalyst nor the surface oxidation(common CNTs post synthesis treatment)affects the adsorbent performances.Therefore it was concluded that non-purified CNTs can be considered as a good alternative for the process,being also their production cost lowered by the absence of any need for post synthesis treatment.Also the adsorption kinetic was studied and the best curve fitting was obtained by using a pseudo-second order model for any of the investigated dye type.In order to gain in generality,the model parameters have been correlated to the main process variables such as the dye initial concentration and the specific mass of CNTs.The assumed parameters functionality allowed to set-up a predictive kinetic model that covers a dye concentration range from 50 to 250 mg·L-1and with a specific mass of CNTS,MCNTs,up to 8 g CNTs·(L wastewater)−1.This model can be used to support process development or assessment of these new materials in already available technological solutions.
Also the equilibrium behaviour was investigated and different models(Langmuir,Freundlich and Temkin)have been applied to adsorption isotherms experimental data.It was concluded that,whatever the dye type,the Temkin model fits in the more accurate way the experimental data.As a general conclusion,a complete characterisation of CNTs as adsorbing materials for dye removal from wastewater has been presented and the measured performances strongly back this material as reliable option for treating and recovery this class of pollutants.
Appendix A.Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.cjche.2016.10.021.
[1]Water for People Water for Life,United Nations World Development Report,UNESCO,2003.
[2]Y.Cai,W.Yue,L.Xu,Z.Yang,Q.Rong,Sustainable urban water resources management considering life-cycle environmental impacts of water utilization under uncertainty,Resour.Conserv.Recycl.108(2016)21–40.
[3]V.K.Gupta,I.Ali,T.A.Saleh,A.Nayak,S.Agawal,Chemical treatment technologies for waste-water recycling— An overview,RSC Adv.2(2012)6380–6388.
[4]J.de Koning,D.Bixio,A.Karabelas,M.Salgot,A.Schäfer,Characterisation and assessment of water treatment technologies for reuse,Desalination1(2008)92–104.
[5]I.Ali,New generation adsorbents for water treatment,Chem.Rev.112(2012)5073–5091.
[6]T.Yao,S.Guo,C.Zeng,C.Wang,L.Zhang,Investigation on efficient adsorption of cationic dyes on porous magnetic polyacrylamide microspheres,J.Hazard.Mater.292(2015)90–97.
[7]R.Pourata,A.R.Khataee,S.Aber,N.Daneshvar,Removal of the herbicide Bentazon from contaminated water in the presence of synthesized nanocrystallite TiO2powders under irradiation of UV-C light,Desalination249(2009)301–307.
[8]E.C.Lima,B.Royer,J.C.P.Vaghetti,N.M.Simon,B.M.da Cunha,F.A.Pavan,E.V.Benvenutti,R.Cataluña-Veses,C.Airoldi,Application of Brazilian pine-fruit shell as a biosorbent to removal of reactive red 194 textile dye from aqueous solution:Kinetics and equilibrium study,J.Hazard.Mater.155(2008)536–550.
[9]A.Pandey,P.Singh,L.Iyengar,Bacterial decolorization and degradation of azo dyes,Int.Biodeterior.Biodegrad.59(2007)73–84.
[10]E.Forgas,T.Cserháti,G.Oros,Removal of synthetic dyes from wastewaters:A review,Environ.Int.30(2004)953–971.
[11]V.K.Gupta,Suhas,Application of low-cost adsorbents for dye removal—A review,J.Environ.Manag.90(2009)2313–2342.
[12]P.C.Vandevivere,R.Bianchi,W.Verstraete,Review:Treatment and reuse of wastewater from textile wet-processing industry:review of emerging technologies,J.Chem.Technol.Biotechnol.72(1998)289–302.
[13]L.Ai,C.Zhang,L.Li,J.Jiang,Iron terephthalate metal–organic framework:Revealing the effective activation of hydrogen peroxide for the degradation of organic dye under visible light irradiation,Appl.Catal.B.149(2014)191–200.
[14]K.Turhan,I.Durukan,S.A.Ozturkcan,Z.Turgut,Decolorization of textile basic dye in aqueous solution by ozone,Dyes Pigments92(2012)897–901.
[15]D.Daâssi,T.Mechichi,M.Nasri,S.Rodriguez-Couto,Decolorization of the metal textile dye Lanaset Grey G by immobilized white-rot fungi,J.Environ.Manag.129(2013)324–332.
[16]J.Altmann,A.S.Ruhl,F.Zietzschmann,M.Jekel,Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment,Water Res.55(2014)185–193.
[17]T.Robinson,G.McMullan,R.Marchant,P.Nigam,Remediation of dyes in textile effluent:A critical review on current treatment technologies with a proposed alternative,Bioresour.Technol.77(2001)247–255.
[18]T.A.Saleh,A.M.Muhammad,B.Tawabini,S.A.Ali,Aminomethylphosphonate chelating ligand and octadecyl alkyl chain in a resin for simultaneous removal of Co(II)ions and organic contaminants,J.Chem.Eng.Data61(9)(2016)3377–3385.
[19]T.A.Saleh,A.A.Al-Saadi,Surface characterization and sorption efficacy of tireobtained carbon:Experimental and semiempirical study of rhodamine B adsorption,Surf.Interface Anal.47(2015)785–792.
[20]Q.Li,S.Mahendra,D.Y.Lyon,L.Brunet,M.V.Liga,D.Li,P.J.J.Alvarez,Antimicrobial nanomaterials for water disinfection and microbial control:Potential applications and implications,Water Res.42(2008)4591–4602.
[21]I.M.Jauris,S.B.Fagan,M.A.Adebayo,F.M.Machado,Adsorption of acridine orange and methylene blue synthetic dyes and anthracene on single wall carbon nanotubes:A first principle approach,Comput.Theor.Chem.1076(2016)42–50.
[22]F.M.Machado,C.P.Bergmann,E.C.Lima,B.Royer,F.E.de Souza,I.M.Jauris,T.Calvete,S.B.Fagan,Adsorption of reactive blue 4 dye from water solutions by carbon nanotubes:Experiment and theory,Phys.Chem.Chem.Phys.14(2012)11139–11153.
[23]M.Migliori,D.Gabriele,R.Di Sanzo,B.De Cindio,S.Correra,Viscosity of multicomponent solutions of simple and complex sugars in water,J.Chem.Eng.Data52(4)(2007)1347–1353.
[24]S.Iijima,Helical microtubules of graphitic carbon,Nature354(1991)56–58.
[25]S.Iijima,T.Ichihashi,Single-shell carbon nanotubes of 1-nm diameter,Nature363(1993)603–605.
[26]D.S.Bethune,C.H.Klang,M.S.De Vries,Cobalt catalysed growth of carbon nanotubes with single-atomic-layer walls,Nature363(2003)605–607.
[27]Y.Wang,Z.Li,J.Wang,J.Li,Y.Lin,Graphene and graphene oxide:Biofunctionalization and applications in biotechnology,Trends Biotechnol.29(2011)205–212.
[28]Y.Li,Q.Du,T.Liu,X.Peng,J.Wang,J.Sun,Y.Wang,S.Wu,Z.Wang,Y.Xia,L.Xia,Comparative study of methylene blue dye adsorption onto activated carbon,graphene oxide,and carbon nanotubes,Chem.Eng.Res.Des.91(2013)361–368.
[29]T.Kuila,S.D.Bose,P.Khanra,A.K.Mishra,N.H.Kim,J.H.Lee,Recent advances in graphene-based biosensors,Biosens.Bioelectron.26(2011)4637–4648.
[30]A.Szabó,C.Perri,A.Csató,G.Giordano,D.Vuono,J.B.Nagy,Synthesis methods of carbon nanotubes and related materials,Materials3(2010)3092–3140.
[31]J.Liu,L.Cui,D.Losic,Graphene and graphene oxide as new nanocarriers for drug delivery applications,Acta Biomater.9(2013)9243–9257.
[32]B.S.Wong,S.L.Yoong,A.Jagusiak,T.Panczyk,H.K.Ho,W.H.Ang,G.Pastorin,Carbon nanotubes for delivery of small molecule drugs,Adv.Drug Deliv.Rev.65(2013)1964–2015.
[33]L.V.Radushkevich,V.M.Lukyanovich,O strukture ugleroda,obrazujucegosja pri termiceskom razlozenii okisi ugleroda na zeleznom kontakte,Zurn.Fisic.Chim.26(1952)88–95.
[34]A.Oberlin,M.Endo,T.Koyama,Filamentous growth of carbon through benzene decomposition,J.Cryst.Growth32(1976)335–349.
[35]K.B.K.Teo,C.Singh,M.Chhowalla,W.I.Milne,in:H.S.Nalwa(Ed.),Catalytic synthesis of carbon nanotubes and nano fibers.Encyclopedia of Nanoscience and Nanotechnology,American Scientific Publisher,Valencia,CA,USA 2003,pp.665–668.
[36]M.J.Yacamàn,M.M.Yoshida,L.Rendon,J.G.Santiesteban,Catalytic growth of carbon microtubules with fullerene structure,Appl.Phys.Lett.6(1993)202–204.
[37]J.W.Seo,A.Magrez,M.Milas,K.Lee,V.Lukovac,L.Forro,Catalytically grown carbon nanotubes:From synthesis to toxicity,J.Phys.D.Appl.Phys.40(2007)109–120.
[38]A.G.Nasibulin,D.P.Brown,P.Queipo,D.Gozalez,H.Jiang,E.Z.Kanppinen,An essential role of CO2and H2O during single-walled CNT synthesis from carbon monoxide,Chem.Phys.Lett.417(2006)179–184.
[39]P.Serp,M.Corrias,P.Kalck,Carbon nanotubes and nano fibers in catalysis,Appl.Catal.A Gen.253(2003)337–358.
[40]Q.H.Yang,P.X.Hou,S.Bai,M.Z.Wang,H.M.Cheng,Adsorption and capillarity of nitrogen in aggregated multi-walled carbon nanotubes,Chem.Phys.Lett.345(2001)18–24.
[41]V.K.K.Upadhyayula,S.Deng,M.C.Mitchell,G.B.Smith,Application of carbon nanotube technology for removal of contaminants in drinking water:A review,Sci.Total Environ.408(2009)1–13.
[42]F.M.Machado,C.P.Bergmann,T.H.M.Fernandes,E.C.Lima,B.Royer,T.Calvete,S.B.Fagan,Adsorption of reactive red M-2BE dye from water solutions by multi-walled carbon nanotubes and activated carbon,J.Hazard.Mater.192(2011)1122–1131.
[43]S.B.Kayiran,D.F.Lamari,D.Levesque,Adsorption properties and structural characterization of activated and nano-carbons,J.Phys.Chem.B108(2004)15211–15215.
[44]T.A.Saleh,The influence of treatment temperature on the acidity of MWCNT oxidized by HNO3or a mixture of HNO3/H2SO4,Appl.Surf.Sci.257(2011)7746–7751.
[45]J.G.Yu,X.H.Zhao,H.Yang,X.H.Chen,Q.Yang,L.Y.Yu,J.H.Jiang,X.Q.Chen,Aqueous adsorption and removal of organic contaminants by carbon nanotubes,Sci.Total Environ.482–483(2014)241–251.
[46]M.H.Dehghani,M.Mosto fi,M.Alimohammadi,G.McKay,K.Yetilmezsoy,A.B.Albadarin,B.Heibati,M.AlGhouti,N.M.Mubarak,J.N.Sahu,High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes:Kinetics,adsorption,mechanism and optimization studies,J.Ind.Eng.Chem.35(2016)63–74.
[47]G.S.Simate,The treatment of brewery wastewater for reuse by integration of coagulation/ flocculation and sedimentation with carbon nanotubes ‘sandwiched’in a granular filter bed,J.Ind.Eng.Chem.21(2015)1277–1285.
[48]X.Sun,H.Ou,C.Miao,L.Chen,Removal of Sudan dyes from aqueous solution by magnetic carbon nanotubes:Equilibrium,kinetic and thermodynamic studies,J.Ind.Eng.Chem.22(2015)373–377.
[49]F.Yu,J.Ma,Y.Wu,Adsorption of toluene,ethylbenzene andm-xylene on multiwalled carbon nanotubes with different oxygen contents from aqueous solutions,J.Hazard.Mater.192(2011)1370–1379.
[50]H.Vijwani,M.N.Nadagouda,V.Namboodiri,S.M.Mukhopadhyay,Hierarchical hybrid carbon nano-structures as robust and reusable adsorbents:Kinetic studies with model dye compound,Chem.Eng.J.268(2015)197–207.
[51]S.Chakma,V.S.Moholkar,Synthesis of bi-metallic oxides nanotubes for fast removal of dye using adsorption and sonocatalysis process,J.Ind.Eng.Chem.37(2016)84–89.
[52]C.H.Wu,Adsorption of reactive dye onto carbon nanotubes:equilibrium,kinetics and thermodynamics,J.Hazard.Mater.144(2007)93–100.
[53]C.Y.Kuo,C.H.Wu,J.Y.Wu,Adsorption of direct dyes from aqueous solutions by carbon nanotubes:Determination of equilibrium,kinetics and thermodynamics parameters,J.Colloid Interface Sci.327(2008)308–315.
[54]T.A.Saleh,Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II):From surface properties to sorption mechanism,Desalin.Water Treat.57(2016)10730–10744.
[55]C.Valderrama,X.Gamisans,X.de Ias Heras,A.Ferran,J.L.Cortina,Sorption kinetics of polycyclic aromatic hydrocarbons removal using granular activated carbon:Intraparticle diffusion coefficients,J.Hazard.Mater.157(2008)386–396.
[56]T.A.Saleh,Mercury sorption by silica/carbon nanotubes and silica/activated carbon:A comparison study,J.Water Supply Res.Technol.64(2015)892–903.
[57]T.A.Saleh,A.M.Muhammad,B.Tawabini,S.A.Ali,Aminomethylphosphonate chelating ligand and octadecyl alkyl chain in a resin for simultaneous removal of Co(II)ions and organic contaminants,J.Chem.Eng.Data61(2016)3377–3385.
[58]T.A.Saleh,G.I.Danmaliki,In fluence of acidic and basic treatments of activated carbon derived from waste rubber tires on adsorptive desulfurization of thiophenes,J.Taiwan Inst.Chem.Eng.60(2016)460–468.
[59]A.K.Mishra,T.Arockiadoss,S.Ramaprabhu,Study of removal ofazo dye by functionalized multi walled carbon nanotubes,Chem.Eng.J.162(2010)1026–1034.
[60]T.A.Saleh,Isotherm,kinetic,and thermodynamic studies on Hg(II)adsorption from aqueous solution by silica-multiwall carbon nanotubes,Environ.Sci.Pollut.Res.22(2015)16721–16731.
[61]T.A.Saleh,V.K.Gupta,Photo-catalyzed degradation of hazardous dye methyl orange by use of a composite catalyst consisting of multi-walled carbon nanotubes and titanium dioxide,J.Colloid Interface Sci.371(2012)101–106.
[62]Y.Yao,F.Xu,M.Chen,Z.Xu,Z.Zhu,Adsorption behavior of methylene blue on carbon nanotubes,Bioresour.Technol.101(2010)3040–3046.
[63]S.Wang,C.W.Ng,W.Wang,Q.Li,Z.Hao,Synergistic and competitive adsorption of organic dyes on multiwalled carbon nanotubes,Chem.Eng.J.197(2012)34–40.
[64]D.Zhao,W.Zhang,C.Chena,X.Wang,Adsorption of methyl orange dye onto multiwalled carbon nanotubes,Procedia Environ.Sci.18(2013)890–895.
[65]D.T.L.Prola,F.M.Machado,C.P.Bergmann,F.E.de Souza,C.R.Gally,E.C.Lima,M.A.Adebayo,S.L.P.Dias,T.Calvete,Adsorption of direct blue 53 dye from aqueous solutions by multi-walled carbon nanotubes and activated carbon,J.Environ.Manag.130(2013)166–175.
[66]V.Grosso,D.Vuono,M.A.Bahattab,G.Di Pro fio,E.Curcio,S.A.Al-Jilil,F.Alsubaie,M.Al fife,J.B.Nagy,E.Drioli,E.Fontananova,Polymeric and mixed matrix polyimide membranes,Sep.Purif.Technol.132(2014)684–696.
[67]F.M.Machado,S.B.Fagan,I.Z.da Silva,M.J.de Andrade,Carbon nanoadsorbents,in:C.P.Bergman,F.M.Machado(Eds.),Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications,Springer International Eds.,2015
[68]G.Limousin,J.P.Gaudet,L.Charlet,S.Szenknect,V.Barthès,M.Krimissa,Sorption isotherms:A review on physical bases,modelling and measurement,Appl.Geochem.22(2007)249–275.
[69]V.K.Gupta,R.Jain,M.N.Siddiqui,T.A.Saleh,S.Agarwal,S.Malati,D.Pathak,Equilibrium and thermodynamic studies on the adsorption of the dye rhodamine-B onto mustard cake and activated carbon,J.Chem.Eng.Data55(2010)5225–5229.
[70]M.Migliori,A.Aloise,E.Catizzone,G.Giordano,Kinetic analysis of methanol to dimethyl ether reaction over H-Mficatalyst,Ind.Eng.Chem.Res.53(2014)14885–14891.
[71]B.H.Hameed,M.I.El-Khaiary,Batch removal of malachite green from aqueous solutions by adsorption on oil palm trunk fibre:Equilibrium isotherms and kinetic studies,J.Hazard.Mater.154(2008)237–244.
[72]W.Cheng,S.G.Wang,L.Lu,W.X.Gong,X.W.Liu,B.Y.Gao,H.Y.Zhang,Removal of malachite green(MG)from aqueous solutions by native and heat-treated anaerobic granular sludge,Biochem.Eng.J.39(2008)538–546.
[73]H.Qiu,L.LV,B.Pan,Q.Zhang,W.Zhang,Q.Zhang,Critical review in adsorption kinetic models,J.Zhejiang Univ.Sci.10(2009)716–724.
[74]A.Policicchio,D.Vuono,T.Rugiero,P.De Luca,J.B.Nagy,Study of MWCNTs adsorption performances in gas processes,J.CO2 Util.10(2015)30–39.
[75]W.Chen,L.Duan,D.Zhu,Adsorption of polar and nonpolar organic chemicals to carbon nanotubes,Environ.Sci.Technol.41(2007)8295–8300.
[76]V.K.Gupta,R.Kumar,A.Nayak,T.A.Saleh,M.A.Barakat,Adsorptive removal of dyes from aqueous solution onto carbon nanotubes:A review,Adv.Colloid Interf.Sci.193(2013)24–34.
[77]M.S.A.Abdelbassit,K.R.Alhooshani,T.A.Saleh,Silica nanoparticles loaded on activated carbon for simultaneous removal of dichloromethane,trichloromethane,and carbon tetrachloride,Adv.Powder Technol.27(2016)1719–1729.
[78]M.Migliori,A.Aloise,G.Giordano,Methanol to dimethylether on H-Mficatalyst:The influence of the Si/Al ratio on kinetic parameters,Catal.Today227(2014)138–143.
 Chinese Journal of Chemical Engineering2017年4期
Chinese Journal of Chemical Engineering2017年4期
- Chinese Journal of Chemical Engineering的其它文章
- Corrosion performance of Al–Al2O3 cold sprayed coatings on mild carbon steel pipe under thermal insulation☆
- Integrated ozone–photo–Fenton process for the removal of pollutant from industrial wastewater☆
- Suppressing secondary reactions of coal pyrolysis by reducing pressure and mounting internals in fixed-bed reactor☆
- Multi-objective regulation in autohydrolysis process of corn stover by liquid hot water pretreatment
- Development of a new cleaner production process for cassava ethanol☆
- Preparation of cross-linked enzyme aggregates of nitrile hydratase ES-NHT-118 from E.coli by macromolecular cross-linking agent☆
