Research progress in the SO2 resistance of the catalysts for selective catalytic reduction of NO x☆
Minhua Zhang,Baojuan Huang,Haoxi Jiang,Yifei Chen*
Key Laboratory for Green Chemical Technology of Ministry of Education,R&D Center for Petrochemical Technology,Tianjin University,Tianjin 300072,China Collaborative Innovation Center of Chemical Science and Engineering,Tianjin 300072,China
1.Introduction
With the growing environmental awareness,the control and prevention of air pollution have attracted extensive attentions in the world.Numerous countries have released stringent regulations to minimize NOxemissions during the past years.As a major atmospheric pollutant,most NOx(~95%)derives from transportation(49%)and power plants(46%)[1].To date,many methods have been used to reduce the emission of NOx,among which selective catalytic reduction(SCR)of NOxstands out as one of the most mature and widely applied post combustion abatement technologies[2].SCR is a process using the reduct ant such as ammonia(NH3)to react with NOxto produce nitrogen and water in the presence of oxygen[3],and in which technology the suitable catalyst plays an important role.V2O5/TiO2catalyst with WO3or MoO3as promoter is a typical and efficient catalyst for SCR process and has already been commercialized[1,2,4].However,these catalysts possess many disadvantages,one of which is SO2oxidization to SO3.In real operating conditions,SO2,excess O2,and vapor are typical impurities present both in the flue gas of power plants and the exhaust gas of vehicles.Among these species,SO2usually plays a detrimental role on the performance of deNOxcatalysts and significantly limits their industrial applications.Accordingly,numerous studies have been conducted in the past few years to develop SO2-tolerant or resistant catalysts.
This review article highlights the recent progress in SO2-tolerant or resistant catalysts of SCR.The paper mainly focuses on the sulfur poisoning mechanism and strategies to reduce sulfur poisoning.In addition,some novel catalysts with good SO2resistance are also mentioned.This review is hoped to guide the design of proper catalysts for SCR with enhanced SO2-tolerance.
2.The Sulfur Poisoning Mechanism
2.1.Metal oxide catalysts
In recent years,Mn-based catalysts have been extensively investigated due to their excellent low-temperature activity for the NH3-SCR reaction.However,Mn-based catalysts are usually prone to deactivation by SO2present in flue gas.Fe-Mn based transition metal oxides are highly active for the low-temperature NH3-SCR reaction[4].However,the NO conversion on the catalyst decreased significantly to 8%-23%when 1000 ppm SO2was added to the feed gas.Mn-Zr mixed oxide catalysts could be poisoned by SO2irreversibly[5].NO conversion over the V2O5-WO3/CeO2-TiO2catalyst also decreased in the presence of 100 ppm SO2over the whole temperature window[6].
In general,about 0.05%-0.3%SO2that exists in the stack gases could have a serious poisoning effect on SCR catalytic activity[7].From a practical point of view,it is very important to understand the effect of SO2on the activity and structure of SCR catalysts,and finally elucidate the deactivation mechanisms for the sake of industrial applications.Many investigations have been carried out to clarify the deactivation mechanisms on different SCR catalysts.
Based on different catalytic systems,various deactivation mechanisms have been reported and few consensuses have been reached.Hence,the discussions of the deactivation mechanisms for specific deNOxcatalysts are of great significance.Pan et al.[7]studied the effect of SO2on the performance of manganese oxides supported on multi walled carbon nanotubes(MnOx/MWCNTs)for low-temperature NH3-SCR,and found that SO2led to the irreversible deactivation of the catalyst.Activity test and characterization results indicated that the main cause of catalyst deactivation was the sulfation of the active center atoms.The formation and deposition of ammonium sulfates which clogged the pore channels and the competitive adsorption between NO and SO2partially contribute to the deactivation of the catalyst.Yamamoto et al.[8]investigated the effect of SO2gas on the activity of the photo-assisted selective catalytic reduction of NO with ammonia over a TiO2photo catalyst.It revealed that the deactivation of the catalyst was due to the deposition of the ammonium sulfate species which reduced the specific surface area and changed the pore structure.Based on the designed TPDC(temperature programmed decomposition)and TPSR(temperature-programmed surface reaction)experiments,Ma et al.[9]proposed a sulfur poisoning mechanism on V-Ti based catalysts which wasillustrated in Fig.1.SO2had little in fluence on the catalytic activities of VTi and VWTicatalysts,which was due to the fact that TiO2can act as a sacrificial agent to protect Vn+sites from sulfation and W modification inhibited sulfation of TiO2.However,compared with TiO2,CeO2was preferential to be sulfated to form sulfates,which could disrupt the redox cycle between Ce3+and Ce4+and break the V-O-Ce bridges.Moreover,the generation of SO3was greatly promoted on CeO2-modified catalyst.All of these caused the sulfation of ceria and the deactivation of V1CeWTi.Li et al.[10]systematically studied the roles of SO2in the NH3-SCR of NO over different V2O5/AC(activated carbon)catalysts and suggested that a tiny amount of VOSO4produced from SO2and V2O5might result in a sharp decrease of NO conversion.Pore blockage was proved to have little influence on the SCR activity of V2O5/AC at temperatures of 200°C and higher.
As a potential candidate for SCR of NOxwith NH3in the medium temperature range,iron titanate catalyst(FeTiOx)has been studied by many researchers.Liu et al.[11]investigated the influence of SO2on the activity of the catalyst and found out that NOxadsorption capability on FeTiOxcatalyst was strongly inhibited by the sulfation process.The active nitrate species could not continuously form on sulfated catalyst surface.This led to the loss of the activity at low temperatures.Mn substitution could obviously enhance the SCR activity of FeTiOxcatalyst at low temperatures[12].However,it also resulted in the reduced SO2-resistant ability.As shown in Fig.2(in red),the SCR reaction pathway was cut off because the formation of surface nitrate species was intensely and irreversibly inhibited by the formation of sulfate species[12].
In general,the sulfur poisoning mechanisms could be different for different metal oxide catalytic systems.The main causes are as follows:sulfation of the active center atoms,formation and deposition of ammonium sulfates on the surface of catalysts clogging the pore channels,and the competitive adsorption between SO2and NOx.
2.2.Zeolite-based catalysts
Zeolite-based catalysts emerged as one of the most important SCR catalysts in recent years due to a number of beneficial characteristics such as their unparalleled hydrothermal stability and high adaptability to high space velocity[13-16].Sulfur tolerance of metal-exchanged zeolite catalysts has also attracted much attention[17,18].Sulfur poisoning of the Cu-zeolite catalyst can occur via different mechanisms with different forms of feed sulfur and different temperature ranges[19].As shown in Fig.3,at lower temperatures,sulfur poisoning was mainly due to the indiscriminate adsorption of SOx(SO2and SO3)species on Cu sites.At higher temperatures,SO3was revealed to have a more significant impact on the activity of the catalyst since the poisoning was irreversible.An investigation into the impact of sulfur oxides on the activity of Cu-SAPO-34 had been conducted by Wijayanti and coworkers[20]to clarify the deactivation mechanism induced by sulfur.The H2-TPR(temperature-programmed desorption)data(Fig.4)confirmed that sulfates or other surface SO2groups were formed during sulfur treatment and there were less available copper sites that could undergo the redox cycle for the sulfated sample compared to the fresh sample.This indicated that pore blockage could be the main reason for the deactivation.Fe-ZSM-5 was also reported to be sensitive to SO2presence[21].Ma and coworkers proved that the competitive adsorption between SO2and NOxduring the SCR reaction led to the decrease NOxconversion on nanosheet Fe-ZSM-5 catalysts[22].

Fig.1.The SO2 deactivation mechanism over different catalysts.(a)V1Ti;(b)V(x)WTi;(c)V1CeWTi[9].

Fig.2.Proposed mechanism of the NH3-SCR reaction over Mn substituted iron titanate catalyst at low temperatures and the influence of SO2 on the reaction pathway(as shown in red)[12].

Fig.3.Sulfur poisoning mechanisms of Cu-zeolite catalysts with different forms of feed sulfur and different temperature ranges.
3.Strategies to Reduce SO2 Poisoning and the Corresponding Mechanisms
The SO2resistance capability of the catalysts in the SCR process is the key factor for its industrialization.Here we summarize several strategies used to reduce the SO2poisoning as well as the corresponding mechanisms.
3.1.Metal modification
Modifying catalysts with a tertiary metal is a common method to solve the problem of sulfur poisoning.Transition metals such as Ce,Sn,Fe,W,Mo,and Co have been used as modifiers with the aim of preparing catalysts with good resistance to SO2.

Fig.4.H2-TPR of fresh and sulfated crushed monolith of Cu-SAPO-34[20].
Cerium is a common rare earth metal and has been discussed vastly due to its wide applications in catalysis.As an oxygen reservoir,cerium can store and release oxygen via the redox cycle shifted between Ce4+and Ce3+.CeO2showed the excellent SCR activity in the presence of SO2at 300-500°C[23].It was proposed that sulfation of CeO2could promote SCR activity,because the formation of sulfated CeO2increased the adsorption of NH3and inhibited the catalytic oxidization of NH3to NO at the same time[23].Xiao et al.[24]studied the effect of SO2on NO reduction over Ce/TiO2by steady state kinetic study.Similarly,SO2showed a promotion on NO reduction over Ce/TiO2at higher temperatures.The mechanism was similar to that mentioned above.
Cerium oxide doping can obviously inhibit the SO2-poisoning of the active manganese oxide species,which is widely believed to be one of the major reasons for the deactivation of manganese-based catalysts[25].In the presence of SO2,ammonium salts usually formed and decreased SCR activities through pore blocking.Wang and coworkers[26]used activated carbon honey comb support MnOx(Mn/ACH)and CeO2-MnOx(CeMn/ACH)as catalysts for NH3-SCR.Compared with Mn/ACH,CeMn/ACH catalyst exhibited better resistance to SO2poisoning.The addition of CeO2inhibited the formation of ammonium sulfates,thus improving the SO2resistance performance.MnOxsupported palygorskite(MnOx/PG)catalyst doped with CeO2was found to improve its SO2resistance by enhancing the stability of the active species via the reduction of manganese sulfate[27].Wang and coworkers[28]also found that the SO2resistance could be greatly enhanced for Ce modified MnOx.The reason was that the formation of Mn(SO4)xwas prevented and the deposition of(NH4)2SO4and NH4HSO4was significantly inhibited by the doping of Ce.The mechanism could also apply to Ce modified Mn/TiO2catalyst for low-temperature NH3-SCR in the presence of SO2[29,30].The addition of Ce could improve the SO2resistance for Sb-V2O5/TiO2catalyst[31,32].Kwon and coworkers[32]investigated the influence of Ce on the catalytic activity of V/Sb/Ce/Ti in the presence of SO2.They proposed that Ce addition to V/Sb/Ti could suppress the formation of NH4HSO4due to the formation of Ce2(SO4)3,and thus show high resistance to SO2.Furthermore,Ce modification could enhance the SO2resistance of V-based catalyst[33].
Ce modification was also found to be an effective method to enhance the SO2resistance of zeolite-based catalysts.Modifying Cu/ZSM-5 catalyst with Ce greatly improved the SO2resistance since it could inhibit the deposition of CuSO4[34].A similar result was also obtained by Lai et al.[35]They revealed that the presence of Ce in the Cu/ZSM-5 catalyst facilitated SO2deposition on the Ce site to form a stable Ce sulfate,thus inhibiting the formation of ammonia sulfate and copper sulfate on the surface in the SCR process,and finally improved the sulfur tolerance of the Ce-Cu/ZSM-5 catalyst.Ce doping Fe/β (β is zeolite with SiO2/Al2O3=40)catalyst was reported to possess outstanding SO2antipoisoning ability,due to that the addition of Ce can reduce the formation of inactive iron sulfate species and then improve SO2resistance[36].
Numerous studies have been conducted to investigate Fe-doped catalysts for NH3-SCR.According to literatures,Fe doping can significantly enhanced the resistance of SO2of the Ce-based[37-39],Mnbased[1,40,41]and Cu-based[42]catalysts.As shown in Fig.5,SO2resistance of Ce/TiO2was significantly enhanced by Fe doping[38].The three dimensional ordered macroporous(3DOM)Ce0.75Zr0.2M0.05O2-δ(M=Fe,Co,Mn,Cu)were synthesized as efficient catalysts for SCR of NOxwith NH3.The SO2tolerance test of the catalysts showed that Ce0.75Zr0.2Fe0.05O2exhibited the best sulfur resistance performance[39].Gao et al.[43]prepared a V2O5-WO3/Fe2O3/TiO2catalyst showed better SO2-resistant ability compared with V2O5-WO3/TiO2and could recovered its activity after cutting off SO2.The activity test results indicated that the SO2resistance of the catalyst was enhanced by the combination effect of V2O5-WO3/TiO2and iron oxide.Teng and coworkers'[44]study demonstrated Fe-doped Ce-Mn/TiO2-ZrO2significantly decreased the formation rate of sulfates and thus enhanced the SO2resistance.Fe-doping also greatly enhanced SO2resistance of Mn-Ce/TiO2catalyst[45].Fang et al.[42]fabricated a monolith deNOxcatalyst based on 3D hierarchical foam-like Fe2O3@CuOx.Their results indicated that the high SO2-tolerance was due to the fact that Fe2O3prevented the generation of both ammonium sulfates and copper sulfates.

Fig.5.NO x conversion of Fe(0.2)-Ce/TiO2 and Ce/TiO2 catalysts in the presence of SO2 at 250°C at two different GHSVs(30000 or 60000 h-1).Reaction conditions:[NO]=[NH3]=1000 ppm,[O2]=3 vol%,[SO2]=500 ppm[38].
Sn also has been used as a modifier to prepare catalysts exhibiting remarkable resistance to SO2poisoning[46-52].Chang et al.[47]revealed that Sn-modified MnOx-CeO2showed remarkably improved SO2tolerance.Sn modification could also reduce the irreversible poisoning effect of sulfation on MnOx-CeO2and Cr-MnOxcatalysts[48,49].
W doping could improve the SO2-tolerance capability which was verified by many studies[53-55].W supported on Ce0.65Zr0.35O2was reported to act as an efficient catalyst for NH3-SCR and showed good SO2tolerance capability[56].A series of WOx-MnOx-CeO2catalysts also exhibited relatively high resistance to SO2poisoning[57].The mechanism of the promoting effect of W addition on the SO2-resistance was proposed.After WO3doping,the SO2oxidation activity of the catalyst was significantly suppressed.The decreased generation of SO3inhibited the formation of ammonium and/or metal sulfates on the acidic catalyst surface,which suggested that Ce and/or Mn sites were less affected by SO2.Therefore,high SO2resistance was achieved on these catalysts.
V modification of SCR catalysts was also investigated[58,59].Yang[60]developed an Fe/AC(activated carbon)catalyst modified by a small amount of V2O5,which achieved good SO2durability at low temperatures.It was found that the vanadium additive promoted the formation of sulfate species,which increased the surface acidity,and hence increased the SO2-tolerance.
According to literatures,TiO2essentially reacts with neither SO2nor SO3above 200°C.Therefore,Ti was also used as a modifier for catalysts to improve the sulfur-poisoning resistance[61].By X-ray photoelectron spectroscopy(XPS)analysis,Xiong et al.[62]verified that the loss of surface Mn species on MnOx/CeO2catalyst was inhibited by doping of Ti,which contributed to improved sulfur durability.
Zhao et al.[63]used different transition metals including Cu,Fe,Mn,and Co to modify V2O5-based catalysts supported on TiO2.The results showed that the Cu modified catalyst Cu-V/TiO2exhibited high activity and N2selectivity as well as outstanding SO2/H2O durability.Ce-Cu-Ti complex oxide catalyst also demonstrated higher SO2-resist ent ability than Ce-Ti oxide catalyst[64].The mechanism of the promoting effect of Cu addition on the SO2-resistance of Ce-Ti oxide catalyst was shown in Fig.6[65].The addition of Cu could prevent Ce4+reduction to Ce3+because of the preferable formation of CuSO4.In addition,SO2adsorption increased the acidity of the catalyst surface.Thus,the preservation of reducibility of Ce4+and the enhanced acidity resulted in the high SO2-resistance of Ce-Cu-Ti oxide.

Fig.6.Graphical description for the effect of Cu addition on the SO2-resistance of a Ce-Ti oxide catalyst[65].

Table 1 Metal modification of deNO x catalysts

Table 1(continued)
There are also studies which introduce Co[66-68],Mo[69,70],Nb[71],and Zr[72-74]as modifiers for active component or support to enhance the SO2resistance of the NH3-SCR catalysts.The metal modification of the deNOxcatalysts in recent researches is summarized in Table 1.
3.2.Structure and morphology control
It has been widely accepted that the structure and morphology of the SCR catalysts have a significant influence on the activity as well as the SO2-resistance of the catalysts[75].The specific morphology was found to be beneficial for SO2-resistance.Shiand coworkers[76]synthesized a hierarchically macro-mesoporous Mn/TiO2(HM-Mn/TiO2)catalyst by sol-gel method and used it for NH3-SCR of NOx.Compared with Mn/TiO2,HM-Mn/TiO2exhibited higher low-temperature activity and better SO2-resistance(Fig.7).The construction of hierarchical transition-metal vanadate nanosheets could also improve the SO2resistance[77].The 3D(three dimensional) flower-like NiMnFe mixed oxides showed great SO2resistant ability[78].The deNOxcatalysts based on hollow porous MnxCo3-xO4nanocages thermally derived from nanocube-like metal-organic frameworks showed improved SO2resistance as compared with the traditionalMnxCo3-xO4nanoparticles[79].

Fig.7.Influence of SO2 on NO x conversion over HM-Mn/TiO2 and Mn/TiO2 at 120°C.Reaction conditions:[NO]=[NH3]=1000 ppm,[O2]=3 vol%,[SO2]=30 ppm,N2 balance,GHSV=30000 h-1[76].
According to the sulfur poisoning mechanisms mentioned above,the reaction of SO2with metal oxides is a main issue for SO2-induced deactivation.After that,the thermodynamically stable sulfate phases were formed and deposited on the catalysts surface,leading to the reduction in the number of chem is or ption sites for NOx.From this aspect,weakening the interaction between SO2and the active component seems to be an effective way to extend the SO2tolerance of the SCR catalysts.
With this in mind,Cai et al.[80]synthesized a multi-shell Fe2O3@MnOx@CNTs catalyst to solve the problem that MnOxcan be easily deactivated by SO2in the flue gas,which has significantly hindered the application of Mn-based catalysts.The activity test results showed that SO2resistance was greatly enhanced due to the additional Fe2O3shell.As shown in Fig.8,the XRD pattern and TG curves indicated that less sulfate species were formed on the surface of the Fe2O3@MnOx@CNTs catalyst,which provided evidence of the antisulfation ability of Fe2O3shell.The results revealed that the overcoated Fe2O3could serve as a protective layer to restrain the formation of manganese sulfate,leading to the desirable SO2resistance of the multi-shell catalyst.MoFe/Beta@CeO2core-shell catalyst using CeO2thin film as the shell could not only suppress the formation of sulfate species and ammonium nitrate but also restrain the generation of iron sulfate,leading to a high SO2tolerance[81].Similarly,a core-shell structured meso-TiO2@MnOx/CNTs catalyst with the mesoporous TiO2layers coated on carbon nanotubes(CNTs)supported MnOxand CeOxnanoparticles also showed excellent SO2-resistant ability[82].Ce,W and Ti-containing mixed oxide catalysts were also prepared with a coreshell structure and employed as NH3-SCR catalysts[83].Compared with Ce-W,the TiO2-coated Ce-W@TiO2catalyst exhibited increased surface W atomic abundance and superior SO2resistance.In contrast,Ce-Ti-W catalyst prepared via a conventional co-precipitation method exhibited inferior SO2resistance to Ce-W.To reduce the SO2poisoning of Ce-based catalysts,Zhang et al.[84]developed a series of inverse TiO2/CeO2catalysts.Compared with the normally discussed CeO2/TiO2catalyst,the inverse TiO2/CeO2catalyst exhibited much better performance in the presence of 200 ppm SO2.Yang and Chen[85]reported that SO2could not access into the body of HPW while NO,NH3,and O2could.Inspired by this,with HPW as the protecting shell,CeO2@HPW catalyst showed improved SO2resistance[86].
3.3.Appropriate support
For industrial applications,supported catalyst is a better candidate compared with the unsupported oxide catalyst,because of its low pressure drop,high thermal and mechanical stability,large surface area,and highly dispersed active sites.The optimization of the interaction between support and active components could probably solve the SO2poisoning problem in NH3-SCR[87].The utilization of supports that possess high-or super-acidity was found to be an effective solution,because of the prevented SO2adsorption[86].

Fig.8.Figure(a)XRD pattern and(b)TGA curves of the catalysts after the SO2 tolerance test[80].
The structure of the support has a great influence on the sulfur tolerance of the deNOxcatalyst.Using three-dimensionally ordered macroporous carbon(3DOMC)as support,a novel MnOx/3DOMC nano-composite catalyst was fabricated[88].Compared with the MnOx/NAC(MnOxsupported on Norit activated carbon)and MnOx/TiO2catalysts,MnOx/3DOMC catalyst exhibited superior SO2resistance.A hexagonal WO3(HWO)supported V-based catalyst V2O5/HWO showed simultaneous resistance to alkalis and sulfur poisoning[89].The combined effect of alkali metal and SO2on the performance of deNOxcatalysts has received much attention because SO2could increase the deactivation induced by the alkali metal[90].As illustrated in Fig.9,the presence of SO2almost had no influence on the SCR activity of the V2O5/HWO catalyst.The reason was that the spontaneous migration of alkal is into the HWO tunnels generates the exposed catalytic active sites.However,under the same conditions,the conventional V2O5/WO3-TiO2catalyst was severely poisoned and completely lost the SCR activity within 4 h.The hexagonal boron nitride as carrier also demonstrated promotional effects on the resistance to SO2[91].Among different supported V-based catalysts(nano-TiO2,meso-TiO2,nano-ZrO2,meso-Al2O3and meso-SiO2),V2O5/meso-TiO2showed the best SO2poisoning resistance due to the higher pore volume of meso-TiO2,which enabled a larger tolerance capacity to ammonium sulfate and sulfite[87].Novel wire-mesh honeycomb-supported Ce-Fe/TiO2(Ce-Fe/WMH)and V2O5/WO3/TiO2/Al2O3/wire-mesh honeycomb(WMH)were also developed as NH3-SCR catalysts.Both of the catalysts showed promising resistance to SO2poisoning,which mightbe attributed to the unique three-dimensional structure[92,93].
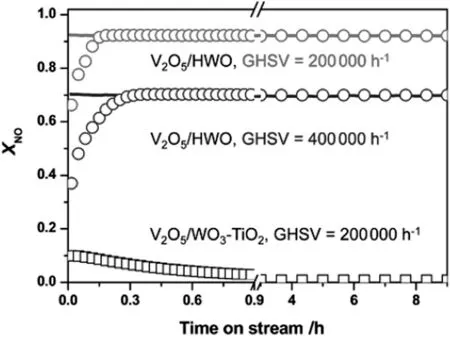
Fig.9.XNO over the V2O5/HWO and the V2O5/WO3-TiO2 catalysts at the reaction temperature of 350°C.Reaction conditions:500 ppm of NO,500 ppm of NH3,3.0 vol%O2,1300 mg·m-3 SO2,and balanced N2,gas flow rate=1000 ml·min-1[89].
The modification of supports has become a hot topic in catalysis due to the exposure of more active facets.The facet effects of supports were also employed to improve the resistance to SO2.Zhao and co-workers[94]used(001)plane-exposed TiO2nanosheets(TiO2-NS)and TiO2nanoparticles(TiO2-NP)which are dominated by(101)facets as supports to develop a novelZrCeVO4/TiO2catalyst for NH3-SCR.The activity testresults showed that the TiO2-NS-supported catalyst exhibited much better SO2durability than the catalyst supported by the TiO2-NP.As illustrated in Fig.10,the deactivation caused by SO2was due to the formation of(NH4)2SO4/NH4HSO4and NH4NO3which covered the active sites on the surface.However,the formation of(NH4)2SO4/NH4HSO4and NH4NO3could be inhibited on the TiO2-NS support.The micromorphology of Ce in Fe2O3/CeO2catalystwas also reported to have effect on the SO2resistance.The Fe2O3/CeO2catalyst supported on CeO2nanorods(CeO2-NR),which mainly exposed(110)and(100)facets,showed excellent catalytic performance and SO2tolerance[95].
Researchers have also utilized graphene(GE)or graphene oxide(GO)as supportto prepare catalysts for NH3-SCR.As a result,the SO2resistance of the catalysts is significantly improved.The introduction of GO prevented the sulfation of the active component due to its presulfuration effects[96].Introduction of GE could also result in enhanced H2O and SO2tolerance ofthe catalysts[97].Xiao etal.[98]had prepared a MnOx-CeO2/GE catalyst and using XPS confirmed that the content of Ce increased after the introduction of GE.CeO2inhibited the formation of manganese sulfate on the catalyst surface and hence demonstrated good resistance to SO2.
The type of catalyst carriers plays a key role in the NH3-SCR and could affect SO2resistance of the catalysts.For instance,in comparison with 3Ag/Al2O3,3Ag/bauxite tended to be more resistant to SO2poisoning in SCR of NOx[99].Synthesized TiO2catalyst loaded with Mn showed better resistance to sulfur poisoning than Mn-loaded commercial TiO2(P25)in the presence of SO2.Subsequent analysis verified that the formation of sulfated titanium and manganese sulfate was significantly inhibited,and the deposition concentration of ammonium sulfate on active sites was low enough,making the cataly stres is tant to SO2poisoning[100].In some research,CeO2was loaded on various supports,including single-component oxides(TiO2,ZrO2,Al2O3,and SiO2)and composite oxides(TiO2-SiO2,TiO2-ZrO2,and TiO2-Al2O3,TiO2-SiO2-Al2O3supports).The results showed that Ce/TiO2-SiO2and Ce/TiO2-SiO2-Al2O3exhibited strong resistance to SO2poisoning[101,102].In summary,the SO2resistance ability can be improved by varying the supports of SCR catalysts.
4.Other Factors Influence SO2-resistance
4.1.Preparation methods and conditions
It is well known that the preparation process has a great effect on the activity of catalysts.Generally,varied preparation methods could significantly affect the physical/chemical properties(including particle morphologies,surface properties,valence states,etc.)of the resulting catalysts.Such varied properties could have a strong influence on the behavior of the catalysts.Thus,the influence of preparation parameters,such as preparation methods and preparation conditions on the catalytic performance and SO2resistance of NH3-SCR catalysts had been investigated systematically.

Fig.10.Promoted SCR performance and the corresponding mechanism of the TiO2 nanosheet-and nanoparticle-based catalysts[94].
Guo and coworkers[103]prepared CeO2/Al2O3catalyst samples by the following three methods:single step sol-gel,impregnation and co-precipitation method.The results indicated that catalyst prepared by sol-gel method had the best SO2-tolerant performance.Larger surface area,higher dispersion of CeO2on Al2O3,higher NH3adsorption capacity and improved redox ability may be accounted for the better SO2resistance of the catalyst prepared by single sol-gel method.Investigations on CeO2-CuO/Al2O3[104],MnOx/TiO2[105],CeO2/TiO2[106]and Ce-O-P[107]catalysts got the same result.The mesopore channels in catalysts prepared by sol-gel method could be able to maintain a dynamic balance between the formation and decomposition of ammonium sulfates in SCR reaction.FTIR analysis indicated that catalyst prepared by sol-gel method was of the weakest sulfation extent among the catalysts prepared with different methods,which agreed well with its good SO2resistance[104].
Hydrothermal method is commonly used to prepare catalysts with strong resistance against SO2.For example,Liu and coworkers[108]prepared Mn-Ce-Ti mixed-oxide catalyst by the hydrothermal method which exhibited excellent SO2resistance with a broad operation temperature window.Ce-Ti-W-Oxcatalyst prepared by the hydrothermal method also exhibited good resistance to SO2and the inhibiting effect of SO2was reversible[109].Moreover,sol vothermal methods such as pyridine-thermal route,ethanol-thermal route were also used to prepare catalysts supported on carbon nanotubes[110-112].Compared with the catalysts prepared by other methods,the highly dispersed metal oxides on carbon nanotubes(CNTs)catalyst prepared by the pyridine-thermal route or ethanol-thermal route presented the best SO2resistance.
Recently,some novel catalyst preparation methods were also adopted to prepare deNOxcatalysts with enhanced SO2resistance.For example,NiMnFe mixed oxides derived from LDH(layered double hydroxide)[78]and the hollow porous MnxCo3-xO4nanocages thermally derived from metal-organic frameworks[79]showed good resistance to SO2.
The preparation parameters during the synthesis process can greatly affect the nano-structures and morphologies,which play a dominant role in the catalytic performance.Wu et al.[113]investigated the effect of pH during co-precipitation on the structural and physico chemical properties of a FeVO4/TiO2-WO3-SiO2catalyst.Characterization results indicated that the catalyst showed the best catalytic activity with high resistance to SO2poisoning when the active component was synthesized at pH=4.5.The pH values adopted during the synthesis of Sb-V/CeO2-TiO2showed the similar effect on the SO2resistance of the catalysts[114].
4.2.Reaction conditions
Reaction conditions such as temperature and composition of the flue gas were also found to be important for the catalytic activity as well as sulfur tolerance of the deNOxcatalysts.
Formation and decomposition of sulfate species on the catalyst surface are directly related to the reaction temperature.Therefore,reaction temperature has a great influence on SO2deactivation of the SCR catalysts[10].For example,the deactivation mechanism for Mn-Ce/TiO2catalyst in NH3-SCR in the presence of SO2at different temperatures was different[115].At 100°C,the catalyst deactivation was mainly due to the formation and deposition of(NH4)2SO4and NH4HSO4.However,at higher temperature(200°C),the irreversible deactivation was caused by sulfated metal sites.The effect of SO2and H2O on the activity of Ce-Ti mixed-oxide catalysts was also bound up with the reaction temperature[116].The Cr0.2-V0.8/TiO2catalyst[117]showed similar behavior with the Ce-Timixed-oxide.As illustrated in Fig.11,in a 12 h test with flue gas containing 100 ppm SO2,the NOxconversion remained at about 99%at 220°C and no signs of sulfur poisoning were observed.However,the introduction of SO2severely decreased the NOxconversion at 180°C.

Fig.11.Resistance to SO2 of Cr0.2-V0.8/TiO2 catalyst.Reaction conditions:220 °C or 180 °C,GHSV=60000 ml·h-1·g-1,[SO2]=100 ppm[117].
Coexisting gases are also essential factors in determining the catalytic performance and sulfur tolerance of the SCR catalysts.The O2concentration,for instance,can significantly affect SO2deactivation and regeneration behavior over SCR catalyst.Li et al.[118]found that the extent of SO2deactivation was O2concentration dependent over CuO-CeO2catalyst.The deactivated catalyst could be reactivated under 5.0 vol%O2,which could be attributed to the involvement of NO2into the reactions.They revealed that NO2could react with NH3/NH4+and promoted the decomposition of sulfate deposited on the catalyst surface.The NO2/NOxratio also reported to had influences on the SO2resistance performance of a vanadium-based catalyst[119].Therefore,by controlling the reaction parameters of the SCR process,we can suppress the SO2induced catalyst deactivation,which is important for the industrial application of SCR catalysts.
4.3.S,P-containing/-treatment
Some S-containing catalysts exhibited good SO2resistance capability.For example,Yu et al.[120]prepared a series of CuSO4/TiO2catalysts and found that SO2had little effect on the activity.Supported Fe and Cu sulfates on Ce-TiOxalso proved to exhibited an enhanced resistance to SO2[121].Marcin et al.proposed that the controlled sulfation of the commercial catalyst prior to the SCR process may improve its SO2resistance[122].Ke et al.[123]found that the Co3O4catalyst was very effective in NH3-SCR after being sulfated by SO2which may be due to the formation of bulk cobalt sulfate.In addition,it exhibited good resistance to SO2under a long term operation condition.Moreover,the S-treated W-CeZr,CeZr oxides[124],Ni-Ce-La composite oxides[125]and sulfated Mn-Co-Ce/TiO2/SiO2[126]were also found to have an enhanced NOxconversion and SO2resistance performance.Phosphates,the solid acid materials,exhibited promising properties for catalysis application.The phosphates Ce-O-P were employed in the SCR process as a novel catalyst and showed a good SO2resistance ability[107].
5.Conclusions and Perspectives
To date,extensive studies have been conducted on the development of deNOxcatalysts and an increasing attention has been paid to the deactivation of the SCR catalysts.Sulfur poisoning,which contributes to the deactivation of the deNOxcatalysts,is usually irreversible.Extensively investigations have been carried out to elucidate the sulfur deactivation mechanisms on different catalysts in order to improve the SO2-tolerant ability of the SCR catalysts.The sulfur poisoning mechanisms may vary on different catalysts.However,there exist several underlying causes:sulfation of the active center atoms,formation and deposition of ammonium sulfates on the catalyst surface clogging the pore channels,and the competitive adsorption between SO2and NOx.To reduce SO2poisoning,there are several strategies including modifying the catalysts with the transition metals,preparing catalysts with specific structure and morphology,and selecting appropriate supports.Moreover,methods used to prepare the catalysts and the reaction conditions could have a great influence on the SO2-resistance of the catalysts.
Although considerable progress has been achieved,the activity test time in the presence of SO2is relatively short.Therefore,it is still a challenge to develop catalysts with good SO2resistance for industrial applications.The rapid development of theoretical calculations can assist the investigation of SO2resistance.Theoretical calculations can help to illustrate the reaction mechanisms that are difficult to access merely by experimental study.It may become a main research area in the field of SCR catalysts in the future.Moreover,it may provide guidance for future research on the development of high SO2resistance catalysts.
Recently,metal-organic frameworks,which are known to be porous crystalline materials with a large surface area were used as SCR catalysts and exhibited good SO2resistance[127-129].Besides,more novel SCR catalysts should be developed to attain good sulfur tolerance.
[1]G.Qi,R.T.Yang,Low-temperature selective catalytic reduction of NO with NH3over iron and manganese oxides supported on titania,Appl.Catal.B 44(2003)217-225.
[2]L.Yan,Y.Liu,H.Hu,H.Li,L.Shi,D.Zhang,Investigations on the antimony promotional effect on CeO2-WO3/TiO2for selective catalytic reduction of NOxwith NH3,Chem Cat Chem 8(2016)2267-2278.
[3]K.Q.Tran,P.Kilpinen,N.Kumar,In-situ catalytic abatement of NOxduring fluidized bed combustion—a literature study,Appl.Catal.B 78(2008)129-138.
[4]R.Q.Long,R.T.Yang,R.Chang,Low temperature selective catalytic reduction(SCR)of NO with NH3over Fe-Mn based catalysts,Chem.Commun.(2002)452-453.
[5]J.Zuo,Z.Chen,F.Wang,Y.Yu,L.Wang,X.Li,Low-temperature selective catalytic reduction of NOxwith NH3over novel Mn-Zr mixed oxide catalysts,Ind.Eng.Chem.Res.53(2014)2647-2655.
[6]K.Cheng,J.Liu,T.Zhang,J.Li,Z.Zhao,Y.Wei,G.Jiang,A.Duan,Effect of Ce doping of TiO2support on NH3-SCR activity over V2O5-WO3/CeO2-TiO2catalyst,J.Environ.Sci.(China)26(2014)2106-2113.
[7]S.Pan,H.Luo,L.Li,Z.Wei,B.Huang,H2O and SO2deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOxwith NH3,J.Mol.Catal.A Chem.377(2013)154-161.
[8]A.Yamamoto,K.Teramura,S.Hosokawa,T.Tanaka,Effects of SO2on selective catalytic reduction of NO with NH3over a TiO2photocatalyst,Sci.Technol.Adv.Mater.16(2015)024901.
[9]Z.Ma,X.Wu,Y.Feng,Z.Si,D.Weng,L.Shi,Low-temperature SCR activity and SO2deactivation mechanism of Ce-modified V2O5-WO3/TiO2catalyst,Prog.Nat.Sci.Mater.Int.25(2015)342-352.
[10]P.Li,Z.Liu,Q.Li,W.Wu,Q.Liu,Multiple roles of SO2in selective catalytic reduction of NO by NH3over V2O5/AC catalyst,Ind.Eng.Chem.Res.53(2014)7910-7916.
[11]F.Liu,K.Asakura,H.He,W.Shan,X.Shi,C.Zhang,In fluence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOxwith NH3,Appl.Catal.B 103(2011)369-377.
[12]F.Liu,H.He,Selective catalytic reduction of NO with NH3over manganese substituted iron titanate catalyst:Reaction mechanism and H2O/SO2inhibition mechanism study,Catal.Today 153(2010)70-76.
[13]A.Kumar,M.A.Smith,K.Kamasamudram,N.W.Currier,A.Yezerets,Chemical deSOx:an effective way to recover Cu-zeolite SCR catalysts from sulfur poisoning,Catal.Today 267(2016)10-16.
[14]W.Shan,H.Song,Catalysts for the selective catalytic reduction of NOxwith NH3at low temperature,Catal.Sci.Technol.5(2015)4280-4288.
[15]D.W.Brookshear,J.g.Nam,K.Nguyen,T.J.Toops,A.Binder,Impact of sulfation and desulfation on NOxreduction using Cu-chabazite SCR catalysts,Catal.Today 258(2015)359-366.
[16]Y.J.Kim,H.J.Kwon,I.Heo,I.S.Nam,B.K.Cho,J.W.Choung,G.K.Y.Moon-Soon Cha,Mn-Fe/ZSM-5 as a low-temperature SCR catalyst to remove NOxfrom diesel engine exhaust,Appl.Catal.B 126(2012)9-12.
[17]K.Wijayanti,K.Leistner,S.Chand,A.Kumar,K.Kamasamudram,N.W.Currier,A.Yezerets,L.Olsson,Deactivation of Cu-SSZ-13 by SO2exposure under SCR conditions,Catal.Sci.Technol.6(2016)2565-2579.
[18]Y.Jangjou,M.Ali,Q.Chang,D.Wang,J.Li,A.Kumar,W.S.Epling,Effect of SO2on NH3oxidation over a Cu-SAPO-34 SCR catalyst,Catal.Sci.Technol.6(2016)2679-2685.
[19]A.Kumar,M.A.Smith,K.Kamasamudram,N.W.Currier,H.An,A.Yezerets,Impact of different forms of feed sulfur on small-pore Cu-zeolite SCR catalyst,Catal.Today 231(2014)75-82.
[20]K.Wijayanti,S.Andonova,A.Kumar,J.Li,K.Kamasamudram,N.W.Currier,A.Yezerets,L.Olsson,Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34,Appl.Catal.B 166-167(2015)568-579.
[21]M.L.M.de Oliveira,C.M.Silva,R.Moreno-Tost,T.L.Farias,A.Jiménez-López,E.Rodríguez-Castellón,Simulation of SCR equipped vehicles using iron-zeolite catalysts,Appl.Catal.A 366(2009)13-21.
[22]L.Ma,H.Qu,J.Zhang,Q.Tang,S.Zhang,Q.Zhong,Preparation of nanosheet Fe-ZSM-5 catalysts,and effect of Fe content on acidity,water,and sulfur resistance in the selective catalytic reduction of NOxby ammonia,Res.Chem.Intermed.39(2013)4109-4120.
[23]S.Yang,Y.Guo,H.Chang,L.Ma,Y.Peng,Z.Qu,N.Yan,C.Wang,J.Li,Novel effect of SO2on the SCR reaction over CeO2:Mechanism and significance,Appl.Catal.B 136-137(2013)19-28.
[24]X.Xiao,S.Xiong,Y.Shi,W.Shan,S.Yang,Effect of H2O and SO2on the selective catalytic reduction of NO with NH3over Ce/TiO2catalyst:Mechanism and kinetic study,J.Phys.Chem.C 120(2016)1066-1076.
[25]D.Zhang,L.Zhang,L.Shi,C.Fang,H.Li,R.Gao,L.Huang,J.Zhang,In situ supported MnOx-CeOxon carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3,Nanoscale 5(2013)1127-1136.
[26]Y.Wang,X.Li,L.Zhan,C.Li,W.Qiao,L.Ling,Effect of SO2on activated carbon honeycomb supported CeO2-MnOxcatalyst for NO removal at low temperature,Ind.Eng.Chem.Res.54(2015)2274-2278.
[27]L.Zhang,X.Zhang,S.Lv,X.Wu,P.Wang,Promoted performance of a MnOx/PG catalyst for low-temperature SCR against SO2poisoning by addition of cerium oxide,RSC Adv.5(2015)82952-82959.
[28]Y.Wang,L.Yang,W.P.Liao,F.Wang,Research of SO2resistance of MnOxcatalyst modified by Ce for low temperature SCR with NH3,Adv.Mater.Res.356-360(2011)529-532.
[29]Z.Wu,R.Jin,H.Wang,Y.Liu,Effect of ceria doping on SO2resistance of Mn/TiO2for selective catalytic reduction of NO with NH3at low temperature,Catal.Commun.10(2009)935-939.
[30]L.Wei,S.Cui,H.Guo,X.Ma,L.Zhang,DRIFT and DFT study of cerium addition on SO2of Manganese-based Catalysts for low temperature SCR,J.Mol.Catal.A Chem.421(2016)102-108.
[31]K.J.Lee,P.A.Kumar,M.S.Maqbool,K.N.Rao,K.H.Song,H.P.Ha,Ce added Sb-V2O5/TiO2catalysts for low temperature NH3SCR:physico-chemical properties and catalytic activity,Appl.Catal.B 142-143(2013)705-717.
[32]D.W.Kwon,K.B.Nam,S.C.Hong,The role of ceria on the activity and SO2resistance of catalysts for the selective catalytic reduction of NOxby NH3,Appl.Catal.B 166-167(2015)37-44.
[33]W.Cha,S.H.Ehrman,J.Jurng,CeO2added V2O5/TiO2catalyst prepared by chemical vapor condensation(CVC)and impregnation method for enhanced NH3-SCR of NOxat low temperature,J.Environ.Chem.Eng.4(2016)556-563.
[34]L.Pang,C.Fan,L.Shao,K.Song,J.Yi,X.Cai,J.Wang,M.Kang,T.Li,The Ce doping Cu/ZSM-5 as a new superior catalyst to remove NO from diesel engine exhaust,Chem.Eng.J.253(2014)394-401.
[35]S.Lai,D.Meng,W.Zhan,Y.Guo,Y.Guo,Z.Zhang,G.Lu,The promotional role of Ce in Cu/ZSM-5 and in situ surface reaction for selective catalytic reduction of NOxwith NH3,RSC Adv.5(2015)90235-90244.
[36]S.Y.Jiang,R.X.Zhou,Ce doping effect on performance of the Fe/β catalyst for NOxreduction by NH3,Fuel Process.Technol.133(2015)220-226.
[37]W.L.Zhen,R.T.Guo,W.G.Pan,Low temperature selective catalytic reduction of NO on CeO2-Fe3O4/TiO2,CeO2/TiO2catalysts prepared by coprecipitation method,Adv.Mater.Res.960-961(2014)234-239.
[38]Y.Shu,H.Sun,X.Quan,S.Chen,Enhancement of catalytic activity over the ironmodified Ce/TiO2catalyst for selective catalytic reduction of NOxwith ammonia,J.Phys.Chem.C 116(2012)25319-25327.
[39]S.Cai,D.Zhang,L.Zhang,L.Huang,H.Li,R.Gao,L.Shi,J.Zhang,Comparative study of 3D ordered macroporous Ce0.75Zr0.2M0.05O2-δ(M=Fe,Cu,Mn,Co)for selective catalytic reduction of NO with NH3,Catal.Sci.Technol.4(2014)93-101.
[40]W.Zhao,C.Li,P.Lu,Q.Wen,Y.Zhao,X.Zhang,C.Fan,S.Tao,Iron,lanthanum and manganese oxides loaded on γ-Al2O3for selective catalytic reduction of NO with NH3at low temperature,Environ.Technol.34(2013)81-90.
[41]X.Tang,J.Hao,H.Yi,J.Li,Low-temperature SCR of NO with NH3over AC/C supported manganese-based monolithic catalysts,Catal.Today 126(2007)406-411.
[42]C.Fang,L.Shi,H.Hu,J.Zhang,D.Zhang,Rational design of 3D hierarchical foamlike Fe2O3@CuOxmonolith catalysts for selective catalytic reduction of NO with NH3,RSC Adv.5(2015)11013-11022.
[43]R.Gao,D.Zhang,X.Liu,L.Shi,P.Maitarad,H.Li,J.Zhang,W.Cao,Enhanced catalytic performance of V2O5-WO3/Fe2O3/TiO2microspheres for selective catalytic reduction of NO by NH3,Catal.Sci.Technol.3(2013)191-199.
[44]Y.X.Teng,C.Y.Song,X.N.Lu,Z.S.Tong,Y.S.Qin,In fluence of Fe doping on Ce-Mn/TiO2-ZrO2catalysts for low-temperature selective catalytic reduction of NO,Adv.Mater.Res.898(2014)447-451.
[45]B.Shen,T.Liu,N.Zhao,X.Yang,L.Deng,Iron-doped Mn-Ce/TiO2catalyst for low temperature selective catalytic reduction of NO with NH3,J.Environ.Sci.22(2010)1447-1454.
[46]M.E.Yu,C.Li,G.Zeng,Y.Zhou,X.Zhang,Y.E.Xie,The selective catalytic reduction of NO with NH3over a novel Ce-Sn-Ti mixed oxides catalyst:promotional effectof SnO2,Appl.Surf.Sci.342(2015)174-182.
[47]H.Chang,J.Li,X.Chen,L.Ma,S.Yang,J.W.Schwank,J.Hao,Effect of Sn on MnOx-CeO2catalyst for SCR of NOxby ammonia:Enhancement of activity and remarkable resistance to SO2,Catal.Commun.27(2012)54-57.
[48]H.Chang,X.Chen,J.Li,L.Ma,C.Wang,C.Liu,J.W.Schwank,J.Hao,Improvement of activity and SO2tolerance of Sn-modified MnOx-CeO2catalysts for NH3-SCR at low temperatures,Environ.Sci.Technol.47(2013)5294-5301.
[49]M.Qiu,S.Zhan,D.Zhu,H.Yu,Q.Shi,NH3-SCR performance improvement of mesoporous Sn modified Cr-MnOxcatalysts at low temperatures,Catal.Today 258(2015)103-111.
[50]X.Li,Y.Li,S.Deng,T.A.Rong,A Ce-Sn-Oxcatalyst for the selective catalytic reduction of NOxwith NH3,Catal.Commun.40(2013)47-50.
[51]P.Zhang,Q.Hou,SnO2modified Ce-Ti-Oxcatalyst for the selective catalytic reduction of NOxwith NH3,React.Kinet.Mech.Catal.117(2016)119-128.
[52]C.Fang,L.Shi,H.Li,L.Huang,J.Zhang,D.Zhang,Creating hierarchically macro-/mesoporous Sn/CeO2for the selective catalytic reduction of NO with NH3,RSC Adv.6(2016)78727-78736.
[53]D.W.Kwon,K.B.Nam,S.C.Hong,In fluence of tungsten on the activity of a Mn/Ce/W/Ti catalyst for the selective catalytic reduction of NO with NH3at low temperatures,Appl.Catal.A 497(2015)160-166.
[54]D.W.Kwon,S.C.Hong,Promotional effect of tungsten-doped CeO2/TiO2for selective catalytic reduction of NOxwith ammonia,Appl.Surf.Sci.356(2015)181-190.
[55]P.Zhang,K.Li,Q.Lei,Enhanced activity of tungsten doped CeAlOxcatalysts for the selective catalytic reduction of NOxwith NH3,React.Kinet.Mech.Catal.116(2015)523-533.
[56]Z.Fang,B.Yuan,T.Lin,H.Xu,Y.Cao,Z.Shi,M.Gong,Y.Chen,Monolith Ce0.65Zr0.35O2-based catalysts for selective catalytic reduction of NOxwith NH3,Chem.Eng.Res.Des.94(2015)648-659.
[57]Z.Ma,X.Wu,Y.Feng,Z.Si,D.Weng,Effects of WO3doping on stability and N2O escape of MnOx-CeO2mixed oxides as a low-temperature SCR catalyst,Catal.Commun.69(2015)188-192.
[58]P.Zhang,D.Li,Selective catalytic reduction of NO with NH3over iron-vanadium mixed oxide catalyst,Catal.Lett.144(2014)959-963.
[59]B.Wu,Effect on addictives adding of Mn-Ce/TiO2selective catalytic reduction NO by NH3at low-temperature,Adv.Mater.Res.955-959(2014)25-29.
[60]W.Yang,F.Liu,L.Xie,Z.Lian,H.He,Effect of V2O5additive on the SO2resistance of a Fe2O3/AC catalyst for NH3-SCR of NOxat low temperatures,Ind.Eng.Chem.Res.55(2016)2677-2685.
[61]Z.Lian,F.Liu,H.He,Enhanced activity of Ti-modified V2O5-CeO2catalyst for the selective catalytic reduction of NOxwith NH3,Ind.Eng.Chem.Res.53(2014)19506-19511.
[62]Y.Xiong,C.Tang,X.Yao,L.Zhang,L.Li,X.Wang,Y.Deng,F.Gao,L.Dong,Effect of metal ions doping(M=Ti4+,Sn4+)on the catalytic performance of MnOx/CeO2catalyst for low temperature selective catalytic reduction of NO with NH3,Appl.Catal.A 495(2015)206-216.
[63]X.Zhao,L.Huang,H.Li,H.Hu,J.Han,L.Shi,D.Zhang,Highly dispersed V2O5/TiO2modified with transition metals(Cu,Fe,Mn,Co)as efficient catalysts for the selective reduction of NO with NH3,Chin.J.Catal.36(2015)1886-1899.
[64]X.Gao,X.S.Du,L.W.Cui,Y.C.Fu,Z.Y.Luo,K.F.Cen,A Ce-Cu-Ti oxide catalyst for the selective catalytic reduction of NO with NH3,Catal.Commun.12(2010)255-258.
[65]X.S.Du,X.Gao,L.W.Cui,Y.C.Fu,Z.Y.Luo,K.F.Cen,Investigation of the effect of Cu addition on the SO2-resistance of a Ce-Ti oxide catalyst for selective catalytic reduction of NO with NH3,Fuel 92(2012)49-55.
[66]J.Qiao,N.Wang,Z.Wang,W.Sun,K.Sun,Porous bimetallic Mn2Co1Oxcatalysts prepared by a one-step combustion method for the low temperature selective catalytic reduction of NOxwith NH3,Catal.Commun.72(2015)111-115.
[67]Q.M.Zhang,C.L.Song,G.Lv,F.Bin,H.T.Pang,J.O.Song,Effect of metal oxide partial substitution of V2O5in V2O5-WO3/TiO2on selective catalytic reduction of NO with NH3,J.Ind.Eng.Chem.24(2015)79-86.
[68]X.Zhang,B.Shen,K.Wang,J.Chen,A contrastive study of the introduction of cobalt as a modifier for active components and supports of catalysts for NH3-SCR,J.Ind.Eng.Chem.19(2013)1272-1279.
[69]X.Li,Y.Li,Molybdenum modified CeAlOxcatalyst for the selective catalytic reduction of NO with NH3,J.Mol.Catal.A Chem.386(2014)69-77.
[70]D.W.Kwon,K.H.Park,S.C.Hong,Enhancement of SCR activity and SO2resistance on VOx/TiO2catalyst by addition of molybdenum,Chem.Eng.J.284(2016)315-324.
[71]S.Ding,F.Liu,X.Shi,H.He,Promotional effect of Nb additive on the activity and hydrothermal stability for the selective catalytic reduction of NOxwith NH3over CeZrOxcatalyst,Appl.Catal.B 180(2016)766-774.
[72]Y.Jiang,Y.Yan,S.B.Huang,X.X.Zhang,X.W.Wang,W.X.Song,Selective catalytic reduction of NO with NH3over a Ce-Zr-Ti oxide catalyst,Adv.Mater.Res.864-867(2014)353-356.
[73]F.Cao,J.Xiang,S.Su,P.Wang,L.Sun,S.Hu,S.Lei,The activity and characterization of MnOx-CeO2-ZrO2/γ-Al2O3catalysts for low temperature selective catalytic reduction of NO with NH3,Chem.Eng.J.243(2014)347-354.
[74]X.Zhao,L.Huang,H.Li,H.Hu,X.Hu,L.Shi,D.Zhang,Promotional effects of zirconium doped CeVO4for the low-temperature selective catalytic reduction of NOxwith NH3,Appl.Catal.B 183(2016)269-281.
[75]M.Aguilar-Romero,R.Camposeco,S.Castillo,J.Marín,V.Rodríguez-González,L.A.García-Serrano,I.Mejía-Centeno,Acidity,surface species,and catalytic activity study on V2O5-WO3/TiO2nanotube catalysts for selective NO reduction by NH3,Fuel 198(2017)123-133.
[76]Y.Shi,S.Chen,H.Sun,Y.Shu,X.Quan,Low-temperature selective catalytic reduction of NOxwith NH3over hierarchically macro-mesoporous Mn/TiO2,Catal.Commun.42(2013)10-13.
[77]L.Huang,X.Zhao,L.Zhang,L.Shi,J.Zhang,D.Zhang,Large-scale growth of hierarchical transition-metal vanadate nanosheets on metal meshes as monolith catalysts for De-NOxreaction,Nanoscale 7(2015)2743-2749.
[78]H.Li,D.Zhang,P.Maitarad,L.Shi,R.Gao,J.Zhang,W.Cao,In situ synthesis of 3D flower-like NiMnFe mixed oxides as monolith catalysts for selective catalytic reduction of NO with NH3,Chem.Commun.48(2012)10645-10647.
[79]L.Zhang,L.Shi,L.Huang,J.Zhang,R.Gao,D.Zhang,Rational design of highperformance DeNOxcatalysts based on MnxCo3-xO4nanocages derived from metal-organic frameworks,ACS Catal.4(2014)1753-1763.
[80]S.Cai,H.Hu,H.Li,L.Shi,D.Zhang,Design of multi-shell Fe2O3@MnOx@CNTs for the selective catalytic reduction of NO with NH3:Improvement of catalytic activity and SO2tolerance,Nanoscale 8(2016)3588-3598.
[81]J.Liu,Y.Du,J.Liua,Z.Zhao,K.Cheng,Y.Chen,Y.Wei,W.Song,X.Zhang,Design of MoFe/Beta@CeO2catalysts with a core-shell structure and their catalytic performances for the selective catalytic reduction of NO with NH3,Appl.Catal.B 203(2017)704-714.
[82]L.Zhang,D.Zhang,J.Zhang,S.Cai,C.Fang,L.Huang,H.Li,R.Gao,L.Shi,Design of meso-TiO2@MnOx-CeOx/CNTs with a core-shell structure as DeNOxcatalysts:promotion of activity,stability and SO2-tolerance,Nanoscale 5(2013)9821-9829.
[83]X.Liu,P.Ning,H.Li,Z.X.Song,Y.C.Wang,J.H.Zhang,X.S.Tang,M.Z.Wang,Q.L.Zhang,Probing NH3-SCR catalytic activity and SO2resistance over aqueous-phase synthesized Ce-W@TiO2catalyst,J.Fuel Chem.Technol.44(2016)225-231.
[84]L.Zhang,L.Li,Y.Cao,X.Yao,C.Ge,F.Gao,Y.Deng,C.Tang,L.Dong,Getting insight into the in fluence of SO2on TiO2/CeO2for the selective catalytic reduction of NOby NH3,Appl.Catal.B 165(2015)589-598.
[85]R.T.Yang,N.Chen,A new approach to decomposition of nitric oxide using sorbent/catalyst without reducing gas:Use of heteropoly compounds,Ind.Eng.Chem.Res.33(1994)825-831.
[86]X.Weng,X.Dai,Q.Zeng,Y.Liu,Z.Wu,DRIFT studies on promotion mechanism of H3PW12O40in selective catalytic reduction of NO with NH3,J.Colloid Interface Sci.461(2016)9-14.
[87]F.Guo,J.Yu,M.Chu,G.Xu,Interaction between support and V2O5in the selective catalytic reduction of NO by NH3,Catal.Sci.Technol.4(2014)2147-2155.
[88]X.Gao,L.Li,L.Song,T.Lu,J.Zhao,Z.Liu,Highly dispersed MnOxnanoparticles supported on three-dimensionally ordered macroporous carbon:A novel nanocomposite for catalytic reduction of NOxwith NH3at low temperature,RSC Adv.5(2015)29577-29588.
[89]Z.Huang,H.Li,J.Gao,X.Gu,L.Zheng,P.Hu,Y.Xin,J.Chen,Y.Chen,Z.Zhang,J.Chen,X.Tang,Alkali-and sulfur-resistant tungsten-based catalysts for NOxemissions control,Environ.Sci.Technol.49(2015)14460-14465.
[90]Q.Li,S.Chen,Z.Liu,Q.Liu,Combined effect of KCl and SO2on the selective catalytic reduction of NO by NH3over V2O5/TiO2catalyst,Appl.Catal.B 164(2015)475-482.
[91]D.Zhou,Z.Ren,B.Li,Z.Ma,X.Zhang,H.Yang,In fluence of hexagonal boron nitride on the selective catalytic reduction of NO with NH3over CuOx/TiO2,RSC Adv.5(2015)31708-31715.
[92]Y.Shu,T.Aikebaier,X.Quan,S.Chen,H.Yu,Selective catalytic reaction of NOxwith NH3over Ce-Fe/TiO2-loaded wire-mesh honeycomb:Resistance to SO2poisoning,Appl.Catal.B 150-151(2014)630-635.
[93]Y.Shu,H.Sun,X.Quan,S.Chen,Improvement of water-,sulfur dioxide-,and dust-resistance in selective catalytic reduction of NOxwith NH3using a wiremesh honeycomb catalyst,Ind.Eng.Chem.Res.51(2012)7867-7873.
[94]X.Zhao,L.Huang,S.Namuangruk,H.Hu,X.Hu,L.Shi,D.Zhang,Morphologydependent performance of Zr-CeVO4/TiO2for selective catalytic reduction of NO with NH3,Catal.Sci.Technol.6(2016)5543-5553.
[95]J.Han,J.Meeprasert,P.Maitarad,S.Nammuangruk,L.Shi,D.Zhang,Investigation of the facet-dependent catalytic performance of Fe2O3/CeO2for the selective catalytic reduction of NO with NH3,J.Phys.Chem.C 120(2016)1523-1533.
[96]W.Su,X.Lu,S.Jia,J.Wang,H.Ma,Y.Xing,Catalytic reduction of NOxover TiO2-graphene oxide supported with MnOxat low temperature,Catal.Lett.145(2015)1446-1456.
[97]X.Lu,C.Song,S.Jia,Z.Tong,X.Tang,Y.Teng,Low-temperature selective catalytic reduction of NOxwith NH3over cerium and manganese oxides supported on TiO2-graphene,Chem.Eng.J.260(2015)776-784.
[98]X.Xiao,Z.Sheng,L.Yang,F.Dong,Low-temperature selective catalytic reduction of NOxwith NH3over a manganese and cerium oxide/graphene composite prepared by a hydrothermal method,Catal.Sci.Technol.6(2016)1507-1514.
[99]X.Wang,L.Jiang,J.Wang,R.Wang,Ag/bauxite catalysts:Improved lowtemperature activity and SO2tolerance for H2-promoted NH3-SCR of NOx,Appl.Catal.B 165(2015)700-705.
[100]E.Park,M.Kim,H.Jung,S.Chin,J.Jurng,Effect of sulfur on Mn/Ti catalysts prepared using chemical vapor condensation(CVC)for low-temperature NO reduction,ACS Catal.3(2013)1518-1525.
[101]C.Liu,L.Chen,J.Li,L.Ma,H.Arandiyan,Y.Du,J.Xu,J.Hao,Enhancement of activity and sulfur resistance of CeO2supported on TiO2-SiO2for the selective catalytic reduction of NO by NH3,Environ.Sci.Technol.46(2012)6182-6189.
[102]W.Zhao,Y.Tang,Y.Wan,L.Li,S.Yao,X.Li,J.Gu,Y.Li,J.Shi,Promotion effects of SiO2or/and Al2O3doped CeO2/TiO2catalysts for selective catalytic reduction of NO by NH3,J.Hazard.Mater.278(2014)350-359.
[103]R.T.Guo,Y.Zhou,W.G.Pan,J.N.Hong,W.L.Zhen,Q.Jin,C.G.Ding,S.Y.Guo,Effect of preparation methods on the performance of CeO2/Al2O3catalysts for selective catalytic reduction of NO with NH3,J.Ind.Eng.Chem.19(2013)2022-2025.
[104]R.T.Guo,W.L.Zhen,W.G.Pan,J.N.Hong,Q.Jin,C.G.Ding,S.Y.Guo,Low temperature selective catalytic reduction of NO on CeO2-CuO/Al2O3catalysts prepared by different methods,Environ.Technol.35(2014)1766-1772.
[105]B.Jiang,Y.Liu,Z.Wu,Low-temperature selective catalytic reduction of NO on MnOx/TiO2prepared by different methods,J.Hazard.Mater.162(2009)1249-1254.
[106]X.Gao,Y.Jiang,Y.Fu,Y.Zhong,Z.Luo,K.Cen,Preparation and characterization of CeO2/TiO2catalysts for selective catalytic reduction of NO with NH3,Catal.Commun.11(2010)465-469.
[107]W.Yao,Y.Liu,X.Wang,X.Weng,H.Wang,Z.Wu,The superior performance of sol-gel made Ce-O-P catalyst for selective catalytic reduction of NO with NH3,J.Phys.Chem.C 120(2016)221-229.
[108]Z.Liu,J.Zhu,J.Li,L.Ma,S.I.Woo,Novel Mn-Ce-Ti mixed-oxide catalyst for the selective catalytic reduction of NOxwith NH3,ACS Appl.Mater.Interfaces 6(2014)14500-14508.
[109]H.Li,G.F.Qu,Y.K.Duan,P.Ning,Q.L.Zhang,X.Liu,Z.X.Song,Performance and characterisation of CeO2-TiO2-WO3catalysts for selective catalytic reduction of NO with NH3,Chem.Pap.69(2015)817-826.
[110]C.Fang,D.Zhang,L.Shi,R.Gao,H.Li,L.Ye,J.Zhang,Highly dispersed CeO2on carbon nanotubes for selective catalytic reduction of NO with NH3,Catal.Sci.Technol.3(2013)803-811.
[111]J.Han,D.Zhang,P.Maitarad,L.Shi,S.Cai,H.Li,L.Huang,J.Zhang,Fe2O3nanoparticles anchored in situ on carbon nanotubes via an ethanol-thermal strategy for the selective catalytic reduction of NO with NH3,Catal.Sci.Technol.5(2015)438-446.
[112]D.Zhang,L.Zhang,C.Fang,R.Gao,Y.Qian,L.Shi,J.Zhang,MnOx-CeOx/CNTs pyridine-thermally prepared via a novel in situ deposition strategy for selective catalytic reduction of NO with NH3,RSC Adv.3(2013)8811-8819.
[113]G.Wu,J.Li,Z.Fang,L.Lan,R.Wang,T.Lin,M.Gong,Y.Chen,Effectively enhance catalytic performance by adjusting pH during the synthesis of active components over FeVO4/TiO2-WO3-SiO2monolith catalysts,Chem.Eng.J.271(2015)1-13.
[114]H.T.Danh,Y.E.Jeong,P.A.Kumar,H.P.Ha,Enhanced NH3-SCR activity of Sb-V/CeO2-TiO2catalyst at low temperatures by synthesis modification,Res.Chem.Intermed.42(2016)155-169.
[115]R.Jin,Y.Liu,Z.Wu,H.Wang,T.Gu,Relationship between SO2poisoning effects and reaction temperature for selective catalytic reduction of NO over Mn-Ce/TiO2catalyst,Catal.Today 153(2010)84-89.
[116]X.Gao,Y.Jiang,Y.Zhong,Z.Luo,K.Cen,The activity and characterization of CeO2-TiO2catalysts prepared by the sol-gel method for selective catalytic reduction of NO with NH3,J.Hazard.Mater.174(2010)734-739.
[117]R.Yang,H.Huang,Y.Chen,X.Zhang,H.Lu,Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NOxwith NH3,Chin.J.Catal.36(2015)1256-1262.
[118]B.Li,Z.Ren,Z.Ma,X.Huang,F.Liu,X.Zhang,H.Yang,Selective catalytic reduction of NO by NH3over CuO-CeO2in the presence of SO2,Catal.Sci.Technol.6(2016)1719-1725.
[119]M.Magnusson,E.Fridell,H.Harelind,Improved low-temperature activity for marine selective catalytic reduction systems,Proc.IMechE Part M J.Eng.Marit.Environ.230(2016)126-135.
[120]Y.Yu,J.Chen,J.Wang,Y.Chen,Performances of CuSO4/TiO2catalysts in selective catalytic reduction of NOxby NH3,Chin.J.Catal.37(2016)281-287.
[121]X.Du,X.Wang,Y.Chen,X.Gao,L.Zhang,Supported metal sulfates on Ce-TiOxas catalysts for NH3-SCR of NO:high resistances to SO2and potassium,J.Ind.Eng.Chem.36(2016)271-278.
[122]M.Kiełtyka,A.P.S.Dias,H.Kubiczek,B.Sarapata,T.Grzybek,The in fluence of poisoning on the deactivation of DeNOxcatalysts,C.R.Chim.18(2015)1036-1048.
[123]R.Ke,J.Li,X.Liang,J.Hao,Novel promoting effect of SO2on the selective catalytic reduction of NOxby ammonia over Co3O4catalyst,Catal.Commun.8(2007)2096-2099.
[124]A.Väliheikki,T.Kolli,M.Huuhtanen,T.Maunula,R.L.Keiski,Activity enhancement of W-CeZr oxide catalysts by SO2treatment in NH3-SCR,Top.Catal.58(2015)1002-1011.
[125]L.Zhang,H.Qu,T.Du,W.Ma,Q.Zhong,H2O and SO2tolerance,activity and reaction mechanism of sulfated Ni-Ce-La composite oxide nanocrystals in NH3-SCR,Chem.Eng.J.296(2016)122-131.
[126]L.Qiu,Y.Wang,D.Pang,F.Ouyang,C.Zhang,SO42--Mn-Co-Ce supported on TiO2/SiO2with high sulfur durability for low-temperature SCR of NO with NH3,Catal.Commun.78(2016)22-25.
[127]P.Wang,H.Zhao,H.Sun,H.Yu,S.Chen,X.Quan,Porous metal-organic framework MIL-100(Fe)as an efficient catalyst for the selective catalytic reduction of NOxwith NH3,RSC Adv.4(2014)48912-48919.
[128]H.Jiang,Q.Wang,H.Wang,Y.Chen,M.Zhang,MOF-74 as an efficient catalyst for the low-temperature selective catalytic reduction of NOxwith NH3,ACS Appl.Mater.Interfaces 8(2016)26817-26826.
[129]P.Wang,H.Sun,X.Quan,S.Chen,Enhanced catalytic activity over MIL-100(Fe)loaded ceria catalysts for the selective catalytic reduction of NOxwith NH3at low temperature,J.Hazard.Mater.301(2016)512-521.
 Chinese Journal of Chemical Engineering2017年12期
Chinese Journal of Chemical Engineering2017年12期
- Chinese Journal of Chemical Engineering的其它文章
- Modified molecular matrix model for predicting molecular composition of naphtha☆
- Simultaneously energy production and dairy wastewater treatment using bioelectrochemical cells:In different environmental and hydrodynamic modes
- Combination of a crude oil-degrading bacterial consortium under the guidance of strain tolerance and a pilot-scale degradation test☆
- A strong adhesive block polymer coating for antifouling of large molecular weight protein☆
- Experimental and modeling investigation of dynamic interfacial tension of asphaltenic-acidic crude oil/aqueous phase containing different ions
- Research on fault detection method for heat pump air conditioning system under cold weather☆
