Influence of Na+,K+,Mg2+,Ca2+,and Fe3+on filterability and settleability of drilling sludge☆
Liyan Liu,Hao Yan,Wei Tan,Guorui Zhu*
School of Chemical Engineering and Technology,Tianjin University,Tianjin 300350,China
1.Introduction
With rapid development of drilling technology,large amount of sludge has been produced during drilling operation.Drilling sludge is aqueous suspension of charged hydrophilic colloid,which consists of swelling clays, fine mineral particles,heavy metals and organic materials such as various surfactants,hydrocarbons.Many of these components are potentially toxic or directly do harm to the environment[1–3].The disposal of large quantities of drilling sludge has become one of the most difficult environmental problems all over the world due to its complex compositions and stability.Thus, finding a cost-effective way to dewater prior to the following harmless treatment process to reduce its disposal cost is very important.Due to the dewatering of drilling sludge is considered to be a vital step in its reduction,sedimentation and mechanical methods,such as filtration and centrifugation,have been widely used to reduce the volume of drilling sludge[4,5].However,mechanical dewatering is very difficult without pretreatment and chemical conditioning.
According to the previous works,the thermal hydrolysis,freezing and thawing,ultrasonication,acid and alkali adjustment,have been put forward to improve the sludge dewaterability[6–9].These pretreatments achieve certain effect on sludge dewatering,but at the same time they present some limits on applicability in industry,such as requiring high amounts of mechanical equipments and energy.Inorganic cations,polyacrylamide and surfactant conditioning have been used in sludge treatment owing to their low cost and operability.Since the main solid component of drilling sludge is small clay particles with a large number of negative charge,the particles have good ion adsorption characteristic.Among different chemical conditioning,the addition of inorganic cations has an advantage in providing more positive charge,which plays an important role in the adsorption onto clay particles to neutralize negative charge and improve the dewaterability of drilling sludge.
Inorganic cations are added to sludge in the coagulation process,and they can change sludge surface charge,particle size and floc strength[10,11].The most typical inorganic cations are trivalent ions.Turchiuliet al.[12]observed that ferric chloride conditioning enhances synthetic sludge dewatering rate as evidenced by the decrease of capillary suction time.At the same time,ferric flocs bind to water and increase bound water content.Besides,the divalent cations are considered as effective chemical conditioning agents in sludge treatment.Guanet al.[13]reported that calcium chloride can improve the dewaterability of activated sludge by interacting with the protein,phenols and O—H functional group in the flocs and neutralizing the surface charge of flocs.Calcium ions compress the colloids double electrode layers,resulting in aggregation phenomena between sludge particles[10].For monovalent ions,the role of which in solid–liquid separation process of sludge is controversial.Karaet al.[14]used potassium and sodium to condition activated sludge,and the results revealed that the addition of monovalent ions has no improvement in settleability of activated sludge.Iritaniet al.[15]found that the synergy effects of sodium chloride addition and ultrasonication promote the sedimentation velocity and decrease activated sludge volume.The recent studies have shown that inorganic cations can affect the flocculation and separation of different sludge in varying degrees.The research about application of inorganic cations conditioning to drilling sludge separation is useful to enrich the variety of sludge treatment.Moreover,it can be seen from the previous works that types of cations have some relation to separation effect.Thus,it is worthwhile to clarify the influence of different inorganic cations on the solid–liquid separation of drilling sludge.
In this paper,the effects of different inorganic cations(Na+,K+,Mg2+,Ca2+,Fe3+)on the filterability and settleability of drilling sludge were investigated systematically and contrastively.The relationships between the cations and floc characteristics were analyzed from the variation of Zeta potential,specific surface area,particle size distribution and Fourier-transformed infrared spectra(FT-IR),which elucidated the change mechanism of filterability and settleability of sludge with different cations conditioning.
2.Materials and Methods
2.1.Materials
The raw drilling sludge used in this research was taken from an oily field located in northeastern China,and it was brown viscous homogeneous mixture with poor settleability.The basic characteristics of the drilling sludge are listed in Table 1.The mineralogical measured by XRD of the raw drilling sludge has been summarized in Fig.1 to investigate the phase composition.The raw drilling sludge included two major stable crystalline phases,which were SiO2and Al2SiO5.It indicated that the main solid content of drilling sludge was clay mineral.The morphological character of raw drilling sludge particle is shown in Fig.2.Aggregate flocks with flake-like surface forming cotton-wool structure present in the micrographs,which is similar to the result previously reported by Liuet al.[16].
The ferric chloride,calcium chloride,magnesium chloride,potassium chloride and sodium chloride were analytical grade,and they were purchased from Xinqiao Chemical Reagent Co.(Tianjin,China).

Table 1Basic characteristics of drilling sludge
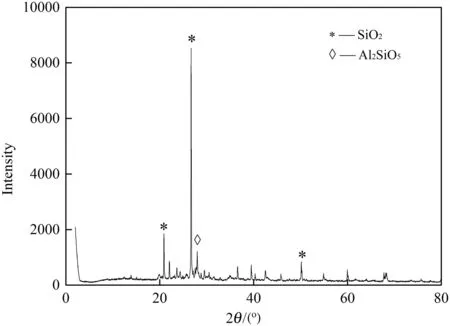
Fig.1.Mineralogical analysis by XRD of raw drilling sludge.
2.2.Experimental procedure
The volume of sludge samples used in the experiment was 50 ml in 150 ml beakers.The inorganic salt powder was dissolved by distilled water to form a homogeneous solution of 50 g·L−1,and gradually added into the sludge using a graduated finnpipette.Then,the mixture was stirred at 250 r·min−1and 50 r·min−1for 2 min and 15 min,respectively.Finally,the raw and treated sludge samples were poured into the experimental apparatus shown in Fig.3 to carry out a series of dewatering experiments.The diameter of the filter chamber was 90 mm.The sludge was dewatered until no filtrate flowed out in 60 s under 0.3 MPa,and the filtrate mass and the filtration time were recorded to calculate filtration rate[17].After the dewatering test,the filter cake was removed,weighed,dried and the water content was calculated.The settling tests of drilling sludge were carried out at room temperature.The settling sample volume was 100 ml,and the diameter of the cylinder was 28 mm.The supernatant volume was recorded every 12 h without disturbance.At the end of settling experiments,the supernatant was collected to determine the turbidity.All the tests were performed in triplicate,and the average values were shown in the paper.
2.3.Analysis method
The water content of filter cake was calculated using the following equation[2,18]:

The basic characteristics of drilling sludge,including water content,volatile suspended solids and pH,were measured according to CJ/T 221-2005(China standard for sludge analysis).The viscosity of the drilling sludge was examined using a rotational viscometer(NDJ-8S,Nirun,China).The identification of drilling sludge crystalline phases was measured by X-ray diffraction equipment(D8-Focus,Bruker S4,Germany).The surface structure of drilling sludge flocs was determined by a scanning electron microscope(SU8010,Hitachi,Japan).The supernatant of the drilling sludge after settling tests was collected for the turbidity measurements using a turbidimeter(WGZ-2,Xinrun,China).The zeta potential of supernatant was tested using Zetasizer Nano ZS90(Malvern,UK).The specific surface area and particle size distribution of drilling sludge particles were analyzed by a Mastersize 2000 analyzer(Malvern,UK)after dispersing drilling sludge samples in distilled water.The drilling sludge filter cake dried at 105°C for 24 h was ground into powder,which was dried again under the same conditions to be used for Fourier transform infrared spectrum studies.The Fourier transform infrared spectrum of sludge powder was obtained using a Nexus 470 spectrometer(Nicolet,USA).

Fig.2.Morphological observation by SEM of raw drilling sludge particle.
3.Results and Discussion
3.1.Effects of different inorganic cations on filterability
The filterability of the drilling sludge depends on not only the extent of dewatering but also the rate of filtration.The water content and filtration rate were measured to directly reflect the drilling sludge filterability.Fig.4 reveals the effects of different inorganic coagulants dosage on the water content of filter cake.As all inorganic coagulants dosage increased from 0 to 1.0%,the water content of drilling sludge treated by monovalent and divalent cations decreased from 30.56%,the minimum water contentofNaCl,KCl,MgCl2and CaCl2were 29.87%,28.71%,27.54%and 26.66%,respectively.The water content of drilling sludge treated with FeCl3increased from 30.56%to 33.05%.Comparing five inorganic cations in terms of their solid–liquid separation performance of drilling sludge,the divalent cations had better separation performance than monovalent and trivalent ones at the same dosage.Under the same valence,calcium ion was superior to magnesium ion,and potassium ion was better than sodium ion.The results might be attributable to their different changes in surface charge and sludge flocs,which will be described in latter parts.Ferric ion failed to decrease water content of filter cake because ferric ion dissolved in solution forming hydrophilic colloidal or amorphous hydroxide precipitateviaa series of hydrolysis processes[19],which was usually positively charged and had a strong hydrophilic property.Therefore,it was held onto the surface of clay particles by adsorption or adhesion to increase water content of filter cake.

Fig.3.Diagram of the pressure filtration dewatering experimental apparatus.
The effects of different inorganic coagulants dosage on drilling sludge filtration rate showed that filtration rate increased as the concentration increased regardless of the inorganic cations in solution(Fig.5).When the inorganic coagulants dosage increased from 0 to 1%,the filtration rate of treated drilling sludge was approximately 1.8–2.7 times that of raw drilling sludge,indicating an improvement of the sludge filterability after inorganic cations addition.Moreover,the filtration rate had a significant increase upon increasing the valence of cations at the same dosage.The acceleration on filtration rate of drilling sludge was FeCl3>CaCl2>MgCl2>KCl>NaCl.The drilling sludge conditioned with trivalent cations dewatered quickly,and drilling sludge conditioned with monovalent cations separated slowly.The multivalent cations could provide more positive charge to neutralize the negative charge on surface of drilling sludge particles,resulting in superior dewaterability[20].In the case of monovalent,the greater filtration rate occurred with K+and less with Na+.For the divalent cations,it can be found that the effect of Ca2+was better than Mg2+on enhancing filtration rate when the dosage was fixed.The results indicated that the filterability of the drilling sludge was influenced by dosage and types of inorganic coagulants.At low dosages,inorganic cations failed to provide more positive charge to reduce the negative charge on surface of sludge particles.The sludge filterability could be improved with the gradual increase of positive charge to the sludge system.The reasons for different influences of cations on filterability were attributed to difference of ionic nature.Due to the negative charge of sludge particles,cations played an important role in flocculation of the sludge.The flocculation was related to the valence and hydrated radius of cations.The increase of the ionic valence enhanced the ability of flocculation.At the same valence,the cations with smaller hydrated radius performed better flocculation.

Fig.4.Effects of dosage on water content of filter cake after filtration.

Fig.5.Effects of dosage on filtration rate.
3.2.Effects of different inorganic cations on settleability
The supernatant volume was used to evaluate settleability of sludge.The settling behavior of the raw drilling sludge and treated drilling sludge samples was monitored for 144 h.Fig.6 illustrates variation of the supernatant volume during the settling process.The settleability of the raw drilling sludge was comparatively poor,indicating it was stable enough without inorganic cations conditioning.In contrast,the settling interface of the treated drilling sludge samples with different inorganic cations was clearer,and the volume of supernatant rose obviously.The settling rate decreased and gradually leveled off along with time.After 144 h of settling process,the supernatant volume of drilling sludge samples treated with NaCl,KCl,MgCl2,CaCl2and FeCl3were 17.0,19.5,22.0,26.0 and 31.0 ml,respectively.Furthermore,the trivalent cation(iron)exhibited the best settleability for all treated sludge samples.In comparison,the settling rate of bivalent cations(calcium and magnesium)was slower.Compared to trivalent and bivalent cations,the settling rate of monovalent cations(potassium and sodium)was slowest,and the settleability followed the order:trivalentcation>bivalentcation>monovalentcation.The phenomenon mightowe to various charge densities of different inorganic cations.The supernatant volume of drilling sludge treated with FeCl3was greater than that of other inorganic coagulants,and approximately 31%could be separated after settling 144 h.Moreover,comparing CaCl2(KCl)and MgCl2(NaCl),the inorganic cations with larger hydrated radius performed worse sedimentation properties,because the hydration membrane resistances weakened settleability.
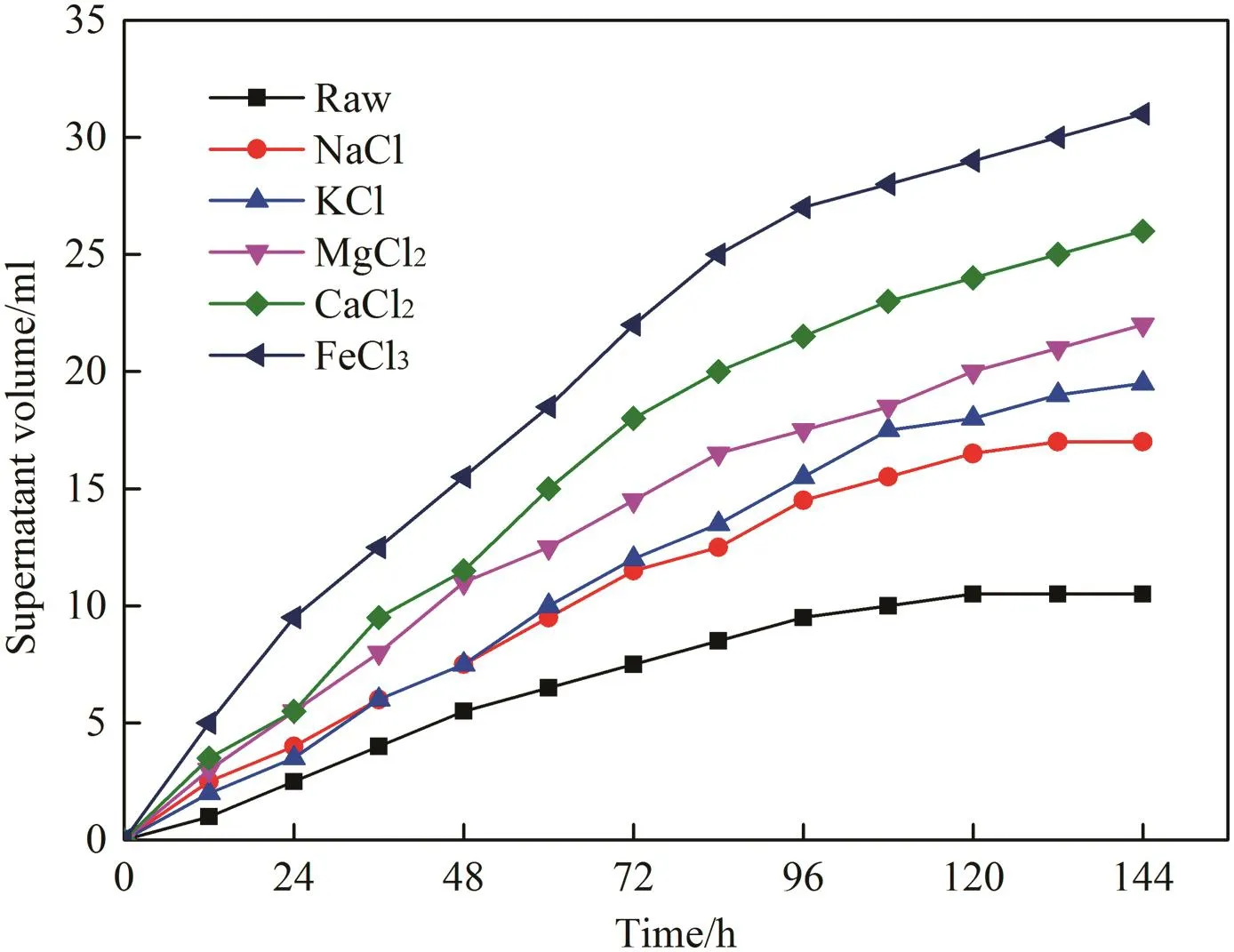
Fig.6.Settling curve of drilling sludge treated with inorganic coagulants at 0.6%dosage.
Supernatant turbidity was also examined to help represent the dewatering and settling performance[21,22].The effects of different inorganic cations on supernatant turbidity are shown in Fig.7.All drilling sludge samples were treated with inorganic coagulants at 0.6%dosage.The supernatant turbidity of raw drilling sludge was 60.44 NTU,and lots of fine particles appeared in the supernatant.The turbidity of supernatant conditioned with inorganic cations had a dramatic difference,which sharply decreased 44%at least.As valence of cations increased from 1 to 3,the supernatant turbidity gradually decreased.The fall of the supernatant turbidity indicated that fine particles in the supernatant decreased.The change of supernatant turbidity corresponded to the settling behavior of drilling sludge.This indicated that inorganic cations were successful in providing positive charge to neutralize the negative charge on surface of drilling sludge particles and forming larger sludge flocs[21].
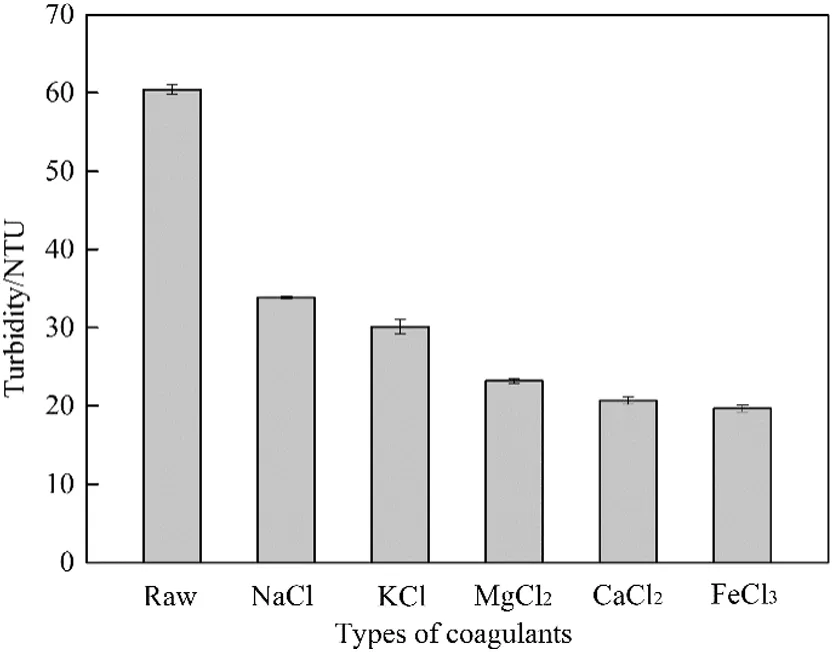
Fig.7.Effect of different inorganic coagulants on supernatant turbidity.
3.3.Possible influence mechanisms of different inorganic cations
3.3.1.FT-IR analysis
Functional groups of sludge flocs affect the sludge dewaterability and stability of flocs[23,24].To determine the interaction between different inorganic cations and sludge flocs,FT-IR analysis of sludge flocs treated with different inorganic cations is given in Fig.8.The bands at 3620 and 3433 cm−1are O—H stretching vibration.The bands at 2925 and 2854 cm−1are linked to asymmetric and symmetric stretching vibration of CH2,respectively[13,25].A band of C=O and C—N stretching variation is at 1622 cm−1.The band at 1428 cm−1is the O—H deformation vibration.The intense band at 1033 cm−1is attributed to vibration of C—O.The wavenumbers less than 1000 cm−1corresponds to phosphate and sulphur groups.The similar functional groups of sludge flocs have been reported previously[26,27].
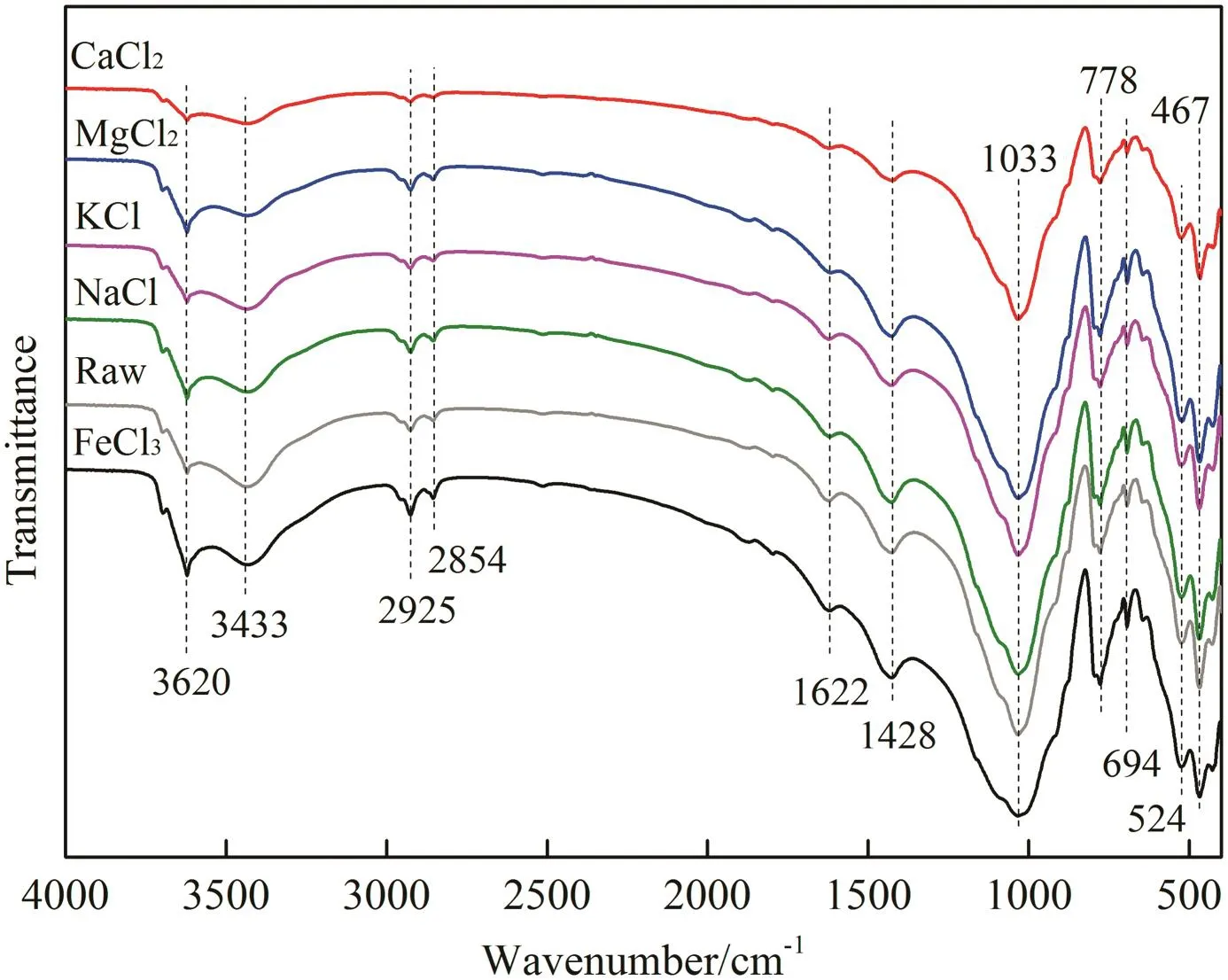
Fig.8.FT-IR spectra of the drilling sludge treated with different inorganic coagulants at 0.6%dosage.
Fig.8 shows that there was no obvious shift of characteristic bands after different inorganic cations conditioning.The results indicated that there was no chemical adsorption between inorganic cations and drilling sludge flocs[27].Absorption includes chemical absorption and physical absorption.Therefore,inorganic cations affected filterability and settleability of drilling sludge by physical adsorption.Due to the negative charge of sludge particles,electrostatic interaction played a major role in the physical absorption.
3.3.2.Surface charge
Zeta potential is a measurement of the sludge surface charge,which affects sludge properties and has a strong relation with sludge dewatering and settling[28].The zeta potential of the drilling sludge treated with different inorganic cations is illustrated in Fig.9.Original zeta potential of raw drilling sludge was negatively charged with−23.2 mV,and the addition of inorganic cations could neutralize negative charge effectively.The value was Fe3+>Ca2+>Mg2+>K+>Na+,which was consistent with the cationic valence order.The effects of multivalent cations on charge neutralization were more conspicuous than monovalent cations.Under the same valence,the drilling sludge treated with sodium(magnesium)ion had higher negative charge value than the sludge treated with potassium(calcium)ion.Similar effect of monovalent cations has been observed previously[14].
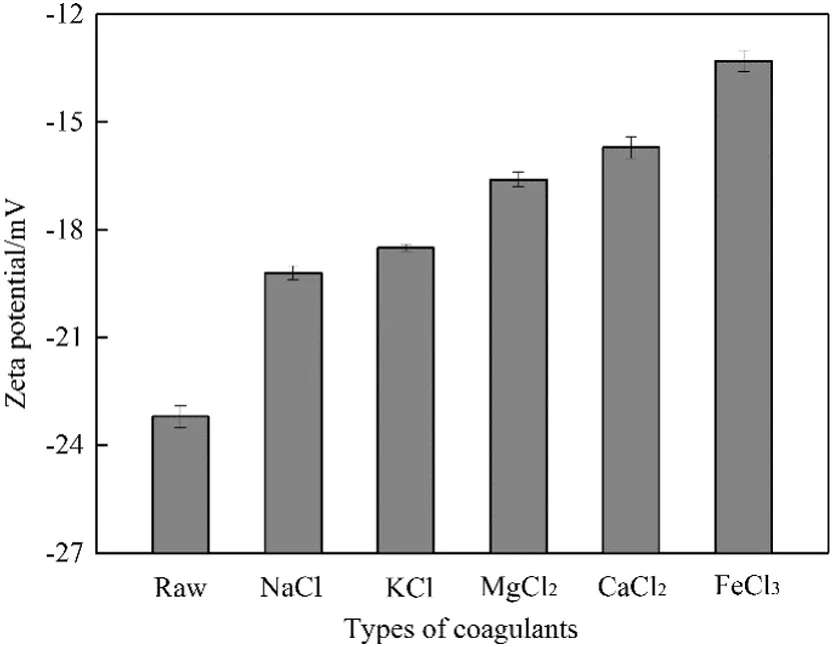
Fig.9.Zeta potential of drilling sludge treated with different inorganic coagulants at 0.6%dosage.
The particles of sludge carry high negative surface charge and an electric double layer has formed surrounding the particle,which results in repulsion of adjacent particles.Therefore,the drilling sludge keeps a relatively stable system,inhibiting flocculation and leading to poor filterability and settleability[29,30].The presence of inorganic cations neutralized the negative charge of sludge.The reduction in sludge negative charge decreased repulsion forces and affected the degree of sludge dispersion[31,32].The colloidal stability was destroyed and sludge particles congregated with each other after the addition of cations.Therefore,inorganic cations improved filterability and settleability by this means.Liuet al.[29]found that the seawater and brine are effective in improving the sludge dewaterability.The improvement is mainly due to the effects of inorganic cations in the seawater and brine.
The differences of sludge surface charge between different inorganic cations conditioning were related to the valence and hydrated radius of cations.As the ionic valence increased,the trivalent cations exhibited a significant advantage on decreasing negative surface charge of sludge.Since trivalent cation had high charge density,it was easier to compress the double layer and decrease zeta potential than bivalent cations and monovalent cations.Comparison of the zeta potential of Ca2+conditioning with that of Mg2+conditioning showed the effect of Ca2+conditioning was slightly better at the same valence.This might be because Mg2+and Ca2+have different hydrated radius.The hydrated radius ofMg2+and Ca2+is 0.428 nmand 0.412 nm,respectively,and the hydrated radius of Mg2+is larger than Ca2+[33].The presence of the hydration layer weakened the electrostatic attraction between cations and particles of sludge,and the larger the hydrated radius was,the more difficult it was for cations to absorb onto the particles.Consequently,these weakened the role of compressing the double layer for cations.Similarly,the result of K+and Na+was consistent with that of Ca2+and Mg2+.
3.3.3.Specific surface area and particle size distribution
In order to further investigate the effects of inorganic cations on physical characteristics of sludge flocs,the specific surface area and the particle size were analyzed(Table 2).Generally,large flocs and small specific surface area denote good dewaterability and settleability[21,34].Fig.10 presents size distribution of particles before and after cations conditioning.The particle size distribution curves of drilling sludge treated with different inorganic cations shift to right.After treatment with NaCl,KCl,MgCl2,CaCl2and FeCl3at 0.6%dosage,the particle size of drilling sludge was increased from 11.358 μm to 13.422,13.917,14.887,14.898 and 15.585 μm,respectively.The particle size increased gradually with cationic valence,while the reverse occurred for the specific surface of sludge particles.
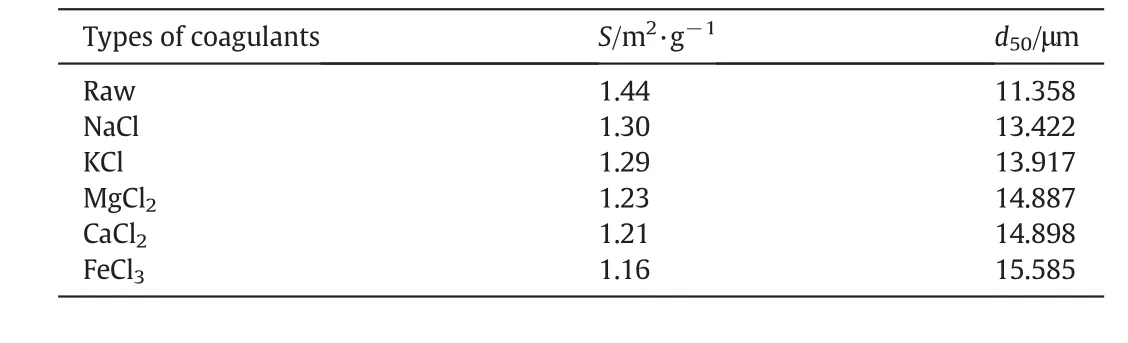
Table 2Specific surface area and particle size of sludge treated with different inorganic coagulants at 0.6%dosage

Fig.10.Particle size distribution of drilling sludge treated with different inorganic coagulants at 0.6%dosage.
According to Fig.10 and Table 2,the particle size of sludge treated with different inorganic cations had varying degrees of increase.The percentage increases of particle size were 18.17%,22.53%,31.07%,31.17%and 37.22%,respectively.For the sludge treated with trivalent cation(iron),the increase of particle size was larger than that of sludge treated with divalent cations(calcium and magnesium).The increase of particle size treated with monovalent cations(potassium and sodium)was found to be the smallest.The difference in the particle size increase attributed to the fact that the types of cations were different.As ionic charge strength increased,the thickness of the double layer decreased,according to double layer theory,which in turn decreased the repulsion between particles and allowed Van der Waals attractive forces to promote aggregation[35].Therefore,trivalent cation achieved the largest increase of particle size.In the case of divalent cations,the greater particle size occurred with calcium conditioning.For the monovalent cations,the greater particle size occurred with potassium conditioning.The increase of particle size presented a consistent change with the zeta potential(Fig.8),and the lower surface charge corresponded to the better aggregation of the particles[34].Besides,some researchers found that the divalent cations can facilitate flocculation and increase flocs size by cation bridging[35,36].The trivalent cations existed in the form of colloidal hydroxide precipitates in the solution,and colloidal particles would be enmeshed in these precipitates[37].As a result,the particles aggregated and formed large flocs,and flocs growth improved the filterability and settleability of drilling sludge.
4.Conclusions
The influence of different inorganic cations on the filterability and settleability of drilling sludge was investigated in this paper.Moreover,the characteristics of drilling sludge flocs were examined to give a comprehensive insight into the essential mechanism.The following conclusions can be drawn from the research:
(1)The solid–liquid separation behavior of drilling sludge was related to concentration and types of cations.The addition of different inorganic cations in drilling sludge could enhance the filterability and settleability in terms of filtration rate,supernatant volume and supernatant turbidity.The effects of inorganic cations were in the following order:Fe3+>Ca2+>Mg2+>K+>Na+.Multivalent cations performed better than monovalent cations.At the same valence,the cations with smaller hydrated radius got superior conditioning results.Besides,although Fe3+conditioning could effectively enhance filtration rate,in reduction of the water content,it was difficult to be competent.
(2)The filterability and settleability were linked to the physical characteristics of sludge flocs,including its surface charge,particle size and specific surface area.The different inorganic cations could neutralize negative surface charge and increase the particle size with different extent.Consequently,the degree of drilling sludge dispersion was reduced and the colloidal system was broken,causing the different improvement of filterability and settleability.
Nomenclature
Across-sectional area,m2
tfiltration time,s
Vfiltrate volume,m3
W1mass of wet filter cake after filtration,g
W2mass of filter cake after drying at 105°C for 24 h,g
Acknowledgements
The authors are grateful to both the provision of drilling sludge from Jereh Environmental Protection Technology Co.,Ltd.and the testing support of Shandong Institute of Tianjin University.
[1]M.Loginov,M.Citeau,N.Lebovka,E.Vorobiev,Electro-dewatering of drilling sludge with liming and electrode heating,Sep.Purif.Technol.104(2013)89–99.
[2]J.Zou,H.Zhu,F.Wang,H.Sui,J.Fan,Preparation of a new inorganic–organic composite flocculant used in solid–liquid separation for waste drilling fluid,Chem.Eng.J.171(1)(2011)350–356.
[3]K.Hu,X.Chen,W.Huang,B.Lu,Y.Chen,Novel sequential treatment methodology for disposal of water-based waste drilling mud,Environ.Eng.Sci.29(7)(2012)669–676.
[4]Y.Qi,K.B.Thapa,A.F.A.Hoadley,Application of filtration aids for improving sludge dewatering properties— A review,Chem.Eng.J.171(2)(2011)373–384.
[5]L.Wang,D.He,Z.Tong,W.Li,H.Yu,Characterization of dewatering process of activated sludge assisted by cationic surfactants,Biochem.Eng.J.91(2014)174–178.
[6]C.Bougrier,J.P.Delgenes,H.Carrere,Effects of thermal treatments on five different waste activated sludge samples solubilisation,physical properties and anaerobic digestion,Chem.Eng.J.139(2)(2008)236–244.
[7]J.Diak,B.Oermeci,C.Proux,Freeze–thaw treatment of RBC sludge from a remote mining exploration facility in subarctic Canada,Water Sci.Technol.63(6)(2011)1309–1313.
[8]X.Feng,J.Deng,H.Lei,T.Bai,Q.Fan,Z.Li,Dewaterability of waste activated sludge with ultrasound conditioning,Bioresour.Technol.100(3)(2009)1074–1081.
[9]S.Guo,G.Li,J.Qu,X.Liu,Improvement of acidification on dewaterability of oily sludge from flotation,Chem.Eng.J.168(2)(2011)746–751.
[10]A.Pevere,G.Guibaud,E.D.van Hullebusch,W.Boughzala,P.N.L.Lens,Effect of Na+and Ca2+on the aggregation properties of sieved anaerobic granular sludge,Colloids Surf.A Physicochem.Eng.Asp.306(1–3)(2007)142–149.
[11]D.C.Sobeck,M.J.Higgins,Examination of three theories for mechanisms of cationinduced bio flocculation,Water Res.36(3)(2002)527–538.
[12]C.Turchiuli,C.Fargues,Influence of structural properties of alum and ferric flocs on sludge dewaterability,Chem.Eng.J.103(1–3)(2004)123–131.
[13]B.Guan,J.Yu,H.Fu,M.Guo,X.Xu,Improvement of activated sludge dewaterability by mild thermal treatment in CaCl2solution,Water Res.46(2)(2012)425–432.
[14]F.Kara,G.C.Gurakan,F.D.Sanin,Monovalent cations and their influence on activated sludge floc chemistry,structure,and physical characteristics,Biotechnol.Bioeng.100(2)(2008)231–239.
[15]E.Iritani,M.Nishikawa,N.Katagiri,K.Kawasaki,Synergy effect of ultrasonication and salt addition on settling behaviors of activated sludge,Sep.Purif.Technol.144(2015)177–185.
[16]H.Liu,J.K.Yang,N.R.Zhu,H.Zhang,Y.Li,S.He,C.Z.Yang,H.Yao,A comprehensive insight into the combined effects of Fenton's reagent and skeleton builders on sludge deep dewatering performance,J.Hazard.Mater.258(2013)144–150.
[17]J.Olivier,J.B.Conrardy,A.Mahmoud,J.Vaxelaire,Electro-dewatering of wastewater sludge:An investigation of the relationship between filtrate flow rate and electric current,Water Res.82(2015)66–77.
[18]Y.Zhang,Z.Miao,J.Zou,A new cation-modified Al-polyacrylamide flocculant for solid–liquid separation in waste drilling fluid,J.Appl.Polym.Sci.132(11)(2015).
[19]G.Lei,J.Ma,X.Guan,A.Song,Y.Cui,Effect of basicity on coagulation performance of polyferric chloride applied in eutrophicated raw water,Desalination247(1–3)(2009)518–529.
[20]C.C.Wu,J.J.Wu,Effect of charge neutralization on the dewatering performance of alum sludge by polymer conditioning,Water Sci.Technol.44(10)(2001)315–319.
[21]Y.Sun,H.Zheng,J.Zhai,H.Teng,C.Zhao,C.Zhao,Y.Liao,Effects of surfactants on the improvement of sludge dewaterability using cationic flocculants,PLoS One9(10)(2014).
[22]Y.Sun,W.Fan,H.Zheng,Y.Zhang,F.Li,W.Chen,Evaluation of dewatering performance and fractal characteristics of alum sludge,PLoS One10(6)(2015).
[23]B.Q.Liao,D.G.Allen,G.G.Leppard,I.G.Droppo,S.N.Liss,Interparticle interactions affecting the stability of sludge flocs,J.Colloid Interface Sci.249(2)(2002)372–380.
[24]X.Zhou,G.Jiang,T.Zhang,Q.Wang,G.-j.Xie,Z.Yuan,Role of extracellular polymeric substances in improvement of sludge dewaterability through peroxidation,Bioresour.Technol.192(2015)817–820.
[25]S.Amir,M.Ha fidi,G.Merlina,J.C.Revel,Sequential extraction of heavy metals during composting of sewage sludge,Chemosphere59(6)(2005)801–810.
[26]O.Gulnaz,A.Kaya,S.Dincer,The reuse of dried activated sludge for adsorption of reactive dye,J.Hazard.Mater.134(1–3)(2006)190–196.
[27]J.Laurent,M.Casellas,M.N.Pons,C.Dagot,Flocs surface functionality assessment of sonicated activated sludge in relation with physico-chemical properties,Ultrason.Sonochem.16(4)(2009)488–494.
[28]L.H.Mikkelsen,K.Keiding,Physico-chemical characteristics of full scale sewage sludges with implications to dewatering,Water Res.36(10)(2002)2451–2462.
[29]J.Liu,G.Zhao,C.Duan,Y.Xu,J.Zhao,T.Deng,G.Qian,Effective improvement of activated sludge dewaterability conditioning with seawater and brine,Chem.Eng.J.168(3)(2011)1112–1119.
[30]K.Luo,Q.Yang,X.-m.Li,S.-y.Zhang,Y.Pang,X.Li,X.-s.Liao,Effect of calcium ions on dewaterability of enzymatic-enhanced anaerobic digestion sludge,Appl.Biochem.Biotechnol.176(8)(2015)2346–2357.
[31]Y.Liu,H.H.P.Fang,Influences of extracellular polymeric substances(EPS)on flocculation,settling,and dewatering of activated sludge,Crit.Rev.Environ.Sci.Technol.33(3)(2003)237–273.
[32]X.Y.Li,S.F.Yang,Influence of loosely bound extracellular polymeric substances(EPS)on the flocculation,sedimentation and dewaterability of activated sludge,Water Res.41(5)(2007)1022–1030.
[33]A.G.Volkov,S.Paula,D.W.Deamer,Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers,Bioelectrochem.Bioenerg.42(2)(1997)153–160.
[34]J.Yu,M.Guo,X.Xu,B.Guan,The role of temperature and CaCl2in activated sludge dewatering under hydrothermal treatment,Water Res.50(2014)10–17.
[35]T.P.Nguyen,N.P.Hankins,N.Hilal,A comparative study of the flocculation behaviour and final properties of synthetic and activated sludge in wastewater treatment,Desalination204(1–3)(2007)277–295.
[36]G.Luo,W.Liang,H.Tan,C.Yao,N.Zhang,L.Lu,Effects of calcium and magnesium addition on the start-up of sequencing batch reactor using biofloc technology treating solid aquaculture waste,Aquac.Eng.57(2013)32–37.
[37]T.Li,Z.Zhu,D.S.Wang,C.H.Yao,H.X.Tang,Characterization of floc size,strength and structure under various coagulation mechanisms,Powder Technol.168(2)(2006)104–110.
 Chinese Journal of Chemical Engineering2017年5期
Chinese Journal of Chemical Engineering2017年5期
- Chinese Journal of Chemical Engineering的其它文章
- Partition coefficient prediction of Baker's yeast invertase in aqueous two phase systems using hybrid group method data handling neural network
- An improved flexible tolerance method for solving nonlinear constrained optimization problems:Application in mass integration
- An optimal filter based MPC for systems with arbitrary disturbances☆
- Measurement and calculation of solubility of quinine in supercritical carbon dioxide☆
- Solubility and metastable zone width measurement of 3,4-bis(3-nitrofurazan-4-yl)furoxan(DNTF)in ethanol+water
- The effect of transition metal ions(M2+=Mn2+,Ni2+,Co2+,Cu2+)on the chemical synthesis polyaniline as counter electrodes in dye-sensitized solar cells☆
