锂同位素示踪大陆风化:进展与挑战
苟龙飞,金章东,贺茂勇
1. 中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061
2. 中国科学院大学,北京 100049
3. 西安交通大学 全球环境变化研究院,西安 710049
锂同位素示踪大陆风化:进展与挑战
苟龙飞1,2,金章东1,3,贺茂勇1
1. 中国科学院地球环境研究所 黄土与第四纪地质国家重点实验室,西安 710061
2. 中国科学院大学,北京 100049
3. 西安交通大学 全球环境变化研究院,西安 710049
大陆风化制约着地表物质循环及其从陆地向湖泊/海洋的迁移,并通过消耗大气CO2调节长时间尺度的全球碳循环和气候变化,因此如何有效示踪大陆风化是地球表生过程研究的重要科学问题之一。锂(Li)的两个同位素(6Li和7Li)拥有巨大相对质量差、无化合价变化,且不受氧化还原条件和生物作用影响等优势,赋予Li同位素体系具备示踪大陆风化的潜力。然而,风化体系中Li的来源和分馏的制约要素争议颇多。本文从储库、风化壳、河流体系、淋滤实验和模型模拟等方面综述了目前Li同位素示踪大陆风化的研究现状和存在的挑战。最后指出,在示踪大陆风化方面,Li同位素具有其独特的作用,建议细化岩石/矿物淋滤实验、大小流域相结合、加强多同位素体系相互补充与验证、以及计算机模型模拟,有望减少Li同位素示踪大陆风化的不确定性。
锂同位素;大陆风化;地球化学示踪;制约要素;综述
锂(Li)同位素的研究起始于McLennan and Ainslie(1922),继Taylor and Urey(1937,1938)讨论了Li同位素分馏之后,由于分析测试方法的限制,Li同位素的研究一直发展缓慢。直至20世纪80年代以来,得益于质谱技术的革新,如热电离质谱仪(TIMS)、二次离子质谱仪(SIMS)、离子探针(Ion Probe),尤其是多接收电感耦合等离子体质谱仪(MC-ICP-MS)的出现(赵葵东和蒋少涌,2001;Su et al,2015),Li同位素的测试精度已达到0.2‰左右(Huang et al,2010;Van Hoecke et al,2015;Lin et al,2016),其SIMS微区原位分析精度也达到1‰左右(李献华等,2015),致使Li同位素的研究迅速发展。目前,Li同位素已被广泛应用于示踪星云、行星的起源和演化(Seitz et al,2004,2007;Liu et al,2009)、壳-幔物质循环(Tomascak et al,2000;Magna et al,2002;Su et al,2014;Zheng et al,2015)、板块俯冲及岛弧演化(Zack et al,2003;Tian et al,2015;万红琼等,2015)、盐湖形成与演化(肖应凯等,1994;Orberger et al,2015)、成矿物质来源(Helvaci et al,2004;苏嫒娜等,2011;Dill and Weber,2013)、重建地质尺度的环境变化(Froelich and Misra,2014;Li and West,2014;Vigier et al,2015)以及行星和大陆风化(Ushikubo et al,2008;Liu and Rudnick,2011;Wang et al,2015;Fairén et al,2015)等。在示踪大陆风化作用方面,Li同位素为化学风化过程和物质循环研究注入了全新的活力(Vigier et al,2009; Misra and Froelich,2012;Dellinger et al,2015)。
由于硅酸盐岩的化学风化被认为是地质时间尺度上最重要的碳汇过程(Berner et al,1983;Kump et al,2000),而Li同位素具有揭示硅酸盐岩风化强度和通量、示踪地壳物质循环(特别是营养盐循环)的潜力(Berner and Kothavala,2001;Vigier et al,2009;Misra and Froelich,2012;Cermeno et al,2015),因此Li同位素被用来示踪岩石/矿物化学风化、全球/区域环境变化、构造运动、生物演替等过程,进而为理解大陆风化及其对构造和气候演化的响应和反馈机制提供了全新的视角。然而,随着对Li同位素示踪大陆风化研究的拓展和深入,流域Li同位素分馏控制要素颇具争议,风化壳Li同位素难以类比等问题愈加突出。为了更好地理解Li同位素在示踪大陆风化方面的优势及局限,本文综述了Li同位素在风化壳、流域风化、岩石实验淋滤、模型模拟等方面的研究进展及其面临的挑战,以期促进Li同位素在示踪大陆风化方面的进一步发展。
1 大陆岩石和风化产物Li含量及其同位素组成
1.1 自然储库中的Li同位素组成
原子序数为3的Li有6Li和7Li两个质量差约17%的稳定同位素,其同位素组成的表达式为:

目前广泛使用的Li的标准物质是美国国家标准与技术研究所合成的碳酸锂(Li2CO3),即NIST-LSVEC,其7Li /6Li = 12.1025 ± 0.0016(Chan et al,2009)。
Li具有中等不相容的性质,Li+半径为0.68 Å,与Mg2+(0.66 Å)、Al3+半径(0.51 Å)和Fe2+(0.74 Å)的半径比较接近,因此Li+可以部分替代铁镁质岩石中的Mg、Fe(刘英俊等,1984)。从基性岩到酸性岩,Li含量升高,例如辉长岩中Li含量是1.5 μg · g-1,橄榄岩中Li的含量是4 μg · g-1(Vils et al,2008),花岗岩的Li含量在6 — 40 μg · g-1(刘英俊等,1984)。不同岩性的δ7Li值变化也较明显,辉长岩中Li同位素约为4.3‰,橄榄岩中Li同位素组成约为4‰(Vils et al,2008),花岗岩的Li同位素组成约为-10‰ — 18‰(Tomascake,2004)。
Li同位素体系具备的特有示踪潜力使其发展迅速,目前已经基本获得了自然储库中Li含量及δ7Li值(图1,表1),这为Li同位素示踪大陆风化的研究奠定了基础(Tomascak,2004)。大洋中脊新鲜玄武岩(MORB)的Li含量及其同位素组成分别是3.1 — 7.5 μg · g-1和1.5‰ — 5.6‰(Chan et al,1992;Liu et al,2011)。在岩浆结晶过程中,Li优先进入流体相(Chan et al,1994;You and Chan,1996;Brenan et al,1998),导致其在地幔亏损,而在地壳富集。大陆地壳主要成分是花岗闪长质,在近地表处接近花岗岩成分(Mason and Moore,1982;Wedepohl,1995;韩呤文等,2003),由此上地壳中Li的平均含量及其同位素比值接近花岗岩,含量约为30.5 μg · g-1,δ7Li约为0.6‰(Tomascak,2004;Misra and Froelich,2012;Sauzéat et al,2015)。
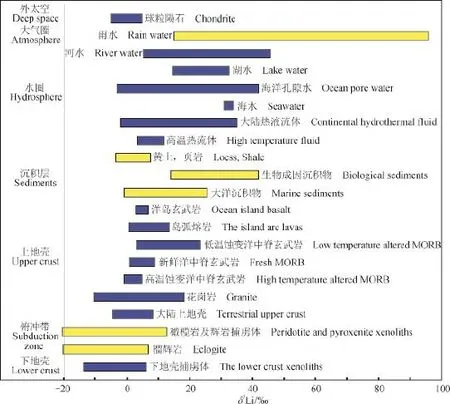
图1 自然储库中Li同位素的分布(据汤艳杰等(2009)补充)Fig.1 Lithium isotopic compositions in natural reservoirs (Modifi ed from Tang et al (2009))

表1 大陆常见物质及相关风化产物的Li含量及其同位素组成Tab.1 Lithium concentrations and isotopic compositions of fresh and weathered crust materials on the continents
1.2 风化产物的Li含量及同位素组成
由于Li是水溶性元素,因此Li将随风化淋溶作用迁移至溶液中。然而,大量研究表明在风化淋溶过程中Li仅仅发生微弱的同位素分馏,或者不发生显著的同位素分馏,Li同位素的分馏主要发生在其搬运过程中(Pistiner and Henderson,2003;Qiu et al,2009;Wimpenny et al,2010a;Verney-Carron et al,2011)。在搬运过程中,6Li优先在次生粘土矿物中富集,因此粘土物质中Li含量较高,平均约为80 μg· g-1,δ7Li值为1.6‰ — 5‰;7Li则主要随流体带入海洋,这些7Li随俯冲过程被带到下地壳/地幔,最后造成上地壳富6Li(Marschall et al,2007a,2007b)。陆源碎屑沉积物中Li平均含量为24 μg· g-1,δ7Li值则变化于0‰ — 6‰;生物成因沉积物Li含量极低,其同位素组成约为6‰ — 32‰;沉积碳酸盐岩中Li含量一般小于5 μg · g-1,其δ7Li值为-1.6‰ — 5‰(Hoefs and Sywall,1997;Chan and Hein,2007;Vils et al,2008)。新生代碳酸盐岩中的Li含量很低,约在0.2—4 μg · g-1,δ7Li值约为-41‰ — 25‰(Hoefs and Sywall,1997)。盐湖中Li含量变化范围则非常大,变化于0 —262 μg · g-1(表1),这与盐湖各自成因及不同的物质来源有关(Yu et al,2013)(表1)。
土壤中Li含量变化范围也比较大,为5 —200 μg · g-1(刘英俊等,1984),δ7Li值在-2.6‰ —14‰(Ryu et al,2014)。页岩中Li含量平均为60 μg · g-1,一些富Li矿物中Li含量可以高达1%(Meier,1982)。黄土中Li平均含量与上地壳基本一致,变化于20—35 μg · g-1,平均值约为30.5 μg · g-1,δ7Li值也较为均一,约为0.6‰(Jia et al,2007;Tsai et al,2014;Sauzéat et al,2015)。
2 Li同位素示踪大陆风化研究进展
自然界中Li一般以Li+离子形式存在(Faure and Mensing,2005),无化合价变化,氧化还原条件对其分馏没有影响。同时Li不是生命元素,生物作用对其分馏也可能没有影响(Rudnick et al,2004;Lemarchand et al,2010;Clergue et al,2015)。所有地质过程中Li同位素的最大分馏发生在大陆风化作用过程中(Rudnick et al,2004;Liu and Rudnick,2011;Henchiri et al,2014)(图1),在自然界已观察到超过110‰的分馏,约90‰的Li同位素分馏发生在地表/浅地表环境(Hoefs and Sywall,1997;Rudnick et al,2004;Yoon,2009;Millot et al,2010a)。与(浅)地表环境下约90‰的Li同位素分馏相比,直接由温度引起的Li同位素分馏则微不足道,仅为2‰(Millot et al,2010b;Wimpenny et al,2010b)。因此,拥有较大相对质量差的6Li和7Li具备示踪大陆风化作用的潜力(Chan et al,1992;Kisakürek et al,2004a;Misra and Froelich,2012;Dellinger et al,2015)。
然而,到底哪些因素影响着由化学风化导致的不同气候带河水Li含量及δ7Li值变化呢?此问题一直争议不断(Huh et al,1998,2001)。为了回答这一问题,早期研究者尝试对风化壳剖面进行剖析,试图为Li同位素示踪大陆风化提供线索。然而,由于风化壳自身成因的复杂性,结果不太理想(见下讨论)。随后,为了认识Li同位素在大陆风化过程中的行为,研究者开始对全球主要大河流和不同气候区小流域进行调查分析,并开展室内淋滤和计算机模拟,以解译大陆风化过程中Li同位素分馏的影响因素。然而,目前所获得的结论观点不一,乃至相互矛盾,致使在运用Li同位素示踪大陆风化方面依然存在诸多争议和不确定性。
2.1 风化壳中Li同位素组成变化复杂
风化壳是岩石/矿物长期风化、雨水混合、次生矿物生成、地下水淋滤运移等过程共同作用下的产物(Huh et al,2002;Pistiner and Henderson,2003;Ryu et al,2014;Verney-Carron et al,2015)。通过同一地区风化壳中的Li同位素分析发现,不同风化壳的Li含量及其同位素表现出复杂的变化,难以相互类比。例如,Huh et al(2002)研究发现,夏威夷地区玄武岩成因土壤中的Li含量是新鲜玄武岩的数倍,δ7Li值从剖面顶部到底部呈有规律的下降(从10‰降低到3‰);而Pistiner and Henderson(2003)的研究则发现该地区剖面土壤中δ7Li值与新鲜玄武岩一致(约4‰);Ryu et al(2014)的研究还发现,从剖面浅部到深部,Li元素含量逐步上升,次生矿物(尤其是高岭土)的生成造成很大的Li同位素分馏。此外,新鲜岩石初始风化时是否产生Li同位素分馏也没有定论(Pistiner and Henderson,2003;Ryu et al,2014)。尽管如此,风化壳中的Li同位素研究取得了以下共识:(1)风化壳形成过程中总伴随着外来物质的加入,这在Li含量和δ7Li值上都有反映(Huh et al,2002;Pistiner and Henderson,2003;Kisakürek et al,2004b;Liu et al,2013;Ryu et al,2014);(2)6Li易被粘土吸附,而7Li易进入流体的性质可用来示踪古地下水水位(Kisakürek et al,2004b;Rudnick et al,2004);(3)次生矿物的生成和溶解是风化壳中δ7Li值的重要制约要素之一(Lemarchand et al,2010;Liu et al,2013;Ryu et al,2014;Verney-Carron et al,2015)。
风化壳形成于一个开放的体系,其物质组成受母岩、气候、水文、生物作用、地形、形成时间、外来物质加入等要素共同制约(Ollier,1988;李德文等,2002)。因此,不同风化壳之间化学组成变化的主导因素可能各不相同,从而造成不同剖面得到不同的认识。
与其他风化壳相比,具有均匀和稳定组成的黄土可能是开展Li同位素示踪大陆风化过程的理想对象。然而,目前针对黄土Li同位素示踪风化过程的研究工作仅有一例。Tsai et al(2014)对渭南黄土-古土壤序列中的Li同位素研究发现,黄土中碎屑物质的Li含量和δ7Li值变化不大,与物源及矿物相关,且与磁化率和粒径对应较好;黄土中碳酸盐岩的Li含量和δ7Li值变化则比较大,并且其δ7Li值与化学风化指数具有较好的相关性。
2.2 水系流域Li同位素的控制要素颇具争议
水系流域中的Li元素主要来源于岩石风化、人类活动和大气降水等(Clergue et al,2015;Dillinger et al,2015;Liu et al,2015;Wang et al,2015;Pogge von Strandmann et al,2016,2017)。目前,各大陆主要流域河水和悬浮物Li同位素组成均已有数据报道(图2)。河水中Li的含量变化巨大,为0.06 — 81.2 μg· L-1,其δ7Li值变化大约在0.8‰ — 45.1‰,全球河水平均δ7Li值为23‰(Huh et al,1998;Liu and Rudnick,2011;Misra and Froelich,2012)。河流悬浮物的δ7Li组成大约在-6.8‰ — 9.5‰,总是低于各自河水中溶解态的δ7Li值。然而,由于大流域河水的Li同位素组成所受影响因素复杂,主控因素各不相同,很多问题还存在争议。近几年,更多的研究转向对小流域Li收支的系统研究,得出了一些重要的认识(Lemarchand et al,2010;Clergue et al,2015),为Li同位素示踪大陆风化注入了活力。
河水中Li的来源及河水δ7Li值的控制要素是目前运用Li同位素示踪大陆风化的争议焦点之一。以Huh等人为代表的观点认为,河水中的Li主要来源于硅酸盐岩的风化,其含量主要反映流域硅酸盐岩风化量;控制河水δ7Li值的最主要因素不是岩性,而是风化过程中原生矿物的溶解与粘土矿物的吸附引起的同位素分馏之间的平衡,因此δ7Li值反映流域硅酸盐岩的风化强度(Huh et al,1998,2001;Kisakürek et al,2005;Vigier et al,2009;Liu and Rudnick,2011;Misra and Froelich,2012;Froelich and Misra,2014;Dellinger et al,2014,2015;Pogge von Strandmann et al,2016)。与此同时,他们提出河水δ7Li值变化还与以下过程有关:(1)次生矿物的形成造成河水高的δ7Li值(Vigier et al,2009;Li and West,2014);(2)冰川作用对河水的δ7Li值影响微弱(Wimpenny et al,2010b);(3)河水的pH对河水δ7Li值有一定影响(Dellinger et al,2015);(4)气候条件可能影响Li的元素行为,具体表现为寒冷气候环境下Li吸附在矿物表面且不发生分馏,而在温暖湿润气候条件下,6Li优先进入次生矿物晶格且发生分馏(Godfrey et al,2013)。然而,更多的研究表明,受水文循环、次生矿物溶解等因素影响,河水δ7Li值与气候带关系不显著(Millot et al,2010c;Liu et al,2015;Pogge von Strandmann et al,2016)。此外,Dellinger et al(2015)提出δ7Li值变化与大陆风化之间非线性的关系。
与Huh等人的观点不同,以Pogge von Strandmann等人为代表的观点则认为,河水中Li的来源受多种因素控制,δ7Li值并不简单反映流域硅酸盐岩风化强度(Yoon,2009;罗超和郑洪波,2011;Liu et al,2011;Henchiri et al,2014;Wang et al,2015;Pogge von Strandmann and Henderson,2015,Pogge von Strandmann et al,2017)。例如:蒸发岩、火山活动带出的热液Li等均会较大程度地影响河流中的Li含量(Yoon,2009;Henchiri et al,2014;Wang et al,2015);蒸发岩则可以影响河水的δ7Li值(Yoon,2009;Wang et al,2015);悬浮物中不同粘土矿物和流体之间不同的分馏系数可能是控制某些流域河水的δ7Li值变化的主因(Yoon,2009;Wang et al, 2015;Pogge von Strandmann et al,2017)。由此,Pogge von Strandmann and Henderson(2015)提出了与Huh等截然相反的观点,认为河水δ7Li值的变化反映的是冲积平原的形成及更多次生矿物的生成,而非化学风化强度。
那么,硅酸盐岩风化是不是控制河水Li同位素变化的主因呢?面对大河体系中Li含量及同位素组成的不确定性和多解性,近年来研究者转而开始研究小尺度水系流域(Catchment)中的Li收支。因为小尺度水系流域具有单一或稳定的岩性、相近的气候,这一研究思路展现出解决争议的巨大潜力,并已成为大陆风化研究的热点。通过小流域地球关键带(Earth’s critical zone)的研究发现,在流域尺度上,Li同位素没有生命效应的重要结论(Lemarchand et al,2010;Clergue et al,2015;Pogge von Strandmann et al,2016)。Li收支定量研究结果则显示,降雨、大气尘降对小流域Li收支有影响,其中对安山岩流域影响很大(Clergue et al,2015),而对花岗岩质流域影响则很小(Lemarchand et al,2010)。这可能主要是由于不同类型岩石Li含量之间数量级差别的“本底”效应造成的。目前,众多针对小流域的Li同位素研究工作正在进行中。
结合对大/小尺度水系流域中的Li同位素研究,大流域河水的Li含量及同位素组成的差异是各种制约要素的共同结果(Misra and Froelich,2012;Wang et al,2015)。为了确定全球尺度上不同制约要素之间的关系及其贡献大小,有必要开展更多小尺度流域水系中Li含量及其同位素组成的时空变化。开展小流域内高分辨率季节性河水化学与Li同位素结合的研究,或成为解决制约Li同位素变化控制要素争议的一个有效途径。
2.3 岩石淋滤实验尚无定论
Li同位素分馏的室内淋滤实验主要采用一些代表性的岩浆岩开展,但尚未获得一致的认识。例如:有的实验结果显示,基性岩石在酸性非平衡条件下淋滤时,没有发生Li同位素分馏(Wimpenny et al,2010a),而在相同条件下酸性岩石淋滤时则发生Li同位素分馏(Pistiner and Henderson,2003;Wunder et al,2005;Millot et al,2010b)。在近平衡条件下中性岩石的溶解实验中,次生矿物形成时Li的类质同象被认为是造成Li同位素分馏的最主要因素(Vigier et al,2008;Wimpenny et al,2010a;Millot et al,2010b)。由于Li是中等不相容性元素,Li在各类型岩石中不同的赋存状态可能是造成观察到上述现象的主要因素。在岩浆分异结晶过程中,Li元素的不相容性使酸性岩具有高的Li含量。重要的是,与基性岩中Li以类质同象方式占据在稳定的晶格位置不同,酸性岩中高Li含量可能使矿物晶体处于高能态,酸性岩发生风化时Li优先被淋滤,而7Li与水优先结合可使水分子处于低能态,从而发生Li同位素分馏(Huh et al,2001);相反,基性岩的淋滤可能是矿物整体溶解的过程,在没有次生矿物形成的情况下,不发生同位素分馏。

图2 已报道的全球主要河流溶解态和悬浮物中δ7Li值和Li质量分数的变化范围Fig.2 Concentrations and isotopic compositions of lithium reported in dissolved and suspended phases in global major rivers
此外,Li吸附到矿物表面是否引起Li同位素分馏也还没有定论,有实验发现吸附会影响Li的分馏,且分馏程度与所吸附的矿物类型有关(Pistiner and Henderson,2003),有些则认为该过程不发生分馏(罗超和郑洪波,2011;Wimpenny et al,2015)。同时,相对于巨大的动力学过程引起的Li同位素分馏,低温条件下(地表环境)扩散所致的平衡分馏效应基本上可忽略(Verney-Carron et al,2011)。
2.4 模型模拟尚处摸索阶段
现代计算机的强大计算功能为定量解决地学问题提供了新的渠道。上述岩石矿物Li同位素淋滤实验获得的基础数据为计算机模拟Li同位素体系创造了条件。目前有关Li同位素分馏的模拟结果并不多,主要提出了多种控制河水Li同位素组成变化要素的组合,例如气候对河水中的Li通量的作用(Vigier and Goddéris,2015)、次生矿物的溶解(Bouchez et al,2013)、粘土和土壤对河水Li的固定(Bouchez et al,2013;Li and West,2014;Wanner et al,2014)等因素分别被不同研究者认为对水体δ7Li值影响很大。由于上述制约Li同位素分馏的不平衡、非线性复杂过程,模型模拟的计算结果由初始状态和边界条件决定,因此实验淋滤结果的好坏直接决定模拟结论的正确与否,反之模拟结论是否合理又需要水系流域、风化壳的研究来佐证。应该看到,模型模拟拓展了Li同位素示踪大陆风化的研究手段,但是室内淋滤数据依然十分有限,这可能造成模拟结果一定的“蝴蝶效应”。
3 问题与挑战
上述可知,对Li同位素示踪大陆风化的研究已取得一些重要成果,在风化壳、水系流域、实验室模拟等方面均取得了重要进展,这些进展极大地促进了对Li同位素在表生地球化学行为的认识以及其示踪大陆风化的可行性。事实上,对Li同位素体系示踪大陆风化的研究始于检验“构造抬升-化学风化-气候变化”假说。该假说认为构造隆升造成大陆岩石风化加强进而导致了晚新生代气候变冷。此前Sr、Nd、Os等多个同位素体系参与验证该假说,但因各自的局限性最终未能获得理想的结果。与这几个同位素体系类似,Li同位素体系也面临着如何定量评估各影响因素,进而示踪大陆硅酸盐岩风化的困境。然而,Li同位素巨大的分馏远比原始风化物质组成范围大得多,只有风化过程才会引起这么大的分馏(Lemarchand et al,2010;Misra and Froelich,2012;Sauzeat et al,2015)。因此,若不细分风化过程中各具体影响因素,那么Li同位素分馏在一定程度上应该可以反映化学风化的变化。基于以上事实,Li同位素的确加深了人们对大陆风化的理解,但是要使Li同位素体系更有效地示踪大陆风化,需要面对以下三大挑战:
(1)如何扣除深部热液的影响。深部热液具有高Li含量,由火山、地震等构造运动带入地表体系的深部热液可能成为Li同位素体系示踪硅酸盐岩风化的一个干扰项。因此,在一些构造活跃的高山流域,在运用Li同位素示踪硅酸盐岩风化强度时,需考虑深部热液的Li贡献及其同位素组成。
(2)如何量化风化过程中的Li同位素分馏的各个控制要素。目前已经认识到的制约Li同位素分馏的要素包括侵蚀、溶解、吸附、次生矿物的形成、pH等(Huh et al,1998,2001;Kisakürek et al,2005;Vigier et al,2009;Liu and Rudnick,2011;Dellinger et al,2014,2015;Froelich and Misra,2014),这些因素对δ7Li值的贡献大小还不能有效量化。由于形成演化过程和形成时间不同,风化壳Li同位素特征难以进行类比,因此风化壳剖面的研究恐难以量化Li同位素分馏的控制要素。相反,对单一岩性流域的连续监测,有望缩小制约要素,进而减少Li同位素示踪风化的不确定性。
(3)如何量化人为活动对Li同位素分馏的贡献。6Li作为重要的化工、电源和核聚变原料(贾小波等,2007;Arikawa et al,2010),目前已有多种人工方法富集分离6Li(Tatenuma et al,2001),使δ7Li值超过自然分馏的百倍以上(Qi et al,1997)。因此,尽管对有些流域尚未构成影响(Négrel et al,2010;Wang et al,2015),但如何量化人类活动对水体δ7Li值的贡献成为Li同位素示踪硅酸盐岩风化需面对的又一大难题(Qi et al,1997;Négrel et al,2010)。
4 研究展望
地表环境下Li的地球化学行为使其同位素在示踪大陆风化方面取得了一些新的认识和进展,同时也面临着诸多挑战。Li同位素体系能否作为大陆风化的示踪指标,还需要加强以下四个方面的研究:
(1)细化岩石/矿物淋滤实验:通过实验室控制实验条件,从单一变量着手,系统评价不同要素贡献,为Li同位素体系示踪大陆风化提供可靠依据;
(2)大小流域相结合:对比研究单一岩性、相同气候条件下、没有人为输入的小流域内Li收支及其同位素行为,结合大流域Li同位素的宏观特征,将进一步明确Li同位素来源和分馏的制约要素。鉴于黄土具有相对均匀的、可代表上地壳的地球化学成分,查明黄土现代风化过程中Li同位素行为有望对认识Li同位素示踪整体大陆风化产生不可替代的推动作用,但这项工作目前尚未引起足够重视;
(3)多同位素体系相互补充与验证:地表风化作用是复杂的,Li同位素获得的认识可能只是风化过程的某一侧面,存在不确定性或多解性,因此需要与其他同位素相互补充与验证,以更全面地认识化学风化过程及其制约要素;
(4)加强计算机模型模拟研究:目前计算机模型模拟Li同位素体系的研究尚处于起步阶段,所得认识还较为有限,但计算机模拟将最终为Li同位素示踪大陆风化的定量研究提供最有益的帮助。
致谢:特别感谢中国科学院地球环境研究所肖军、张飞、邓丽和中国科学技术大学孙贺在论文写作过程中的有益讨论。
韩呤文, 马振东, 张宏飞, 等. 2003. 地球化学[M]. 北京: 地质出版社: 30 – 53. [Han Y W, Ma Z D, Zhang H F, et al. 2003. Geochemistry [M]. Beijing: Geological Publishing House: 30 – 53.]
贾小波, 杨永伟, 周志伟, 等. 2007. 聚变堆氦冷固态包层结构和6Li富集度对产氚率的影响[J]. 原子能科学技术, 41(3): 335 – 338. [Jia X B, Yang Y W, Zhou Z W, et al. 2007. Influence of helium cooled solid blanket structure and6Li enrichment on tritium breeding ratio in fusion reactor [J]. Atomic Energy Science and Technology, 41(3): 335 – 338.]
李德文, 崔之久, 刘耕年. 2002. 风化壳研究的现状与展望[J]. 地球学报, 23(3): 283 – 288. [Li D W, Cui Z J, Liu G N. 2002. Present situation and prospects of researches on weathering crust [J]. Acta Geoscientia Sinica, 23(3): 283 – 288.]
李献华, 刘 宇, 汤艳杰, 等. 2015. 离子探针Li同位素微区原位分析技术与应用[J]. 地学前缘, 22(5): 160 – 170. [Li X H, Liu Y, Tang Y J, et al. 2015. In situ Li isotopic miscroanalysis using SIMS and its applications [J]. Earth Science Frontiers, 22(5): 160 – 170.]
刘英俊, 曹励明, 李兆麟, 等. 1984. 元素地球化学[M]. 北京:科学出版社: 125 – 136. [Liu Y J, Cao L M, Li Z L, et al. 1984. Element geochemistry [M]. Beijing: Science Press: 125 – 136.]
罗 超, 郑洪波. 2011. 锂同位素作为硅酸盐风化强度的指标——关于其可行性的讨论[J]. 矿物岩石地球化学通报, 30(S1): 505. [Luo C, Zheng H B. 2011. Lithium isotope as a proxy of silicate weathering intensity—discussion on its feasibility [J]. Bulletin of Mineralogy, Petrology and Geochemistry, 30(S1): 505.]
苏嫒娜, 田世洪, 侯增谦, 等. 2011. 锂同位素及其在四川甲基卡伟晶岩型锂多金属矿床研究中的应用[J]. 现代地质, 25(2): 236 – 242. [Su A N, Tian S H, Hou Z Q, et al. 2011. Lithium isotope and its application to Jiajika pegmatite type lithium polymetallic deposit in Sichuan [J]. Geoscience, 25(2): 236 – 242.]
汤艳杰, 张宏福, 英基丰. 2009. 锂同位素分馏机制讨论[J].
地球科学(中国地质大学学报), 34(1): 43 – 55. [Tang Y J, Zhang H F, Ying J F. 2009. Discussion on fractionation mechanism of lithium isotopes [J]. Earth Science (Journal of China University of Geosciences), 34(1): 43 – 55.]
万红琼, 孙 贺, 刘海洋, 等. 2015. 俯冲带Li同位素地球化学: 回顾与展望[J]. 地学前缘, 22(5): 29 – 43. [Wan H Q, Sun H, Liu H Y, et al. 2015. Lithium isotopic geochemistry in subduction zones: Retrospects and prospects [J]. Earth Science Frontiers, 22(5): 29 – 43.]
肖应凯, 祁海平, 王蕴慧, 等. 1994. 青海大柴达木湖卤水、沉积物和水源水中的锂同位素组成[J]. 地球化学, 23(4): 329 – 338. [Xiao Y K, Qi H P, Wang Y H, et al. 1994. Lithium isotopic compositions of brine sediments and source water in Da Qaidam Lake, Qinghai China [J]. Geochemica, 23(4): 329 – 338.]
赵葵东, 蒋少涌. 2001. Li同位素研究进展及其地质应用[J].高校地质学报, 7(4): 390 – 398. [Zhao K D, Jiang S Y. 2001. Recent advances in research on lithium isotopes and its geological applications [J]. Geological Journal of China Universities, 7(4): 390 – 398.]
Arikawa Y, Yamanoi K, Nagai T, et al. 2010. Note: Light output enhanced fast response and low afterglow6Li glass scintillator as potential down-scattered neutron diagnostics for inertial confinement fusion [J]. Review of Scientific Instruments, 81(10): 3.
Berner R A, Kothavala Z. 2001. Geocarb Ⅲ: A revised model of atmospheric CO2over Phanerozoic time [J]. American Journal of Science, 301(2): 182 – 204.
Berner R A, Lasaga A C, Garrels R M. 1983. The carbonatesilicate geochemical cycle and its effect on atmospheric carbon-dioxide over the past 100 million years [J]. American Journal of Science, 283(7): 641 – 683.
Bouchez J, von Blanckenburg F, Schuessler J A. 2013. Modeling novel stable isotope ratios in the weathering zone [J]. American Journal of Science, 313(4): 267 – 308.
Brenan J M, Neroda E, Lundstrom C C, et al. 1998. Behaviour of boron, beryllium, and lithium during melting and crystallization: Constraints from mineral-melt partitioning experiments [J]. Geochimica et Cosmochimica Acta, 62(12): 2129 – 2141.
Cermeno P, Falkowski P G, Romero O E, et al. 2015. Continental erosion and the Cenozoic rise of marine diatoms [J]. Proceedings of the National Academy of Sciences of the United States of America, 112(14): 4239 – 4244.
Chan L H, Edmond J M, Thompson G, et al. 1992. Lithium isotopic composition of submarine basalts—Implications for the lithium cycle in the oceans [J]. Earth and Planetary Science Letters, 108(1/ 2 /3): 151 – 160.
Chan L H, Gieskes J M, You C F, et al. 1994. Lithium isotope geochemistry of sediments and hydrothermal fl uids of the Guaymas Basin, Gulf of California [J]. Geochimica et Cosmochimica Acta, 58(20): 4443 – 4454.
Chan L H, Hein J R. 2007. Lithium contents and isotopic compositions of ferromanganese deposits from the global ocean [J]. Deep Sea Research Part Ⅱ-Topical Studies in Oceanography, 54(11/ 12/ 13): 1147 – 1162.
Chan L H, Lassiter J C, Hauri E H, et al. 2009. Lithium isotope systematics of lavas from the Cook-Austral Islands: Constraints on the origin of HIMU mantle [J]. Earth and Planetary Science Letters, 277(3/4): 433 – 442.
Clergue C, Dellinger M, Buss H L, et al. 2015. Influence of atmospheric deposits and secondary minerals on Li isotopes budget in a highly weathered catchment, Guadeloupe (Lesser Antilles) [J]. Chemical Geology, 414: 28 – 41.
Dellinger M, Gaillardet J, Bouchez J, et al. 2014. Lithium isotopes in large rivers reveal the cannibalistic nature of modern continental weathering and erosion [J]. Earth and Planetary Science Letters, 401: 359 – 372.
Dellinger M, Gaillardet J, Bouchez J, et al. 2015. Riverine Li isotope fractionation in the Amazon River basin controlled by the weathering regimes [J]. Geochimica et Cosmochimica Acta, 164: 71 – 93.
Dill H G, Weber B. 2013. Gemstones and geosciences in space and time: Digital maps to the “Chessboard classification scheme of mineral deposits” [J]. Earth-Science Reviews, 127: 262 – 299.
Fairén A G, Losa-Adams E, Gil-Lozano C, et al. 2015. Tracking the weathering of basalts on Mars using lithium isotope fractionation models [J]. Geochemistry Geophysics Geosystems, 16(4): 1172 – 1197.
Faure G, Mensing T M. 2005. Isotopes: principles and applications (third edition) [M]. New Jersey: John Wiley & Sons: 859 – 863.
Froelich F, Misra S. 2014. Was the late Paleocene-early Eocene hot because earth was fl at? An ocean lithium isotope view of mountain building, continental weathering, carbon dioxide, and earth's Cenozoic climate [J]. Oceanography, 27(1): 36 – 49.
Godfrey L V, Chan L H, Alonso R N, et al. 2013. The role of climate in the accumulation of lithium-rich brine in the central Andes [J]. Applied Geochemistry, 38: 92 – 102.
Helvaci C, Mordogan H, Colak M, et al. 2004. Presence and distribution of lithium in borate deposits and some recent lake waters of west-central Turkey [J]. International Geology Review, 46(2): 177 – 190.
Henchiri S, Clergue C, Dellinger M, et al. 2014. The infl uence of hydrothermal activity on the Li isotopic signature ofrivers draining volcanic areas [J]. Procedia Earth and Planetary Science, 10: 223 – 230.
Hoefs J, Sywall M. 1997. Lithium isotope composition of Quaternary and Tertiary biogene carbonates and a global lithium isotope balance [J]. Geochimica et Cosmochimica Acta, 61(13): 2679 – 2690.
Huang K F, You C F, Liu Y H, et al. 2010. Low-memory, small sample size, accurate and high-precision determinations of lithium isotopic ratios in natural materials by MC-ICPMS [J]. Journal of Analytical Atomic Spectrometry, 25(7): 1019 – 1024.
Huh Y, Chan L H, Chadwick O. 2002. Lithium isotopes as a probe of weathering processes: Hawaiian soil climosequence [J]. Geochimica et Cosmochimica Acta, 66(15A): A346.
Huh Y, Chan L H, Edmond J M. 2001. Lithium isotopes as a probe of weathering processes: Orinoco River [J]. Earth and Planetary Science Letters, 194(1/2): 189 – 199.
Huh Y, Chan L H, Zhang L, et al. 1998. Lithium and its isotopes in major world rivers: Implications for weathering and the oceanic budget [J]. Geochimica et Cosmochimica Acta, 62(12): 2039 – 2051.
Jia Y F, Huang C C, Pang J L, et al. 2007. Variation of the lithium-barium ratio in the Holocene loess-paleosol profiles in the south of the Chinese Loess Plateau: Implications for pedogenic weathering intensity [J]. Soil Science, 172(11): 925 – 940.
Kisakürek B, James R H, Harris N B W, 2004a. Utility of lithium isotopes as tracers of continental weathering processes [J]. Geochimica et Cosmochimica Acta, 68(11): A420.
Kisakürek B, James R H, Harris N B W. 2005. Li and δ7Li in Himalayan rivers: Proxies for silicate weathering? [J]. Earth and Planetary Science Letters, 237(3 / 4): 387 – 401. Kisakürek B, Widdowson M, James R H. 2004b. Behaviour of Li isotopes during continental weathering: the Bidar laterite profile, India [J]. Chemical Geology, 212(1/2): 27 – 44.
Kump L R, Brantley S L, Arthur M A. 2000. Chemical weathering, atmospheric CO2, and climate [J]. Annual Review of Earth and Planetary Sciences, 28: 611 – 667.
Lemarchand E, Chabaux F, Vigier N, et al. 2010. Lithium isotope systematics in a forested granitic catchment (Strengbach, Vosges Mountains, France) [J]. Geochimica et Cosmochimica Acta, 74(16): 4612 – 4628.
Li G J, West A J. 2014. Evolution of Cenozoic seawater lithium isotopes: Coupling of global denudation regime and shifting seawater sinks [J]. Earth and Planetary Science Letters, 401: 284 – 293.
Lin J, Liu Y, Hu Z, et al. 2016. Accurate determination of lithium isotope ratios by MC-ICP-MS without strict matrixmatching by using a novel washing method [J]. Journal of Analytical Atomic Spectrometry, 31(2): 390 – 397.
Liu C Q, Zhao Z Q, Wang Q L, et al. 2011. Isotope compositions of dissolved lithium in the rivers Jinshajiang, Lancangjiang, and Nujiang: Implications for weathering in Qinghai-Tibet Plateau [J]. Applied Geochemistry, 26: S357 – S359.
Liu M C, McKeegan K D, Goswami J N, et al. 2009. Isotopic records in CM hibonites: Implications for timescales of mixing of isotope reservoirs in the solar nebula [J]. Geochimica et Cosmochimica Acta, 73(17): 5051 – 5079.
Liu X M, Rudnick R L, McDonough W F, et al. 2013. Influence of chemical weathering on the composition of the continental crust: Insights from Li and Nd isotopes in bauxite profi les developed on Columbia river basalts [J]. Geochimica et Cosmochimica Acta, 115: 73 – 91.
Liu X M, Wanner C, Rudnick R L, et al. 2015. Processes controlling δ7Li in rivers illuminated by study of streams and groundwaters draining basalts [J]. Earth and Planetary Science Letters, 409: 212 – 224.
Liu X M, Rudnick R L. 2011. Constraints on continental crustal mass loss via chemical weathering using lithium and its isotopes [J]. Proceedings of the National Academy of Sciences of the United States of America, 108(52): 20873 – 20880.
Magna T, Wiechert U, Halliday A N. 2002. Lithium isotopes and crust-mantle interaction [J]. Geochimica et Cosmochimica Acta, 66(15A): A474.
Marschall H R, Pogge von Strandmann P A E, Seitz H M, et al. 2007a. Heavy lithium in subducted slabs [J]. Geochimica et Cosmochimica Acta, 71(15): A625.
Marschall H R, Pogge von Strandmann P A E, Seitz H M, et al. 2007b. The lithium isotopic composition of orogeniceclogites and deep subducted slabs [J]. Earth and Planetary Science Letters, 262(3 / 4): 563 – 580.
Mason B, Moore C B. 1982. Principles of geochemistry (fourth edition) [M]. New York: John Wiley & Sons: 1 – 344.
McLennan J C, Ainslie D S. 1922. On the structure of the line λ= 6708 Å. of the isotopes of lithium [J]. Proceedings of the Royal Society of London Series A, 101(711): 342 – 348. Meier A L. 1982. Determination of lithium isotopes at natural abundance levels by atomic-absorption spectrometry [J]. Analytical Chemistry, 54(13): 2158 – 2161.
Millot R, Petelet-Giraud E, Guerrot C, et al. 2010a. Multiisotopic composition (δ7Li-δ11B-δD-δ18O) of rainwaters in France: Origin and spatio-temporal characterization [J]. Applied Geochemistry, 25(10): 1510 – 1524.
Millot R, Scaillet B, Sanjuan B. 2010b. Lithium isotopes in island arc geothermal systems: Guadeloupe, Martinique (French West Indies) and experimental approach [J]. Geochimica et Cosmochimica Acta, 74(6): 1852 – 1871.
Millot R, Vigier N, Gaillardet J. 2010c. Behaviour of lithium and its isotopes during weathering in the Mackenzie Basin, Canada [J]. Geochimica et Cosmochimica Acta, 74(14): 3897 – 3912.
Misra S, Froelich F. 2012. Lithium isotope history of Cenozoic seawater: Changes in silicate weathering and reverse weathering [J]. Science, 335(6070): 818 – 823.
Négrel P, Millot R, Brenot A, et al. 2010. Lithium isotopes as tracers of groundwater circulation in a peat land [J]. Chemical Geology, 276(1/2): 119 – 127.
Ollier C D. 1988. Deep weathering, groundwater and climate [J]. Geografiska Annaler Series A, Physical Geography, 70(4): 285 – 290.
Orberger B, Rojas W, Millot R, et al. 2015. Stable isotopes (Li, O, H) combined with brine chemistry: powerful tracers for Li origins in salar deposits from the Puna region, Argentina [J]. Procedia Earth and Planetary Science, 13: 307 – 311.
Pistiner J S, Henderson G M. 2003. Lithium-isotope fractionation during continental weathering processes [J]. Earth and Planetary Science Letters, 214(1/2): 327 – 339.
Pogge von Strandmann P A E, Burton K W, James R H, et al. 2010. Assessing the role of climate on uranium and lithium isotope behaviour in rivers draining a basaltic terrain [J]. Chemical Geology, 270: 227 – 239.
Pogge von Strandmann P A E, Burton K W, Opfergelt S, et al. 2016. The effect of hydrothermal spring weathering processes and primary productivity on lithium isotopes: Lake Myvatn, Iceland [J]. Chemical Geology, 445: 4 – 13. Pogge von Strandmann P A E, Frings P J, Murphy M J. 2017. Lithium isotope behaviour during weathering in the Ganges Alluvial Plain [J]. Geochimica et Cosmochimica Acta, 198: 17 – 31.
Pogge von Strandmann P A E, Henderson G M. 2015. The Li isotope response to mountain uplift [J]. Geology, 43(1): 67 – 70.
Pogge von Strandmann P A E, Reynolds B C, Porcelli D, et al. 2006. Assessing continental weathering rates and actinide transport in the Great Artesian Basin [J]. Geochimica et Cosmochimica Acta, 70(18): A497.
Qi H P, Coplen T B, Wang Q Z, et al. 1997. Unnatural isotopic composition of lithium reagents [J]. Analytical Chemistry, 69(19): 4076 – 4078.
Qiu L, Rudnick R L, McDonough W F, et al. 2009. Li and δ7Li in mudrocks from the British Caledonides: Metamorphism and source influences [J]. Geochimica et Cosmochimica Acta, 73(24): 7325 – 7340.
Rudnick R L, Tomascak P B, Njo H B, et al. 2004. Extreme lithium isotopic fractionation during continental weathering revealed in saprolites from South Carolina [J]. Chemical Geology, 212(1/2): 45 – 57.
Ryu J S, Vigier N, Lee S W, et al. 2014. Variation of lithium isotope geochemistry during basalt weathering and secondary mineral transformations in Hawaii [J]. Geochimica et Cosmochimica Acta, 145: 103 – 115.
Sauzéat L, Rudnick R L, Chauvel C, et al. 2015. New perspectives on the Li isotopic composition of the upper continental crust and its weathering signature [J]. Earth and Planetary Science Letters, 428: 181 – 192.
Seitz H M, Brey G P, Lahaye Y, et al. 2004. Lithium isotopic signatures of peridotite xenoliths and isotopic fractionation at high temperature between olivine and pyroxenes [J]. Chemical Geology, 212(1 /2): 163 – 177.
Seitz H M, Brey G P, Zipfel J, et al. 2007. Lithium isotope composition of ordinary and carbonaceous chondrites,and differentiated planetary bodies: Bulk solar system and solar reservoirs [J]. Earth and Planetary Science Letters, 260(3/4): 582 – 596.
Su B X, Zhang H F, Deloule E, et al. 2014. Lithium elemental and isotopic variations in rock-melt interaction [J]. Chemie der Erde-Geochemistry, 74(4): 705 – 713.
Su B X, Gu X Y, Deloule E, et al. 2015. Potential orthopyroxene, clinopyroxene and olivine reference materials for in situ lithium isotope determination [J]. Geostandards and Geoanalytical Research, 39(3): 357 – 369.
Tatenuma K, Kato E, Nanjo Y, et al. 2001. Lithium isotope separation method for nuclear fusion reactors, involves applying electric field to lithium ion conductor and separating lithium isotope based on difference between ion conductivities of isotopes [P]. Japan Atomic Energy Research Institute (JAAT-C), EP1186337-A2.
Taylor T I, Urey H C. 1937. On the electrolytic and chemical exchange methods for the separation of the lithium isotopes [J]. Journal of Chemical Physics, 5(7): 597 – 598. Taylor T I, Urey H C. 1938. Fractionation of the lithium and potassium isotopes by chemical exchange with zeolites [J]. Journal of Chemical Physics, 6(8): 429 – 438.
Tian S H, Hou Z Q, Su A N, et al. 2015. The anomalous lithium isotopic signature of Himalayan collisional zone carbonatites in western Sichuan, SW China: Enriched mantle source and petrogenesis [J]. Geochimica et Cosmochimica Acta, 159: 42 – 60.
Tomascak P B. 2004. Developments in the understanding and application of lithium isotopes in the Earth and planetary sciences [J]. Reviews in Mineralogy & Geochemistry, 55: 153 – 195.
Tomascak P B, Ryan J G, Defant M J. 2000. Lithium isotope evidence for light element decoupling in the Panama subarc mantle [J]. Geology, 28(6): 507 – 510.
Tsai P H, You C F, Huang K F, et al. 2014. Lithium distribution and isotopic fractionation during chemical weathering and soil formation in a loess profi le [J]. Journal of Asian Earth Sciences, 87: 1 – 10.
Ushikubo T, Kita N T, Cavosie A J, et al. 2008. Lithium in Jack Hills zircons: Evidence for extensive weathering of Earth’s earliest crust [J]. Earth and Planetary Science Letters, 272 (3 / 4): 666 – 676.
Van Hoecke K, Belza J, Croymans T, et al. 2015. Singlestep chromatographic isolation of lithium from wholerock carbonate and clay for isotopic analysis with multicollector ICP-mass spectrometry [J]. Journal of Analytical Atomic Spectrometry, 30(12): 2533 – 2540.
Verney-Carron A, Vigier N, Millot R. 2011. Experimental determination of the role of diffusion on Li isotope fractionation during basaltic glass weathering [J]. Geochimica et Cosmochimica Acta, 75(12): 3452 – 3468.
Verney-Carron A, Vigier N, Millot R, et al. 2015. Lithium isotopes in hydrothermally altered basalts from Hengill (SW Iceland) [J]. Earth and Planetary Science Letters, 411: 62 – 71.
Vigier N, Decarreau A, Millot R, et al. 2008. Quantifying Li isotope fractionation during smectite formation and implications for the Li cycle [J]. Geochimica et Cosmochimica Acta, 72(3): 780 – 792.
Vigier N, Gislason S R, Burton K W, et al. 2009. The relationship between riverine lithium isotope composition and silicate weathering rates in Iceland [J]. Earth and Planetary Science Letters, 287(3/4): 434 – 441.
Vigier N, Goddéris Y. 2015. A new approach for modeling Cenozoic oceanic lithium isotope paleo-variations: The key role of climate [J]. Climate of the Past, 11(4): 635 – 645.
Vigier N, Rollion-Bard C, Levenson Y, et al. 2015. Lithium isotopes in foraminifera shells as a novel proxy for the ocean dissolved inorganic carbon (DIC) [J]. Comptes Rendus Geoscience, 347(1): 43 – 51.
Vils F, Pelletier L, Kalt A, et al. 2008. The Lithium, Boron and Beryllium content of serpentinized peridotites from ODP Leg 209 (Sites 1272A and 1274A): Implications for lithium and boron budgets of oceanic lithosphere [J]. Geochimica et Cosmochimica Acta, 72(22): 5475 – 5504.
Wang Q L, Chetelat B, Zhao Z Q, et al. 2015. Behavior of lithium isotopes in the Changjiang River system: Sources effects and response to weathering and erosion [J]. Geochimica et Cosmochimica Acta, 151: 117 – 132.
Wanner C, Sonnenthal E L, Liu X M. 2014. Seawater δ7Li: A direct proxy for global CO2consumption by continental silicate weathering? [J]. Chemical Geology, 381:154 – 167.
Wedepohl K H. 1995. The composition of the continental crust [J]. Geochimica et Cosmochimica Acta, 59(7): 1217 – 1232.
Wimpenny J, Colla C A, Yu P, et al. 2015. Lithium isotope fractionation during uptake by gibbsite [J]. Geochimica et Cosmochimica Acta, 168: 133 – 150.
Wimpenny J, Gíslason S R, James R H, et al. 2010a. The behaviour of Li and Mg isotopes during primary phase dissolution and secondary mineral formation in basalt [J]. Geochimica et Cosmochimica Acta, 74(18): 5259 – 5279.
Wimpenny J, James R H, Burton K W, et al. 2010b. Glacial effects on weathering processes: New insights from the elemental and lithium isotopic composition of West Greenland rivers [J]. Earth and Planetary Science Letters, 290(3/4): 427 – 437.
Wunder B, Meixner A, Romer R L, et al. 2005. Temperaturedependent isotopic fractionation of lithium between clinopyroxene and high-pressure hydrous fluids [J]. Contributions to Mineralogy and Petrology, 151(1): 112 – 120.
Yoon J. 2009. Lithium as a silicate weathering proxy: Problems and perspectives [J]. Aquatic Geochemistry, 16(1): 189 – 206.
You C F, Chan L H. 1996. Precise determination of lithium isotopic composition in low concentration natural samples [J]. Geochimica et Cosmochimica Acta, 60(5): 909 – 915.
Yu J Q, Gao C L, Cheng A Y, et al. 2013. Geomorphic, hydroclimatic and hydrothermal controls on the formation of lithium brine deposits in the Qaidam Basin, northern Tibetan Plateau, China [J]. Ore Geology Reviews, 50: 171 – 183.
Zack T, Tomascak P B, Rudnick R L, et al. 2003. Extremely light Li in orogenic eclogites: The role of isotope fractionation during dehydration in subducted oceanic crust [J]. Earth and Planetary Science Letters, 208(3/4): 279 – 290.
Zheng J P, Lee C T A, Lu J G, et al. 2015. Refertilizationdriven destabilization of subcontinental mantle and the importance of initial lithospheric thickness for the fate of continents [J]. Earth and Planetary Science Letters, 409: 225 – 231.
Using lithium isotopes traces continental weathering: Progresses and challenges
GOU Longfei1,2, JIN Zhangdong1,3, HE Maoyong1
1. State Key Laboratory of Loess and Quaternary Geology, Institute of Earth Environment, Chinese Academy of Sciences, Xi’an 710061, China
2. University of Chinese Academy of Sciences, Beijing 100049, China
3. Institute of Global Environmental Change, Xi’an Jiaotong University, Xi’an 710049, China
Background, aim, and scope Since weathering processes constrain the cycle of elements and their transportation from the continents to the lake/ocean reservoirs, shape the topography of continents, and regulate global carbon cycle and therefore climate changes by consuming atmospheric carbon dioxide over geological time-scales, how to effectively trace continental weathering processes is one of key scientifi c topics of supergene geochemistry. Being one of the most promising silicate-weathering tracers, lithium (Li) isotopes have long been exploded to trace continental weathering and thus to reconstruct secular weathering scenarios. To acquire effective research methodology, review of establishing Li isotopes to be a silicate-weathering tracer is thus essential. Materials and methods This paper reviewed current major developments, problems and challenges of using Li isotopes to trace weathering with respects to its major reservoirs, weathered crust, riverine systems,leaching experiments, and modeling simulation which have progressively developed in the past decades. Results Owing to large mass difference between6Li and7Li, one valence, and no effect of redox conditions and living beings, if not all, at least at catchment scale, Li isotopes own potential to trace continental weathering processes. However, its sources and controlling factors of Li fractionation during weathering processes are controversial. Discussion Changes in Li isotopic compositions from weathered crust to fresh bedrocks are complex and even reverse, whereas controlling factors for Li isotopic variations in riverine system are various and controversial, in particular for large river systems. Leaching experiments and modeling simulation for Li isotopic compositions are at an early stage. More attentions are focused on seasonal variation in riverine Li isotopic compositions at small, monolithological catchment. Conclusions We pointed out that Li isotope system does have its potential and uniqueness in tracing continental weathering, especially for silicate weathering. Recommendations and perspectives In order to reduce the uncertainty of tracing silicate weathering processes using Li isotopes, elaborating leaching experiments of rocks/minerals, marrying large and small catchments, enhancing computation simulation, and coupling of multi-isotopes are suggested to be needed in future.
Li isotopes; continental weathering; geochemical tracing; controlling factors; review
JIN Zhangdong, E-mail: zhdjin@ieecas.cn
2016-11-15;录用日期:2017-02-14
Received Date: 2016-11-15; Accepted Date: 2017-02-14
国家自然科学基金项目(41225015);国家重点基础研究发展计划项目(2013CB956402)
Foundation Item: National Natural Science Foundation of China (41225015); National Basic Research Program of China (2013CB956402)
金章东,E-mail: zhdjin@ieecas.cn
苟龙飞,金章东,贺茂勇. 2017. 锂同位素示踪大陆风化:进展与挑战[J]. 地球环境学报, 8(2): 89 – 102.
: Gou L F, Jin Z D, He M Y. 2017. Using lithium isotopes traces continental weathering: Progresses and challenges [J]. Journal of Earth Environment, 8(2): 89 – 102.

