Efficient decolorization of dye-containing wastewater using mycelial pellets formed of marine-derived Aspergillus niger☆
Tao Lu,Qilei Zhang,Shanjing Yao*
Key Laboratory of Biomass Chemical Engineering of Ministry of Education,College of Chemical and Biological Engineering,Zhejiang University,Hangzhou 310027,China
1.Introduction
Dye-containing wastewater is one of the common industrial effluents and is very difficult to be treated.For instance,from the textile industry,around 150 million tons of colored waste waters is discharged per annum[1].Many dyes could have been toxic in human cells and lead to dysfunctions of many organs[2].Moreover,it is recognized that color is the first obvious sign of effluents and the existence of even trace amounts of dyes will be easily appreciable and unacceptable[3].The presence of dyes will also reduce sunlight penetration and weaken photosynthesis and cause chemical toxicity to aquatic life[4].Therefore,the treatment of dye-stuff wastewater is extremely important.
Various methods such as ion exchange,solvent extraction,advanced oxidation processes,coagulation,membrane separation and adsorption were used for the dye removal of wastewater[4,5].Adsorption has been proved to be an effective process of dye wastewater treatment.Activated carbon is an effective adsorbent which some industries employ to reduce dye and other hazardous pollutants in effluent with prominent advantages such as high adsorption capacity and the large surface area.However,the large scale application of activated carbon is still limited due to its high running cost[6].Many literatures expounded the feasibility of utilizing all kinds of low-cost adsorbents,which were derived from microorganism biomass,agricultural byproducts and natural materials[7–10].Various physical or chemical pretreatments were used to enhance their adsorption efficiency of the raw adsorbents,which were also reported by many researchers[4,11].
When it comes to practical use,however,there still exist some important issues needed to be addressed,such as clogging problems,rapid solid–liquid separation after adsorption and the reusability of adsorbent[12].These problems sometimes could be settled by immobilization using supporting materials,but the added process would also increase costs and influence mass transfer efficiency.Meanwhile,selfimmobilization of biomass shows broad application prospects in solving these problems.For instance,it is generally known that many fungi,likePenicilliumsp.,Aspergillussp.andRhizopussp.can form stable mycelial pellets to achieve the self-immobilization process[13–15].The pellet formation of filamentous fungi has a lot of advantages like efficient solid–liquid separation,considerable mechanical strength and larger specific surface area[16,17].Therefore,the application of the fungal biomass for dye-removing is an attractive alternative to the dye-containing effluent treatment[18].Both living and dead fungal biomasses have been used in research.Early research mainly focused on dead biomass,because the process is free from nutrient supply and its biosorption efficiency is not affected by dye toxicity[19].However,as biological adsorbents,one of the important characteristics of mycelial pellets is biological activity.In recent years,more and more researchers transfer the research emphasis to live biomasses or even growing biomass for colored wastewater treatments[20].So far,the main contents of these studies are influencing factors,kinetics and the isotherm of the adsorption[21],but few investigations were available on the mechanism of dye biosorption by mycelial pellets,biomass utilization rate and application feasibility.
In our previous work[12,22]a high-efficiency mycelial pellet formed by a marine-derived fungusPenicilliumjanthinellumP1 was used for dye adsorption from aqueous solutions.The mycelial pellet had excellent solid–liquid separation effect and the decolorization rate could still reach 91.3%after the 5 batches of re-utilization.The pellet had also been successfully applied to the treatment of paper mill wastewater combining with fungusPestalotiopsissp.J63,which possesses strong lignin degradation ability[23].
In this paper,another efficient mycelial pellet would be prepared using a novel marine-derived microorganismAspergillus nigerZJUBE-1 which has an excellent adsorption capacity for a variety of dyes.And an azo dye,namely Congo Red would be chosen as a model dye to investigate the decolorization efficiency byA.nigerZJUBE-1 pellets.This fungus has several outstanding advantages,including i)strong salt and acid tolerance;ii)high decolorization efficiency after multiple reutilization;iii)low cost,because ZJUBE-1 is an enzyme-producing strain for β-glucosidase.The objectives of the present work are i)to investigate the natural attributes of ZJUBE-1 mycelial pellets using micrography,SEM and FT-IR;ii)to examine decolorization performance including influencing factors and adsorption kinetics;iii)to enhance the effectiveness of re-utilization by adding nutrition supplement during the adsorption process or subculture the pellets before reuse;and iv)to study mechanisms behind the highly efficient batch decolorization and discuss the application feasibility.
2.Materials and Methods
2.1.Strain,media and dye
The marine-derivedA.nigerZJUBE-1 collected from the East China Sea was identified as a moderately halophilic fungus for producing cellulose[24].It is currently collected in the Center for Type Culture Collection of China(CCTCC),with a conservation number CCTC CM2010132.The culture media forA.nigerhad a formulation of:10 g glucose,2 g NH4Cl,2 g KH2PO4,0.5 g MgSO4and 2 g yeast extract in 1 L water(pH 5.5).The Potato Dextrose Agar(PDA)slant was used for regular maintenance of the fungus and harvest of the spores.All chemicals above were of analytical grade purchased from Sangon Biotech(Shanghai)Co.,Ltd.The azo dye Congo red was purchased from Aladdin Reagent(Shanghai)Co.,Ltd.
2.2.Preparation of mycelial pellets
Spore suspensions with concentration of approximately 107spores per milliliter were used and 1 ml spore suspension was inoculated into 100 ml culture media in 250 ml Erlenmeyer flasks.These inoculated flasks were stored in rotary shakers with a shaking speed of 160 r·min−1and temperature of 28°C.Mycelial pellets were harvested after cultivation of 48 h.
2.3.Decolorization experiments
The processes of decolorization were performed in 150 ml Erlenmeyer flasks.Each flask contained 50 ml Congo red solution(100 mg·L−1)and 4.0 g wet mycelial pellets and were kept in rotary shakers at 120 r·min−1,28 °C for 12 h.The supernatant was collected for dye concentration measurement with a UV spectrometer(UV-1800,Shimadzu,Japan)to calculate the dye decolorization rate(R).In the experiments of influencing factor study,different initial pH values were adjusted and different amounts of NaCl were added for each concentration.All experiments were performed in triplicate.
2.4.Adsorption kinetics
The initial dye concentrations of Congo red solution were chosen as 25,50,75,100,150,200 and 300 mg·L−1.These solutions(50 ml)were added to flasks,which were loaded with 4.0 g wet mycelial pellets and kept in shakers at120 r·min−1,28°C.The Congo red concentration was measured at 0,1,2,3,6,12 and 24 h.The unit of adsorbing capacity is mg·g−1.The experimental results were fitted with Langmuir models to calculate the maximum adsorption capacities.Pseudo-second-order model was also used to study the kinetics of dye adsorption.
2.5.Batch decolorization
In the control group,4.0 g of wet mycelial pellets was added into 50 ml Congo red solution(50 mg·L−1)in an Erlenmeyer flask(150 ml)and kept in a shaker at 120 r·min−1,28 °C.Dye concentration was measured after 12 h.The solution after biosorption was discharged while used mycelial pellets were retained.New Congo red solution(50 mg·L−1)was added into the flask for another decolorization batch.The experiment had a total of six batches.The whole experiment process was conducted in open condition without aseptic operation.
In the nutrition supplement(NS)group,a small amount of C and N sourcesi.e.0.025 g glucose and 0.005 g NH4Cl was added into the flask at the beginning of each batch to support the cell metabolism of mycelial pellets.All other operations were the same as the control group.
In the Re-culture(ReC)group,mycelial pellets after biosorption were transferred to a 4-fold diluted culture medium for cultivation for 12 h before their usage of the next decolorization batch.All other operations were the same as those of the control group.
2.6.Characterization
Mycelia pellets were lyophilized and the morphology was observed with a stereo microscope(Phenix-XTL,165-VT,China)and recorded with a digital camera(Cannon S5 IS).The lyophilized pellet sample was also coated with platinum and examined by scanning electron microscopy(SIRION-100,Philips,Netherlands).Fourier Transform Infrared(FT-IR)spectral analysis was performed by Nicolet 5700(USA).Pellets after adsorption were lyophilized and ripped open with dissecting needles to observe the distribution of dye.Hyphae after dye adsorption were avulsed from the surface of mycelial pellets and observed with an optical microscope(Nikon ECLIPSE E200,Japan).
3.Results and Discussion
3.1.Mycelial pellet characterization
Under given conditions,A.nigerZJUBE-1 can form white,rounded mycelial pellets with a mean diameter of 3 mm(Fig.1A)and its growth status is stable.The yield of mycelial pellets is approximately 360 g·L−1in wet mass and 7.2 g·L−1in dry mass.Fig.1B shows that the surface of pellets is hairy.This feature makes the pellet have a large surface area.The image of SEM(Fig.1C)shows that the pellet has a loose network structure which may be favorable for the mass transfer.The FT-IR analysis is conducted to analyze surface functional groups of mycelial pellets.The result(Fig.1D)shows several typical absorption peaks of fungal cell wall structures.For example,the broad peak from 3500 to 2900 cm−1is due to the overlapping stretching vibration of N--H and O--H groups[12].The peaks at 1634 cm−1and 1015 cm−1are related to the stretch vibrations of C=O bonds of amide groups and asymmetric vibration of C--O--C,respectively.These functional groups on the mycelial surface are quite important in processes of biosorption,as they provide binding sites for dye molecules and the specific binging interaction between biomass and dyes may determine the adsorption capacity[22].
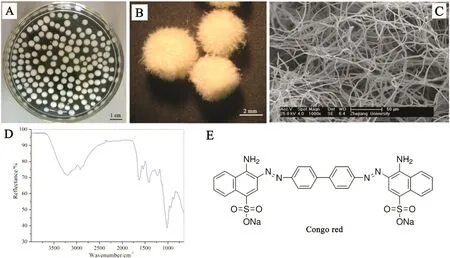
Fig.1.Characterization of mycelial pellets and the model dye.(A)Mycelial pellets in water showed by an optical picture;(B)lyophilized pellets observed with a stereo microscope;(C)internal structure of a pellet observed with SEM;(D)FT-IR spectra of mycelial biomass;(E)the molecular structure of the model dye Congo red.
3.2.In fl uencing factors of decolorization processes
3.2.1.Influences of the initial pH
The azo dye Congo red was chosen as a model dye to investigate influencing factors of decolorization efficiency.The molecular structure of Congo red was as shown in Fig.1E.The pH of solution affects the active sites on the biomass surface as well as properties of dyes.The pH of Congo red solution was adjusted from 2.0 to 10.0 in order to examine the influence of initial pH value.The results show that the biosorption capacity was quite steady among pH 2.0 to pH 5.0,and always staying above 98.7%(Fig.2A).This value was then declined gradually with the increase of pH,and dropped to 88.66%at pH 10.0.The Congo red biosorption capacity of another marine fungiP.janthinellumP1 declines[12]when the solution environment becomes acidic whileA.nigerZJUBE-1 still possesses decolorization rate of 98.7%at pH 2.0.This phenomenon may be due to ZJUBE-1 which is an acid-fast fungus and its mycelial activity,which has important influences on dye decolorization capacity,is kept at a relatively higher level in acid environment.
3.2.2.Effects of salt concentration
As a marine-derived fungus,A.nigerZJUBE-1 possesses moderately halophilic attributes[24].Fig.2B shows that the dye decolorization rate had a slight enhancement when NaCl concentration increases from 0 to 20 g·L−1.This may be because ZJUBE-1 is a marine-derived fungus and certain concentration of NaClis conducive to hold its normal physiological activities[12].When the NaCl concentration increased continuously,the high decolorization percentage is also maintained to 95.6%at 150 g·L−1.Normally,this salt concentration is unfavorable to survive for a majority of microorganisms.The decolorization percentage declined slightly to 90.6%and 87.6%even though NaCl concentration reached 200 and 300 g·L−1,respectively.In summary,ZJUBE-1 mycelial pellets were provided with a strong tolerance to high salt concentrations and had great potential in decoloring treatment of high salt waste waters.
3.3.Biosorption kinetics
The adsorption curve(Fig.3A)shows biosorption characteristics of ZJUBE-1 mycelial pellets at different dye concentrations.The equilibrium dye concentrations at 24 h are fitted by the Langmuir isotherm model and agreed well(Table 1).The linear form is given by Eq.(1):

whereqeis the equilibrium absorption capacity(mg·g−1),qmaxwas maximum adsorption capacity(mg·g−1),Ceis the equilibrium dye concentration(mg·L−1)andKis the Langmuir constant(L·mg−1).The value ofqmaxis calculated as 263.2 mg·g−1.
Pseudo-second-order model[25]is used to fit the biosorption data in order to research the mechanism of dye biosorption.The model is given by Eq.(2):

whereqeis the equilibrium absorption capacity(mg·g−1),qtis adsorption amount at time oft,k2is the rate constant of pseudo-second-order kinetic(g·mg−1·h−1).Fig.3B shows the fitting plot.The date is well fitted(R2>0.99)with Eq.(3).The calculation adsorbing capacities from the model(qcal)and the experimental ones(qexp)were compared in Table 2.Therefore,the biosorption behavior of Congo red by ZJUBE-1 mycelial pellets can be explained using this model,which indicated that dye biosorption efficiency was mainly restricted by the chemical adsorption process,rather than boundary layer resistance[25].The competitions for the active sites increase simultaneously with the dye concentration increases[26].

Fig.2.Influencing factors in decolorization process.(A)Effects of initial solution pH;(B)effects of NaCl concentrations.
3.4.Batch decolorization
3.4.1.Experimental design
The reusability is a very important indicator for the application of biosorbent,so batch decolorization studies need to be performed.We have found in the preliminary experiments that the decolorization rate of the second or later batches were dependent on the supply of nutrients in a certain degree.Therefore,three groups of experiments were designed to verify the mycelial pellets reuse efficiency and to investigate whether energy supply makes contributions to batch decolorization rate.The experimental operation diagram is shown in Fig.4.The control group had no nutrition addition.In nutrition supplement(NS)group,C and N sources are added to maintain normal fungal physiological metabolism.The addition amount was very littlei.e.glucose(0.5 g·L−1)and NH4Cl(0.1 g·L−1).In the Re-culture(ReC)group,mycelial pellets were cultivated again before the next batch of decolorization.
3.4.2.Batch decolorization efficiency
Fig.5 shows the batch decolorization results.The controlgroup has a high decolorization percentage(99.1%)only in the first batch.In subsequent 4 batches,the decolorization percentage dropped to 91.7%,89.8%,83.6%and 75.2%,respectively.In the sixth batch,the decolorization percentage was only 51.5%.Meanwhile,it was exciting that the decolorization rate of 6 batches of NS group and ReC group was maintained at a high level(R>98.5%)from beginning to end.Therefore,operations in NS group and ReC group are both feasible for efficient bath decolorization.
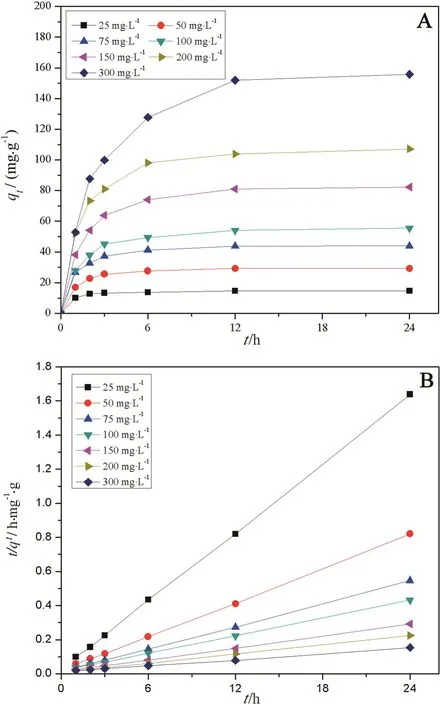
Fig.3.(A)The adsorption curve shows biosorption characteristics of ZJUBE-1 mycelial pellets at different Congo red concentrations.(B)The plot for fitting the model of pseudo-second-order kinetic.

Table 1Parameters in Langmuir isotherm model for Congo red adsorption by mycelial pellets of Aspergillus niger ZJUBE-1
3.4.3.Status of pellets after batch reuses
After 6 batches of decolorization,the mycelial pellets were collected and in-depth observations were conducted.Three groups showed major differences.Fig.6A reveals mycelial pellets along with the dye solution after the sixth decolorization batch.The control group just had very low decolorization efficiency at the sixth batch and the decolored solution still presented red obviously.Pellets of NS group were dark red and the decolored solution was clarified,indicating the high efficiency of decolorization.The solution of ReC group also presented was clarified,and its pellets showed apparently larger diameters than the other two groups.

Table 2Parameters in pseudo-second-order kinetic model for Congo red adsorption rate expressions by mycelial pellets
Fig.6B shows the distribution of dye inside single mycelial pellets.In order to obtain clear observation results,the pellets after adsorption were lyophilized and ripped open with dissecting needles.The whole pellet in control group presented red and the color of central mycelium was a little bit lighter than exterior ones.The outboard mycelium of the NS group pellet shows dark red,however,the innermycelium appeared clear white while the whole pellet still possessed high decolorization ability.This phenomenon indicates that the decolorization efficiency of pellets in NS group was only supported by their outboard mycelium.The inner mycelium of ReC group was also white.Interestingly,the outboard mycelium of pellets in this group presented white-and-red,which was similar to the growth ring.It is because that the mycelialpellet got secondary growth in the process of re-culture and was covered with a layer of fresh mycelium,which played a decisive role in the next decolorization batch.In NS and ReC groups,obviously,the Congo red could hardly be adsorbed by the inner biomass of mycelial pellets,although the clearance among mycelium seems to be large(Fig.1C).As inactivation mycelium also has considerable adsorption ability(data did not show),the inner mycelium in pellet could easily achieve adsorption process if the dye molecules permeated into the pellet interior,even in the case that nutrients and oxygen are both in short supply.Thus,this phenomenon is basically because of the mass transfer resistance to dye molecules caused by the structure of mycelial pellets.In the control group,the Congo red concentration in the solution was always maintained at a higher level,which made the dye molecules permeate to the inner pellet and the inner biomass was utilized.Nonetheless,the decolorization rate in this case was in a low value which could not meet the needs of wastewater treatment.

Fig.5.The batch decolorization rates of Congo red by mycelial pellets in three groups.

Fig.4.The experimental operation diagram for batch decolorization in three groups.
Fig.6C shows mycelium after dye adsorption,which are avulsed from the surface of pellets.It is obvious that Congo red not only colors the cell wall,but penetrates through it and moves into hyphal cytoplasm.A similar result was obtained by usingP.janthinellumpellet[12].Mycelium from control group and NS group are deep red,indicating that they have absorbed a mass of dye molecules.Mycelium from ReC group presented light erred.This is due to fresh mycelium produced by the re-culture process which was enough to deal with the next batch and their adsorption for Congo red was not saturated.

Fig.6.Status of mycelialpellets after batch reuses.(A)Pellets along with the dye solution after the sixth decolorization batch;(B)the distribution ofdye inside a single pellet observed with a stereo microscope;(C)mycelium avulsed from the surface of pellets observed with an optical microscope.
3.4.4.Mass variation of mycelial pellets
In addition to dye distributions,the wet mass of mycelial pellets,as shown in Fig.7,also had a big difference among three groups.Comparing to the initial amount(4.0 g),the pellet biomass in control group(2.2 g)had a significant reduction,which might be because of the mycelial autolysis caused by combined action of dye toxicity and nutrition deficiency.The wet mass of NS group mycelial pellets rose to 5.3 g,demonstrating that mycelium still possessed growth ability during dye adsorption process with the support of a certain amount of nutrition.The wet mass of pellets in ReC group(8.8 g)was more than doubled over the initial amount,indicating that pellets after adsorption could still obtain good state of growth in an appropriate environment.
3.4.5.Spectral analysis of UV–Vis
UV–Vis spectra results of decolorization solutions before and after the sixth batch are as shown in Fig.8.The spectra of untreated solution had a main peak at488 nm and two other peaks at 236 nm and 336 nm,respectively.After having treated by pellets of control group,the absorbance declined by half and no new peak emerged.In the NS group,the original three peaks all vanished and there appeared a new peak at 270 nm.The change is similar in the ReC group,and the absorbance of new peak was even higher.The decolorization process achieved by living biomass may contain both biodegradation and biosorption[27].The appearance of new peak proves that biodegradation happens together with biosorption process.However,the biodegradation process only made a small contribution to the decolorization of NS group because used pellets were still in dark red(Fig.6A).
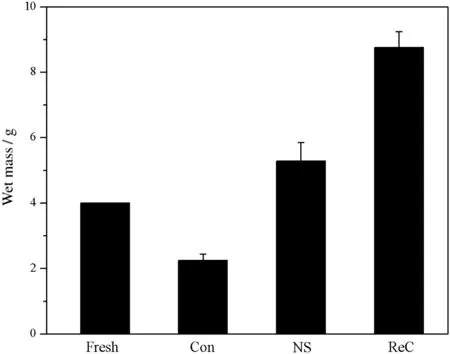
Fig.7.Wet mass of mycelial pellets after 6 batch reuses.
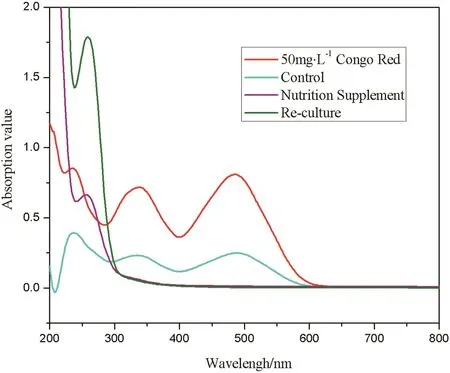
Fig.8.UV–Vis spectra of the decolorization solutions before and after the sixth batch.
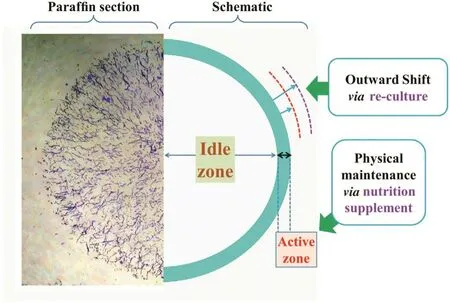
Fig.9.Visualization of decolorization active zone in the section of a mycelialpellet.Paraf fin section is used to show the cross section of the ZJUBE-1 mycelialpellet.The active zone can be enhanced by nutrition supplement or be shifted outward by a re-culture process.
3.5.Decolorization mechanisms
The decolorization mechanisms to Congo red byA.nigerZJUBE-1 mycelial pellets contain both biodegradation and biosorption.In accordance with the relevance to mycelial metabolisms,the mechanisms of fungal biosorption could be classified into two kinds:i)passive reaction,which is non-metabolism dependent;and ii)active uptake,which is metabolism dependent and comprises an energy driven process[28].Nutrition supplement could maintain normal physiological metabolism of mycelium,which would constantly update the micro environment around mycelium.And this micro environment can influence a variety of passive reactions,such as(i)physical adsorption,(ii)ion exchange,(iii)complexation and(iv)precipitation[29],which are the main ways of biosorption.Meanwhile,carbon source could ensure the energy support of active transport,which makes possible the maximization of biosorption.Nutrition supplement could also maintain the enzyme production,which is a critical factor of biodegradation.In conclusion,through multiple avenues,nutrition supplement could maintain the efficiency of biological decolorization of Congo red by ZJUBE-1 mycelial pellets.Because of the mass transfer restrictions caused by mycelial pellet structure,only the outer region can be effectively supplied with oxygen and nutrients[30],Therefore,the efficient biosorption,which depends on the nutrition supply,only happens in the outside active zone of the mycelial pellet.This situation is similar in the fermentation process[31].
Accordingly,we put forward the concept of decolorization active zone in a schematic diagram as shown in Fig.9.Paraffin section is used to show the cross section of the ZJUBE-1 mycelial pellet.Due to the restrictions of mass transfer,efficient biodegradation and biosorption processes are conducted merely in the active zone of the pellet,which accounts for only a small part of the total volume.The rest areas are all idle.The comparative results between control group and NS group(Fig.5)indicates that the maintenance of decolorization rate by nutrition is necessary and effective.The efficiency of the first batch in Control group is satisfactory and the dye distribution also concentrated upon the outer mycelium,as the fresh mycelium of active zone initially possesses certain amounts of nutrients.The adsorptive situation of ReC group in each batch were similar as the first batch of Control group,because the reculture process made the pellet grow again and shift the active zone outward.Then the active zone in the last batch was covered and turned into part of idle zone.
There is still a problem,however,the biosorption behavior of Congo red fits well with the pseudo-second-order kinetic model(Table 2),which considers the rate-determining step as chemical adsorption,and it does not agree with the concept in Fig.9.That may be because the biological decolorization process is not only achieved by the combination of dye molecules and binding sites on mycelium,but also participated by mycelial physiological activities,which contain active transport and even biodegradation,making the whole process complicated.Other model fitting,such as Langmuir isotherm(Table 1),could also be helpful to the understanding of the adsorption process and calculate the maximum adsorption capacity.But this value does not mean as much,because the internal mycelium is often unable to use for adsorption.When mycelium in idle zone also starts the adsorption process,the decolorization rate tends not to meet the needs of wastewater treatment.
Pragmatically,of course,it may not be necessary to make clear what mechanism is running if the prime research goal is to obtain an efficient decolorization system[28].
3.6.Application feasibility and project for ful fillment
Low cost and easy operation are core issues of wastewater treatment.General advantages of mycelium pellets are the good solid–liquid separation property and high efficiency absorption to certain pollutant,such as dyes,heavy metals and phenolic substance[32,33].The special advantage ofA.nigerZJUBE-1 mycelial pellets is the good salt tolerance,which is helpful to the treatment of high salinity wastewater and does not require additional desalination processing.Its tolerance to acid environment is also considerable.In addition,because ZJUBE-1 is enzyme-producing and the utilization of the biomass has double benefitsi.e.effluent treatment and fermentation byproduct management.In batch processes,nutrition supplementis necessary for efficient decolorization.The additive amount need to be further optimized to avoid over-nutrition,which may lead to an increase of COD value.The medium used in re-culture experiments consists of glucose,inorganic salt and yeast extraction,which are not cost-effective.Marine-derivedA.nigerZJUBE-1 could use a variety of agriculture byproducts as C,N sources,includingEichhornia crassipes,which is a disaster for water pollution[34].Thus,there is a lot of room for the lower cost of reculture process.In our previous work,the mycelial pellet combined two fungi[23],P.janthinellumP1 andPestalotiopsissp.J63,was successfully applied to the treatment of paper mill wastewater,henceA.nigerZJUBE-1 could also be researched for some combination to exercise more functions in wastewater treatment.
4.Conclusions
Mycelial pellets formed by marine-derivedA.nigerZJUBE-1 holds great promise in efficient batch decolorization of Congo red from aqueous solution.The pellets have strong salt and acid tolerances in biosorption process.Batch decolorization study shows that mycelial pellets could possess the efficient and continuous decolorization ability with the supplement of certain amounts of C,N sources or through a reculture process between every two batches.UV–Vis spectral result indicates that the decolorization process may both contain biodegradation and biosorption.Mechanism analysis indicates that efficient decolorization relies on the active zone on the surface of the pellet,which can be enhanced by nutrition supplement or be shifted outward by a re-culture process.The structure of pellet could be renovated by altering culture conditions to increase the biomass utilization rate in decolorization processes.
[1]A.W.M.Ip,J.P.Barford,G.Mckay,A comparative study on the kinetics and mechanisms of removal of Reactive Black 5 by adsorption onto activated carbons and bone char,Chem.Eng.J.157(2–3)(2010)434–442.
[2]A.R.Dincer,Y.Gunes,N.Karakaya,E.Gunes,Comparison of activated carbon and bottom ash removal of reactive dye from aqueous solution,Bioresour.Technol.98(4)(2007)834–839.
[3]T.Robinson,G.McMullan,R.Marchant,P.Nigam,Remediation of dyes in textile ef fluent:A critical review on current treatment technologies with a proposed alternative,Bioresour.Technol.77(3)(2001)247–255.
[4]M.T.Yagub,T.K.Sen,S.Afroze,H.M.Ang,Dye and its removal from aqueous solution by adsorption:A review,Adv.Colloid Interface Sci.209(2014)172–184.
[5]S.T.Liu,H.L.Song,S.Z.Wei,Q.J.Liu,X.N.Li,X.W.Qian,Effect of direct electrical stimulation on decolorization and degradation of azo dye reactive brilliant red X-3B in bio film-electrode reactors,Biochem.Eng.J.93(2015)294–302.
[6]X.J.Xiong,X.J.Meng,T.L.Zheng,Biosorption of CI Direct Blue 199 from aqueous solution by nonviableAspergillus niger,J.Hazard.Mater.175(1–3)(2010)241–246.
[7]S.Dawood,T.K.Sen,Removal of anionic dye Congo red from aqueous solution by raw pine and acid-treated pine cone powder as adsorbent:Equilibrium,thermodynamic,kinetics,mechanism and process design,Water Res.46(6)(2012)1933–1946.
[8]S.A.Figueiredo,J.M.Loureiro,R.A.Boaventura,Natural waste materials containing chitin as adsorbents for textile dyestuffs:Batch and continuous studies,Water Res.39(17)(2005)4142–4152.
[9]V.K.Gupta,Suhas,application of low-cost adsorbents for dye removal—A review,J.Environ.Manag.90(8)(2009)2313–2342.
[10]N.Sivarajasekar,R.Baskar,Biosorption of basic violet 10 onto activatedGossypium hirsutumseeds:Batch and fixed-bed column studies,Chin.J.Chem.Eng.23(10)(2015)1610–1619.
[11]Y.Zhou,Z.L.Dai,D.Zhai,C.J.Gao,Surface modification of polypiperazine-amide membrane by self-assembled method for dye wastewater treatment,Chin.J.Chem.Eng.23(6)(2015)912–918.
[12]M.X.Wang,Q.L.Zhang,S.J.Yao,A novel biosorbent formed of marine-derivedPenicillium janthinellummycelial pellets for removing dyes from dye-containing wastewater,Chem.Eng.J.259(2015)837–844.
[13]T.Wucherpfennig,K.A.Kiep,H.Driouch,C.Wittmann,R.Krull,Morphology and rheology in filamentous cultivations,Adv.Appl.Microbiol.72(2010)89–136.
[14]R.Krull,C.Cordes,H.Horn,I.Kampen,A.Kwade,T.R.Neu,B.Nortemann,Morphology of filamentous fungi:Linking cellular biology to process engineering usingAspergillus niger,Adv.Biochem.Eng.Biotechnol.121(2010)1–21.
[15]M.Papagianni,Fungal morphology and metabolite production in submerged mycelial processes,Biotechnol.Adv.22(3)(2004)189–259.
[16]R.Krull,T.Wucherpfennig,M.E.Esfandabadi,R.Walisko,G.Melzer,D.C.Hempel,I.Kampen,A.Kwade,C.Wittmann,Characterization and control of fungal morphology for improved production performance in biotechnology,J.Biotechnol.163(2)(2013)112–123.
[17]E.Borras,P.Blanquez,M.Sarra,G.Caminal,T.Vicent,Trametes versicolor pellets production:Low-cost medium and scale-up,Biochem.Eng.J.42(1)(2008)61–66.
[18]P.Sharma,H.Kaur,M.Sharma,V.Sahore,A review on applicability of naturally available adsorbents for the removal of hazardous dyes from aqueous waste,Environ.Monit.Assess.183(1–4)(2011)151–195.
[19]S.Rangabhashiyam,E.Suganya,N.Selvaraju,L.A.Varghese,Significance of exploiting non-living biomaterials for the biosorption of wastewater pollutants,World J.Microbiol.Biotechnol.30(6)(2014)1669–1689.
[20]B.P.Xin,G.Chen,W.C.Zheng,Bioaccumulation of Cu-complex reactive dye by growing pellets ofPenicillium oxalicumand its mechanism,Water Res.44(12)(2010)3565–3572.
[21]X.L.Ren,L.Yang,M.Liu,Kinetic and thermodynamic studies of acid scarlet 3R adsorption onto low-cost adsorbent developed from sludge and straw,Chin.J.Chem.Eng.22(2)(2014)208–213.
[22]Q.L.Zhang,T.Lu,D.M.Bai,D.Q.Lin,S.J.Yao,Self-immobilization of a magnetic biosorbent and magnetic induction heated dye adsorption processes,Chem.Eng.J.284(2016)972–978.
[23]H.Y.Chen,Y.X.Guan,S.J.Yao,A novel two-species whole-cell immobilization system composed of marine-derived fungi and its application in wastewater treatment,J.Chem.Technol.Biotechnol.89(11)(2014)1733–1740.
[24]D.S.Xue,H.Y.Chen,Y.R.Ren,S.J.Yao,Enhancing the activity and thermostability of thermostable beta-glucosidase from a marineAspergillus nigerat high salinity,Process Biochem.47(4)(2012)606–611.
[25]Y.S.Ho,G.McKay,Pseudo-second order model for sorption processes,Process Biochem.34(5)(1999)451–465.
[26]P.S.Kumar,S.Ramalingam,C.Senthamarai,M.Niranjanaa,P.Vijayalakshmi,S.Sivanesan,Adsorption of dye from aqueous solution by cashew nut shell:Studies on equilibrium isotherm,kinetics and thermodynamics of interactions,Desalination261(1–2)(2010)52–60.
[27]A.R.Khataee,G.Dehghan,A.Ebadi,M.Zarei,M.Pourhassan,Biological treatment of a dye solution by macroalgaeCharasp.:Effect of operational parameters,intermediates identification and artificial neural network modeling,Bioresour.Technol.101(7)(2010)2252–2258.
[28]G.M.Gadd,Biosorption:Critical review of scientific rationale,environmental importance and significance for pollution treatment,J.Chem.Technol.Biotechnol.84(1)(2009)13–28.
[29]G.M.Gadd,C.White,Microbial treatment of metal pollution—A working biotechnology,Trends Biotechnol.11(8)(1993)353–359.
[30]E.J.Espinosa-Ortiz,E.R.Rene,K.Pakshirajan,E.D.van Hullebusch,P.N.L.Lens,Fungal pelleted reactors in wastewater treatment:Applications and perspectives,Chem.Eng.J.283(2016)553–571.
[31]H.Driouch,B.Sommer,C.Wittmann,Morphology engineering ofAspergillus nigerfor improved enzyme production,Biotechnol.Bioeng.105(6)(2010)1058–1068.
[32]Z.Aksu,Application of biosorption for the removal of organic pollutants:A review,Process Biochem.40(3–4)(2005)997–1026.
[33]Y.Sag,Biosorption of heavy metals by fungal biomass and modeling of fungal biosorption:A review,Sep.Purif.Methods30(1)(2001)1–48.
[34]D.S.Xue,H.Y.Chen,D.Q.Lin,Y.X.Guan,S.J.Yao,Optimization of a natural medium for cellulase by a marineAspergillus nigerusing response surface methodology,Appl.Biochem.Biotechnol.167(7)(2012)1963–1972.
 Chinese Journal of Chemical Engineering2017年3期
Chinese Journal of Chemical Engineering2017年3期
- Chinese Journal of Chemical Engineering的其它文章
- A novel green inhibitor for C-steel corrosion in 2.0 mol·L−1 hydrochloric acid solution
- An ecofriendly approach for corrosion control of 6061 Al-15%(v)SiC(P)composite and its base alloy
- Polymorphism of D-mannitol:Crystal structure and the crystal growth mechanism☆
- Bulk and bubble-scale experimental studies of influence of nanoparticles on foam stability
- Simulation and optimization of an industrial gas condensate stabilization unit to modify LPGand NGL production with minimizing CO2 emission to the environment
- Characterization of pyrolytic lignins with different activities obtained from bio-oil☆
