锐钛矿型TiO2担载的Pd催化剂用于乙炔选择加氢的催化性能及其表征
高晓平 郭章龙 周亚男 敬方梨 储伟,*
(1四川大学化学工程学院,成都610065;2四川大学新能源与低碳技术研究院,成都610207)
锐钛矿型TiO2担载的Pd催化剂用于乙炔选择加氢的催化性能及其表征
高晓平1,2郭章龙1,2周亚男1,2敬方梨1储伟1,2,*
(1四川大学化学工程学院,成都610065;2四川大学新能源与低碳技术研究院,成都610207)
采用水热法合成了含有89%{101}晶面的TiO2纳米锭(TiO2-101)和77%{001}晶面的TiO2纳米片(TiO2-001),将其用作载体来制备担载钯催化剂;研究了上述制备的TiO2纳米材料对Pd/TiO2-101和Pd/TiO2-001催化剂用于乙炔选择加氢制聚合级乙烯催化性能的影响。结果表明,Pd/TiO2-101催化剂表现出更好的乙炔转化率和乙烯收率。通过氢气程序升温脱附(H2-TPD)、氢气程序升温还原(H2-TPR)、透射电子显微镜(TEM)、CO化学吸附、X射线光电子能谱(XPS)和热重分析仪(TGA)等对催化剂进行了结构表征和分析。TEM和CO化学吸附结果表明,Pd纳米颗粒(NPs)在TiO2-101载体上有较小的颗粒尺寸(1.53 nm)和较高的分散度(15.95%);而Pd纳米颗粒在TiO2-001载体上的颗粒尺寸是4.36 nm和9.06%的分散度。Pd/TiO2-101催化剂上较小的Pd颗粒尺寸及其较高的分散度使催化剂具有更多的反应活性位点,这促进了其反应的催化活性。
Pd/TiO2催化剂;乙炔选择加氢;锐钛矿型TiO2;{101}晶面;结构表征
1 Introduc tion
Pd-based catalyst is industrially used for the acetylene selective hydrogenation to remove traceamountof acetylene from ethylene feed stream in the commercial production of polymer-grade polyethylene1,2.However,mostof the supported Pd catalystsshow poor selectivity and stability due to strong adsorption of reactant and producton contiguous Pd sites3,4.Severalattemptshave been considered to improve itsselectivity and stability such as(i)inducing the strong metal-support interaction(SMSI)effect to weaken the adsorption strength of ethylene on Pd surface5,6,(ii) adding a secondmetal(e.g.Ag7-11,Zn12,Ga13,14,In15,16)toform alloywith Pd or to suppress themulti-coordination sitesof the Pd surface,(iii)inducting inertmaterial(e.g.carbonaceous deposits formed by pretreatment with feed gases17),(iv)pretreating by plasma18-20.Previousstudieshave showed that Ti3+specieson the support surface could have contact w ith the Pd nanoparticle surfaces,hereby leading to the SMSIeffect21,22.Mobility of the Ti3+from the lattice of TiO2to the surface of Pd particles is usually facilitated by reduction athigh temperature.According to reports in the literature,the specifically exposed planesof support play a crucial role in determining themetal-support interaction and catalytic behavior due to rather differentatomic species,electron density and coordination environment of various facets23.Some publicationshave clarified that the exposed facets of the support nanocrystals could exert a profound influence on the catalytic performances24,25.
Recent progress in the synthesis of anatase TiO2nanomaterials enables to select theexposureof desirable crystalplanes,and thus benefitsmore detailed studies on the catalytic behavior of supportedmetalnanoparticleson TiO2.Forexample,Ru nanoparticles loaded on{101}facets of TiO2nanoparticles exhibited almost double higher turnover frequency in CO2methanation than those over{001}facets of TiO2nanosheets26.The{101}planes displayed amuch stronger interaction w ith Ru nanoparticles than the {001}planes,which enhanced the adsorption and activation of CO2and H2molecules.TiO2nanosheets and nanospindleswere applied to disperse vanadia speciesaswell.The{001}facets of TiO2nanosheets benefited the deposition of octahedral vanadia species,whereas the{101}facetsof TiO2nanospindles resulted in the generation of tetrahedral vanadia species.Octahedrally coordinated vanadia specieson TiO2nanosheets show ed amuch higher activity in selective reduction of NO with NH3mainly becauseof theexistenceofmoreV=O sitesand V―O―V links27. The{100}facets of TiO2promoted activation of O2and the formation of Auδ+and improved the catalytic activity for CO oxidation28.A theoretical study based on density functional theory calculationsw ith a Hubbard U correction(DFT+U)reveals that the catalytic activity of selective hydrogenation of acetylene on oxygen defectsurface ismuchhigher than on the perfectonewhen Pd4cluster supported on theanatase TiO2(101)surfaces29.However,the influence of the exposed crystalplanes of TiO2on the catalytic behavior of Pd nanoparticles for acetylene selective hydrogenation to ethylene,which iskey to enhance the catalytic efficiency of noble mental from the viewpoint of electronic structure,havebeen not reported.
The objective of this reportw as composed in three aspects: firstly to synthesize TiO2nanosheetsmainlywith the{001}facets or nanospindlesmainlyw ith the{101}facets in the presence ofasmorphology-directing agents,respectively;secondly to load Pd NPs on the different shapes of TiO2carriers and characterization of theas-prepared supportsand catalysts;thirdly to investigate theeffectson acetylene selectivehydrogenation to ethylene on these catalysts,and supporting characterization evidences forexplanation of the better performanceof Pd/TiO2-101 sample.In detail,the sampleswere characterized by X-ray diffraction(XRD),Raman spectra,electron spin resonance(ESR), high-resolution transm ission electronm icroscope(HRTEM),X-ray photoelectron spectroscopy(XPS),N2adsorption-desorption, and hydrogen temperature-programmed desorption(H2-TPD), hydrogen temperature-programmed reduction(H2-TPR).In addition,the connection between surface properties of catalystsand catalytic behaviorwasalso investigated.
2 Experim en tal
2.1 Catalysts p reparation
The chemicals(Chengdu Kelong ChemicalReagentCo.,Ltd.) are analyticalgrade and are utilized w ithout further purification. The dom inated{001}facets of anatase TiO2nanosheets were fabricated by hydrothermalmethod as reported in literature30. Typically,25m Lof Ti(OBu)4and 4mL of HFsolution(40%(w, mass fraction))werem ixed in a dried Teflon-lined autoclavew ith a volumeof 100mLand then keptat180°C for24 h.Afterbeing cooled down to ambient temperature,thehydrothermal product waswashedwith 0.1mol·L-1NaOH aqueoussolution to remove fluorine.Then,the white powder was filtrated,washed w ith ethanolaswellasdeionizedwater several times,and dried at80°C for6 h.Finally,theobtained samplewasdenoted asTiO2-001.
Anatase TiO2nanoparticleswith exposed{101}facetswere prepared by amethod reported by Liu etal.28.TiCl4(6.6m L)was added dropw ise into a0.43mol·L-1aqueoussolution of HCl in an ice bath withmagnetic stirring.The resulting clear TiCl4solution was then added into NH3·H2O solution(5.5%(w))to generate thew hite precipitate Ti(OH)4.This system was kept pH around 6-7 by theaddition of 10mLaqueousNH3·H2O(4%(w)). This precipitatewas recovered by filtration and washing,after aging for 2 h at ambient tem perature.The resulting Ti(OH)4precursor and 0.4 g NH4Cldissolved in am ixture containing 30 mL ofwaterand 30m L of isopropyl alcohol.A suspension was gained after treating with stirring and ultrasonic.Then,the suspension w as transferred to a 100m L Teflon-lined autoclave and kept for 24 h at180°C.Finally,the powerswere filtrated and washed by w ater asw ellasethanoluntil therew asno Clin the mother solution determined by aqueousAgNO3(0.05mol·L-1). Theobtained samplewasnamed asTiO2-101.
Pd nanoparticleswere loaded onto TiO2nanocrystals(TiO2-101 and TiO2-001)through impregnation.The loading of Pd is1%(w).TiO2nanocrystals(1 g)were put into an aqueousof PdCl2(0.0229 mol·L-1,4.15m L)atambient temperature for4 hw ith stirring. After drying at60°C for12 h,the derived powerswere treated at 400°C for 3 h under N2atmosphere.The gained catalystswere labeled to asPd/TiO2-001 and Pd/TiO2-101,respectively.Elemental analysis by inductively coupled plasma-atom ic em ission spectroscopy(ICP-AES)reveals that thecontentof Pd is0.56%(w)for Pd/TiO2-001 sam ple and 0.57%(w)for Pd/TiO2-101 sam ple, which are lower than theoriginal theoretical content.
2.2 Characterization of catalysts
The power X-ray diffraction patternsw ere recorded on DX-2700 diffractometer(Haoyuan,China)using Cu Kαradiation at40 kV and 30mA.The 2θscanning rangewas from 10°to 80°with a scan step of 0.03(°)·s-1in a continuousmode.
Morphologieswere analyzed on a TecnaiG2F20 transmission electronm icroscope(TEM).The lattice fringes of the catalysts were characterized through ahigh-resolution transmission electron microscope(HRTEM).The sampleswere crushed and dispersed in ethanol,and the resulting suspensionsw ere allow ed to dry on carbon film supported on coppergrids.
The N2adsorption-desorption isotherms were measured at-196°C using an automated surface area&pore size analyzer (Quantachrome NOVA 1000eapparatus).The specific surfacearea was calculated by the Brunauer-Emmett-Teller(BET)equation.
The TiO2sampleswerealso investigated by Raman spectroscopy.Radiation of 532 nm from an argon-ion laserwasused for excitation.The instrumentversion is LabRAM HR800.
Electron spin resonance spectroscopy was conducted under vacuum at-150°C using a JES-FA200 electron spin resonance spectrometer.Itwas performed to qualitativelymonitor the Ti3+specieson the surfaceof the TiO2.
The hydrogen temperature-programmed reductionmeasurement wasperformed in a fixed-bed reactor atatmospheric pressure.50 mg samplewas loaded in them iddleof reactor tube,and the reductivegasof 5%H2/N2with a totalgas flow rateof 30mL·min-1was introduced.The system was keptat30°C for1 h until the baselinewas stable,and then itwas heated linearly from 30 to 600°C ata heating rate of 10°C·m in-1.The H2uptake amount during the reductionwas recorded by gas chromatograph(SC-200) equipped w ith a thermal conductivity detector(TCD).Prior to hydrogen temperature-programmed desorption(H2-TPD),100mg of catalystswere heated at120°C for 2 h in nitrogen and then placed in H2with a flow rateof 23mL·m in-1for0.5 h at30°C. TPD was carried out in a stream of nitrogenwith a flow rateof 40 m L·m in-1and a temperature ramp of 10°C·m in-1.
Pulse CO chemisorption was employed to determ ine Pd dispersion on ChemStar TPx chemisorption analyzer.Prior to CO adsorptionmeasurements,the Pd/TiO2sampleswere purged in helium at room temperature for30min.The system wasswitched to H2(30m L·m in-1)and heated to 400°C w ith a heating rate of 5°C·min-1.CO pulseswere injected(50μL of 10%of CO in helium)from a calibrated loop over the Pd/TiO2catalystsat30°C and repeated until the desorption peakswere constant.Thenumber of exposed Pd atoms on the Pd/TiO2catalyst surface was calculated by the totalamountof CO adsorption.In this paper,the CO/ Pd stoichiometry of1 isused for calculation.
The X-ray photoelectron spectroscopy datawere collected on XSAM 800 spectrometerw ith an A l Kα(hν=1486.6 eV)X-ray source.Thechargingeffectswerecorrected byadjusting thebinding energy ofC 1s peak from carbon contam ination to 284.6 eV.
The amountof carbonaceous deposited on used catalystswas determ ined by running thermo-gravimetric analysis(on TGA Q500)in airatmosphere(40m L·m in-1).The temperaturewas first keptat30°C for30min,and then increased to 700°Cwitha ramp of 10°C·m in-1.Results of derivative thermogravimetry(DTG) wereobtained from the thermo-gravimetric analysis(TGA)curves by differentiating the latterwith respect to temperature.
2.3 Se lec tive hyd rogenation of acetylene
Acetylene selective hydrogenation reactionwas performed in a fixed-bed reactor in the tem perature range from 40 to 80°C at theatmospheric pressurewith agashourly spacevelocity(GHSV) of 30000mL·g-1·h-1.Thegaseous feedw ith a total flow of 50 m L·m in-1contained 1%C2H2,2%H2and the balance N2.0.1 g catalystwasdiluted by 0.4 g quartz sand for the sakeof avoiding temperatureand concentration gradients.Prior to the reaction,the catalystswere pretreated inhydrogen(20mL·min-1)at400°C for 2 h.Before sampling,the reaction temperaturewas keptconstant for 0.5 h before being raised to the next one.The reactants and productswere detected by gas chromatography(GC)equipped with a flame ionization detector(FID).For the sake of reproducible data,five testswere carried out.Conversion of acetylene, selectivity to ethylene,selectivity to ethane,yield towards ethylene,and carbon balance(Bc)are calculated as follow s31:
3 Resu lts and discussion
3.1 Crystalline s tructu re of TiO2nanopartic les
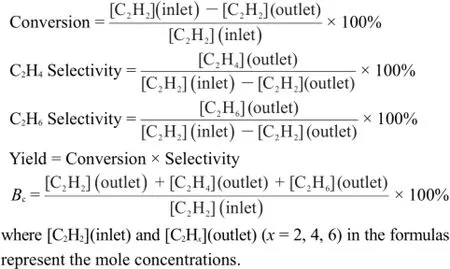
To demonstrate the crystal structure of the TiO2materialsand the Pd/TiO2catalysts,XRD analysis was carried out and the corresponding resultsareexhibited in Fig.1.Allsamplesdisplayed several typical characteristic peaks attributed to theanatase TiO2phase(JCPDS#21-1272,space group:I41/amd(141))32,33.Obviously,the sample TiO2-001(curve C)exhibits relatively stronger diffraction peak at(200)than thatat(004)reflection,indicating a predominantexposureof the{001}facets30,34.Whereas the TiO2-101(curveA)displays a decrease in the(200)reflection and anim provement on intensity of diffraction peak at(004),imp lying theoccupancy of the{101}planes27.Moreover,noobvious change in reflections of Pd/TiO2-101(curve B)and Pd/TiO2-001 catalysts (curve D)is observed w hen com pared w ith the corresponded pristine supports,implying the TiO2nanoparticles remained in the originalstructure andmorphology26.Itshould benoted that there is no X-ray diffractions of Pd species,which is because of the comparatively low loading amount(1%(w),below the detection lim itof XRD)of Pd.The TiO2materialsare further analyzed by Raman spectra(Fig.S1(in Supporting Information))and the resultswere consistentwith the results from XRD analysis.
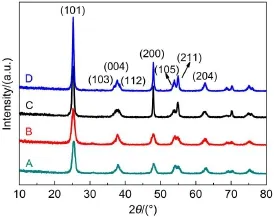
Fig.1 XRD patternsof TiO2-101(A),Pd/TiO2-101(B), TiO2-001(C)and Pd/TiO2-001(D)
Themorphology of the TiO2nanoparticles isstudied by TEM. As shown in Fig.2,TiO2-001 sample exhibits uniform sheet-like shape while the samp le TiO2-101 shows spindle-like shape.The average thickness and side length for the sample TiO2-001 are approximately 5 and 40 nm(Fig.2(a)),respectively.Fig.2(b) shows thatawell-defined sheetstructurewasobserved,which had an interplane spacing of 0.235 nm.A ll of these features implied that the exposed p lanes of anatase TiO2is the{001}facets.In contrast,TiO2-101 samplehasa spindle-likeshapewithan average size of 15.7 nm long and 10 nm w ide(Fig.2(c));from the side view,the interplanar spacing of 0.35 nm is corresponded with the {101}planes of anatase TiO2(Fig.2(d)).On the basis ofWulff construction(Fig.S2(a)and Fig.S2(b)(in Supporting Information)),the percentage of each crystalline facet in the applied samples is calculated.In signal-crystalline TiO2nanospindles,the {101}facetsare the dominant facetswith the ratio of 89%and the other 11%is the{001}facets,while the proportion of{001} planesasw ell as{101}planes in TiO2nanosheets are 77%and 23%,respectively.Hence,in our case,the percentage of each crystalline facet is beyond 75%,demonstrating that the applied TiO2nanomaterials can serveasmodelsupports28.

Fig.2 TEM,HRTEM im ages of TiO2-001(a,b)and TiO2-101(c,d)
3.2 Catalytic performances in selec tive hyd rogena tion of acety lene
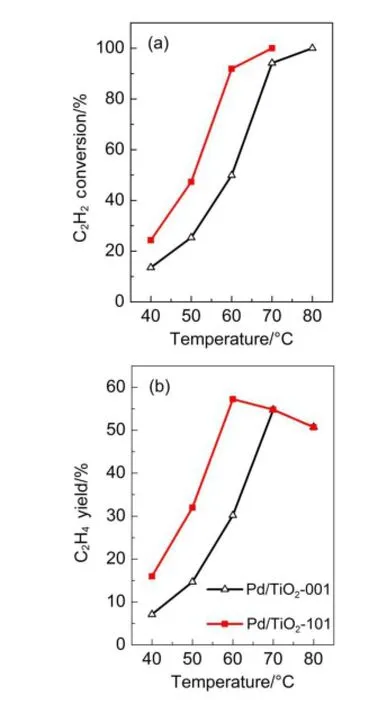
Fig.3 Acetylene conversion asa function of tem perature(a)and ethylene yield versus reaction tem perature(b)over Pd/TiO2-001 and Pd/TiO2-101 catalystsata total flow rate of 50m L·m in-1w ith varying reaction tem peratures from 40 to 80°C
The catalytic behavior of the Pd/TiO2-101 and Pd/TiO2-001 catalystswasevaluated by using partialhydrogenation of acetylene to ethylene as probe reaction under the employed reaction conditions.The catalytic performances of both samples are represented in Fig.3,show ing globally an increase of acetylene conversion with the increasing reaction temperature from 40 to 80°C(Fig.3(a)).The Pd/TiO2-101 catalystexhibits higher acetylene conversion until the reaction temperature reached at70°C (100%for Pd/TiO2-101 vs94%for Pd/TiO2-001)afterwhich full conversion was obtained over the two samples.It isworth noting that the conversion of acetylene isashigh as92%over Pd/TiO2-101 while only 50%is gotten over Pd/TiO2-001.Ow ing to its preferred catalytic performance,Pd/TiO2-101 catalyst surely displays theexcellentyield in ethyleneof 57%at60°C,which is 1.9 timeshigher than thoseover Pd/TiO2-001 catalyst(Fig.3(b)). The preferable catalytic activity of the Pd/TiO2-101 catalystm ight be assigned to its large specific surface area35,high Pd dispersion36,37leading to the formation of more active centers.The selectivity toward ethylene on both catalysts decreased with the increasing conversion(Fig.S3(in Supporting Information)),which is due to the fact that the ethylene is produced asan intermediate in acetylene semi-hydrogenation reaction.Furthermore,Pd/TiO2-101 catalyst shows higher selectivity in ethylenewhen compared with that of the Pd/TiO2-001 catalyst on the basis of equal conversion.The selectivity to ethane(Fig.S4(a)(in Supporting Information))increasesw ith the increasing temperature,especially after60°C.As shown in Fig.S4(b)(in Supporting Information), the carbon balance in both cases is close to 100%.
3.3 Tex ture p roperties of the cata lys ts
In order to explain the difference in the catalytic activity of the two Pd/TiO2catalysts,the structure and size of the Pd nanoparticleswere characterized by TEM and HRTEM,and the resultsare shown in Fig.4.The HRTEM image of Pd/TiO2-101(Fig.4(d)) shows that Pd NPs distribute homogeneously w ithout apparent accumulation,while some Pd NPs on the Pd/TiO2-001 catalyst accumulate after reduction(Fig.4(a)).More than 100 Pd nanoparticlesare obtained from different regions,random ly selected for the sakeof getting theaverage sizeof Pd and the results aredisplayed in thehistograms(Fig.4(c,f)).Aswe can see,thePd/ TiO2-101 catalysthas anarrow size distribution in the range from 1.00 to 2.20 nm and the average size of Pd particles is 1.53 nm which is rather small than thatof Pd/TiO2-001 catalyst(4.36 nm). It is obviously observed that the dispersion of the Pd/TiO2-101 catalystishigher than thatof thePd/TiO2-001 catalyst.This result isw ellagreementw ith theCO chem isorption(Table 1).Remarkably,thenarrower size distribution,smalleraverage particle size and higher dispersion of the Pd/TiO2-101 catalyst can be ascribed to twofactors.The specific surface areas of TiO2-101 were significantly larger than thatof TiO2-001.The BET specific surface areas,nitrogen adsorption-desorption isothermsand BJH pore-size distributionsof the samp lesare given in Fig.S5 and Table S1(in Supporting Information).Generally,thehigher surface areaof the TiO2-101 could contribute to higher dispersion of Pd NPs,w hich facilitates theenhancementof the catalytic activity38,39.This result was accordancew ith the tendency of catalytic behavior in Fig.3. Besides,as reported in the literature,high dispersion of Pd NPs can also be attributed to the strongmetal-support interaction effect40,41.The follow ing section will demonstrate this effectof the Pd/TiO2-101 catalyst.
Based on the reported results in literature42,we can conjecture that Ti3+species combined w ith Pd surface in the interfacem ight play the part of a new reaction sitewhich can greatly improve hydrogen activation aswellas itsdissociation.Although H2-TPD isunable to give direct information about the hydrogen activation/ dissociation ability of the catalysts,itcan supply evidenceon the recombination of atom ic hydrogen,quantity and which kind of hydrogen desorption.Therefore,H2-TPD tests over the two cat-

Fig.4 TEM imagesand the corresponding Pd particlessizedistributionsof Pd/TiO2catalysts(a,b,c)Pd/TiO2-001 and(d,e,f)Pd/TiO2-101
Tab le 1 Proper ties of the Pd/TiO2-001 and Pd/TiO2-101 catalysts
Sample Pd/TiO2-001 Pd/TiO2-101 Pd loadinga/%
0.56
0.57 Pd/Ti 0.025 0.018adeterm ined by ICP-AES;bBET surface area;cdeterm ined by HRTEM;ddeterm ined by CO pu lse;ebased on XPS resu lts Surface areab/(m2·g-1)
51
89 Particlesizec/nm
4.36
1.53 Pd dispersiond/%
9.06
15.95 Surfaceatomic compositione/% Pd
0.72
0.48
Ti 28.64 26.91 alystswere performed and the resultsare delivered in Fig.5.Both catalystsshow twomain peaks(α,β)of desorbed H2,indicating that at least two types of active centers exist on the catalysts surface.Theβ-peak athigher temperature isassociatedw ith the desorption of hydrogen adsorbed strongly,while theα-peak at low tem perature that ismore w eakly bounded to catalysts surface arises from the desorption of physically adsorbed hydrogen43-45. With regard to Pd/TiO2-101 catalyst,the intensity ofβ-peak remarkably increases comparing w ith Pd/TiO2-001 sample,suggesting thatH2dissociation/activation occursmoreeasily on the Pd/TiO2-101 catalyst.
As reported in the literature46,the Ti3+species in TiO2materials are produced by trapping of electronsatdefectivesitesof TiO2and the quantity of gathered electronsm ight reflect the amount of defectsites.Nakaoka and Nosaka47reported six signals of ESR technology occurring on the surfaceof TiO2:(i)Ti4+OTi4+OH-
,(ii) surface Ti3+,(iii)adsorbed oxygen(O2-),(iv)Ti4+O2-Ti4+O2-,(v) inner Ti3+,and(vi)adsorbedwater.Therefore,ESR experiments were carried out over TiO2-001 and TiO2-101 catalysts for qualitatively studying the Ti3+defects,asshown in Fig.6.In our study, it isobviously seen thatboth TiO2-001 and TiO2-101 supportsshow only one strong signalat g valuesof 1.997(less than 2),which can beascribed to Ti3+(3d1)on the surface48.Moreover,the relatively higher intensity of the Ti3+signalover the TiO2-101 support than thatof the TiO2-001 implied significantamountof surface Ti3+defects on TiO2-101 support.In view of the catalytic results,the Pd NPs loaded on TiO2-101 withmore Ti3+defective sites presentedmuch higheractivity than thoseon the TiO2-001 support which indicated that the presence of Ti3+defectsmay contribute to the catalytic performance.
H2-TPR experimentswere performed to investigate the influence of the supportmaterialson the reducibility of Pd speciesand the results are depicted in Fig.7.A negative peak is observed at about 80°C on both catalysts,which was attributed to the decomposition of the palladium hydride formed by exposure to hydrogen atambient temperature49.Noticeably,on Pd/TiO2-101 catalyst,this negative peak w ith slightly lower peak area occurs at slightly lower temperature and a small H2consumption peaks located at105°Cwasobserved.This resultsuggests that there is higher dispersion of Pd nanoparticles on the TiO2-101 supportand implied that the interaction between Pd nanoparticles and TiO2-101 supportm ightbe stronger than thatbetween Pd nanoparticles and TiO2-001 support50,51.Aswell-known,palladium hydride is related to the particle sizeof Pd,and palladium hydride decreases w ith increasing in dispersion of Pd(decreasing Pd crystallite size)52-54.The previous study indicated that the improvement in dispersion of Pdmay be correlatedwith the presence of abundant Ti3+specieson TiO2-101 support55.Considering the reduction peak of TiO2at high temperature,the broad peak between 300 and 450°C isbecause of the reduction of Ti4+(nearby or interactingwith the Pd nanoparticles)to Ti3+.Asdiscussed in the references56, the dissociative hydrogen chem isorbed on palladium may transfer from Pd nanoparticle surface to TiO2supportand thus reduce Ti4+to Ti3+.
Fig.5 H2-TPD profilesof the Pd/TiO2-001 and Pd/TiO2-101 catalysts

Fig.6 ESR spectra of TiO2-001 and TiO2-101 catalysts

Fig.7 H2-TPR profilesof catalysts(a)Pd/TiO2-001 and (b)Pd/TiO2-101
The surface atom ic compositionsof Pd/TiO2-001 and Pd/TiO2-101 catalystswere analyzed by XPSmeasurements.The results of Pd/TiO2-001 and Pd/TiO2-101 sampleswere given in Table1. Itcan be seen thatPd/TiO2-101 catalystgivesa lower atomic ratio of Pd/Ti(0.018)than thatof Pd/TiO2-001(0.025),which could be due to decoration of Pd0metal surface by themobile reducible TiO2.Reducible TiO2can be reduced at high temperature and consequentlymigrate onto the Pd surface,which enhanced the Pd electron density and thenweakened theethyleneadsorption,thus the selectivity of ethylene is improved19,50,57,58.
The catalystswere characterized using CO pulse chem isorption to determ ine the palladium dispersion(Table 1).The dispersion of Pd/TiO2-101 is15.95%,which is higher than thatof Pd/TiO2-001(9.06%).Thisbetter dispersion of Pd/TiO2-101 catalyst implied a largernumberof active sites than thaton the Pd/TiO2-001 samp le,w hich is considered as one of the key reasons for the catalytic performanceenhancement.
3.4 Catalytic stabilitym easu rem en t
The catalyststabilitywas tested on both Pd/TiO2-001 and Pd/ TiO2-101 samplesat70°C.From the results in Fig.8,theacetylene conversion decreased from 100%to 97.1%for Pd/TiO2-101 catalystsw ith reaction time on stream of 900min.While thatwas from 95.5%to 92.4%for the Pd/TiO2-001 sample after reaction for900m in.
The deactivation of Pd-based catalysts for selective hydrogenation of acetylene was mainly caused by accumulation of hydrocarbon specieswhich hindered notonly the pathway of H2and C2H2to the active sitesbutalso the release of C2H459.Thus TGA wasperformed to study the carbon deposition on the spentcatalystsafter the stability experiment,asshown in Fig.9.For the Pd/ TiO2-001 sam ple,the weight loss below 165°C can be assigned to the loss of water,while theweight loss after 165°C can be attributed to the oxidation or decomposition of carbonaceous deposits formed on the catalysts.The quantitative calculation is done based on the weight loss between 165 and 440°C.The amountof deposited carbonaceousspecies for theused Pd/TiO2-001 was13.6 g·g-1(gram carbonaceous per gram catalyst),and thatwas11.55 g·g-1(gram carbonaceouspergram catalyst)for the Pd/TiO2-101 catalysts.
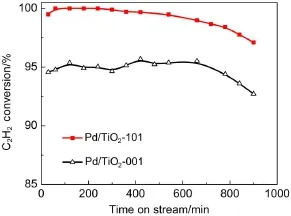
Fig.8 Durability test for Pd/TiO2-001 and Pd/TiO2-101 catalystsat70°C

Fig.9 TGA/DTG resultsof(a)Pd/TiO2-001 and(b)Pd/TiO2-101 after the durability tests at 70°C for 900m in
Moreover,the corresponding DTG results of Pd/TiO2-001 catalyst comprises two peaksat299 and 355°C,which imply two types of carbonaceousspecies.However,the DTG profiles of the spent Pd/TiO2-101 catalyst is sim ilar but less resolved after reaction of 900min.According to the literature60-62,the peak at 281°C is associated w ith the combustion of trapped hydrocarbons absorbed in catalyst pores or on the catalyst surface.The peak centered at343°C can be assigned to the combustion of amorphous carbon located on or in the vicinity of Pd NPs,a precursor of graphitic carbon thathasa structure of oligomeric hydrocarbon, CxHy,which decreases theutilizability of H2and/or C2H2.What′s more,thepeak areaof the Pd/TiO2-101 catalystathigh temperature issignificantly smaller than thatof Pd/TiO2-001,implying higher resistance against carbonaceous compound deposition and hence possessing better stability.
4 Conc lusions
We have synthesized two types of Pd/TiO2catalysts w ith differentcrystal-planeof TiO2supports({101}and{001}facets)and investigated the effects on catalytic properties in the selective hydrogenation of acetylene to ethylene reaction.The characterization resultsshowed thata smaller particles and higher Pd NPs dispersion on Pd/TiO2-101 catalyst than thaton the Pd/TiO2-001 catalyst.Pd/TiO2-101 catalystpresentsignificantly higher catalyticactivity than thatof Pd/TiO2-001 catalyst.The catalytic behavior is dependenton the exposed facetsof TiO2supports.These results notonlymanifested that the structureand catalytic properties of Pd/TiO2catalysts can be tuned by controlling the crystal-planeof the TiO2support,butalso greatly deepened the understanding of the selective hydrogenation of acetylene reaction by Pd/TiO2catalysts.
Acknow ledgment:We would like to thank LIU Ming(Sichuan University),LIU Yue-Feng(Institute of Metal Research, CAS),and ZHENG Jian(Southwest University of Science and Technology)for their assistanceson TEM and Ramanmeasurement;meanwhile,wealso thank CHENMin(Sichuan University), LIAO Xue-Mei(Xihua University),DENG Jie(Chengdu University),and ZHENG Jian foruseful discussion and helps.GAO Xiao-Ping thanks the China ChengDa Engineering Co.,Ltd for scholarship.
Suppo rting In form a tion:available free of charge via the internetathttp://www.whxb.pku.edu.cn.
(1)Kuhn,M.;Lucas,M.;Claus,P.Ind.Eng.Chem.Res.2015,54, 6683.doi:10.1021/acs.iecr.5b01682
(2)Studt,F.;Abild-Pedersen,F.;Bligaard,T.;Sørensen,R.Z.; Christensen,C.H.;Nørskov,J.K.Science 2008,320,1320. doi:10.1126/science.1156660
(3)Kim,S.K.;Kim,C.;Lee,J.H.;Kim,J.;Lee,H.;Moon,S.H. J.Catal.2013,306,146.doi:0.1016/j.jcat.2013.06.018
(4)Crespo-Quesada,M.;Yarulin,A.;Jin,M.;Xia,Y.;Kiw i-M insker,L.J.Am.Chem.Soc.2011,133,12787.doi:10.1021/ ja204557m
(5)Hong,J.;Chu,W.;Chen,M.;Wang,X.;Zhang,T.Catal. Commun.2007,8,593.doi:10.1016/j.catcom.2006.08.010
(7)Pei,G.X.,Liu,X.Y.;Wang,A.;Lee,A.F.;Isaacs,M.A.;Li,L.; Pan,X.;Yang,X.;Wang,X.;Tai,Z.;Wilson,K.;Zhang,T.ACS Catal.2015,5,3717.doi:10.1021/acscatal.5b00700
(8)Lee,J.H.;Kim,S.K.;Ahn,I.Y.;Kim,W.J.;Moon,S.H. Catal.Commun.2011,12,1251.doi:10.1016/j. catcom.2011.04.015
(9)Wang,Z.Q.;Zhou,Z.M.;Zhang,R.;Li,L.;Cheng,Z.M.Acta Phys.-Chim.Sin.2014,30,2315.[王沾祺,周志明,张锐,李莉,程振民.物理化学学报,2014,30,2315.]doi:10.3866/PKU. WHXB201410152
(10)Gu,H.;Xu,B.L.;Zhou,J.;Li,Y.Z.;Fan,Y.N.Acta Phys.-Chim.Sin.2006,22,712.[顾虹,许波连,周静,李远志,范以宁.物理化学学报,2006,22,712.]doi:10.3866/ PKU.WHXB20060613
(11)Guo,Z.L.;Huang,L.Q.;Chu,W.;Luo,S.Z.Acta Phys.-Chim. Sin.2014,30,723.[郭章龙,黄丽琼,储伟,罗仕忠.物理化学学报,2014,30,723.]doi:10.3866/PKU.WHXB201402242
(12)Kontapakdee,K.;Panpranot,J.;Praserthdam,P.Catal. Commun.2007,8,2166.doi:10.1016/j.catcom.2007.03.003
(13)He,Y.;Liang,L.;Liu,Y.;Feng,J.;Ma,C.;Li,D.J.Catal. 2014,309,166.doi:10.1016/j.jcat.2013.09.017
(14)Osswald,J.;Giedigkeit,R.;Jentoft,R.;Armbruster,M.; Girgsdies,F.;Kovnir,K.;Ressler,T.;Grin,Y.;Schlogl,R. J.Catal.2008,258,210.doi:10.1016/j.jcat.2008.06.013
(15)Neumann,M.;Teschner,D.;Knop-Gericke,A.;Reschetilowski, W.;A rmbrüster,M.J.Catal.2016,340,49.doi:10.1016/j. jcat.2016.05.006
(16)Gao,Z.;Zhang,Y.;Li,D.;Werth,C.J.;Zhang,Y.;Zhou,X. J.Hazard.Mater.2015,286,425.doi:10.1016/j. jhazmat.2015.01.005
(17)Teschner,D.;Borsodi,J.;Wootsch,A.;Révay,Z.;Hävecker,M.; Knop-Gericke,A.;Jackson,S.D.;Schlögl,R.Science2008, 320,86.doi:10.1126/science.1155200
(18)Chen,M.H.;Chu,W.;Dai,X.Y.;Zhang,X.W.Catal.Today 2004,89,201.doi:10.1016/j.cattod.2003.11.027
(19)Li,Y.;Jang,B.W.L.Appl.Catal.A 2011,392,173.doi:10.1016/ j.apcata.2010.11.008
(20)Chu,W.;Xu,J.;Hong,J.;Lin,T.;Khodakov,A.Catal.Today 2015,256,41.doi:10.1016/j.cattod.2015.05.024
(21)Panpranot,J.;Nakkararuang,L.;Ngamsom,B.;Praserthdam,P. Catal.Lett.2005,103,53.doi:10.1007/s10562-005-6502-x
(22)Panpranot,J.;Kontapakdee,K.;Praserthdam,P.Appl.Catal.A 2006,314,128.doi:10.1016/j.apcata.2006.08.024
(23)Wang,N.;Qian,W.;Chu,W.;Wei,F.Catal.Sci.Technol.2016, 6,3594.doi:10.1039/c5cy01790d
(24)Si,R.;Flytzani-Stephanopoulos,M.Angew.Chem.Int.Ed. 2008,47,2884.doi:10.1002/anie.200705828
(25)Liu,L.;Yao,Z.;Deng,Y.;Gao,F.;Liu,B.;Dong,L. ChemCatChem 2011,3,978.doi:10.1002/cctc.201000320
(26)Wang,F.;Zhang,S.;Li,C.;Liu,J.;He,S.;Zhao,Y.;Yan,H.; Wei,M.;Evans,D.G.;Duan,X.RSCAdv.2014,4,10834. doi:10.1039/c3ra47076h
(27)Shi,Q.;Li,Y.;Zhou,Y.;M iao,S.;Ta,N.;Zhan,E.;Liu,J.; Shen,W.J.Mater.Chem.A 2015,3,14409.doi:10.1039/ c5ta02897c
(28)Liu,L.;Gu,X.;Cao,Y.;Yao,X.;Zhang,L.;Tang,C.;Gao,F.; Dong,L.ACSCatal.2013,3,2768.doi:10.1021/cs400492w
(29)Yang,J.;Cao,L.X.;Wang,G.C.J.Mol.Model.2012,18, 3329.doi:10.1007/s00894-011-1337-4
(30)Han,X.;Kuang,Q.;Jin,M.;Xie,Z.;Zheng,L.J.Am.Chem. Soc.2009,131,3152.doi:10.1021/ja8092373
(31)He,Y.;Fan,J.;Feng,J.;Luo,C.;Yang,P.;Li,D.J.Catal.2015, 331,118.doi:10.1016/j.jcat.2015.08.012
(32)Tan,Z.;Sato,K.;Takami,S.;Numako,C.;Umetsu,M.;Soga, K.;Nakayama,M.;Sasaki,R.;Tanaka,T.;Ogino,C.;Kondo, A.;Yamamoto,K.;Hashishin,T.;Ohara,S.RSCAdv.2013,3,19268.doi:10.1039/c3ra43383h
(33)Zheng,J.;Liu,Z.;Liu,X.;Yan,X.;Li,D.;Chu,W.J.Alloy. Compd.2011,509,3771.doi:10.1016/j.jallcom.2010.12.152
(34)Tian,F.;Zhang,Y.;Zhang,J.;Pan,C.J.Phys.Chem.C 2012, 116,7515.doi:10.1021/jp301256h
(35)Komhom,S.;Mekasuwandum rong,O.;Praserthdam,P.; Panpranot,J.Catal.Commun.2008,10,86.doi:10.1016/j. catcom.2008.07.039
(36)Sárkány,A.;Schay,Z.;Frey,K.;Széles,É.;Sajó,I.Appl.Catal. A 2010,380,133.doi:10.1016/j.apcata.2010.03.042
(37)M enezes,W.G.;A ltmann,L.;Zielasek,V.;Thiel,K.;Bäumer, M.J.Catal.2013,300,125.doi:10.1016/j.jcat.2012.12.023
(38)Vincent,M.J.;Gonzalez,R.D.Appl.Catal.A 2001,217,143. doi:10.1016/S0926-860X(01)00586-5
(39)Wang,N.;Xu,Z.;Deng,J.;Shen,K.;Yu,X.;Qian,W.;Chu,W.; Wei,F.ChemCatChem 2014,6,1470.doi:10.1002/ cctc.201300720
(40)Douidah,A.;Marécot,P.;Szabo,S.;Barbier,J.Appl.Catal.A 2002,225,21.doi:10.1016/S0926-860X(01)00627-5
(41)Dole,H.A.E.;Safady,L.F.;Ntais,S.;Couillard,M.;Baranova, E.A.J.Catal.2014,318,85.doi:10.1016/j.jcat.2014.07.003
(42)Panagiotopoulou,P.;Kondarides,D.I.J.Catal.2009,267,57. doi:10.1016/j.jcat.2009.07.014
(43)Yu,W.Y.;Mullen,G.M.;Mullins,C.B.J.Phys.Chem.C 2013, 117,19535.doi:10.1021/jp406736b
(44)Huang,L.;Chu,W.;Zhang,T.;Yin,Y.;Tao,X.J.Nat.Gas Chem.2009,18,35.doi:10.1016/S1003-9953(08)60082-1
(45)Han,X.;Chu,W.;Ni,P.;Luo,S.Z.;Zhang,T.J.Fuel Chem. Technol.2007,35,691.doi:10.1016/S1872-5813(08)60004-3
(46)Ikeda,S.;Sugiyama,N.;Murakami,S.Y.;Kominami,H.;Kera, Y.;Noguchi,H.;Uosaki,K.;Torimoto,T.;Ohtani,B.Phys. Chem.Chem.Phys.2003,5,778.doi:10.1039/b206594k
(47)Nakaoka,Y.;Nosaka,Y.J.Photochem.Photobiol.A 1997,110, 299.doi:10.1016/S1010-6030(97)00208-6
(48)Salama,T.M.;Hattori,H.;Kita,H.;Ebitani,K.;Tanaka,T. J.Chem.Soc.Faraday Trans.1993,89,2067.doi:10.1039/ FT9938902067
(49)M cCue,A.J.;M cKenna,F.M.;Anderson,J.A.Catal.Sci. Technol.2015,5,2449.doi:10.1039/c5cy00065c
(50)Riyapan,S.;Boonyongmaneerat,Y.;Mekasuwandum rong,O.; Praserthdam,P.;Panpranot,J.Catal.Today 2015,245,134. doi:10.1016/j.cattod.2014.07.017
(51)Neyertz,C.;Volpe,M.Co lloids Surf.A 1998,136,63. doi:10.1016/S0927-7757(97)00249-5
(52)Ziemecki,S.B.;M ichel,J.B.;Jones,G.A.Reac.Solids1986, 2,187.doi:10.1016/0168-7336(86)80082-1
(53)Gómez-Quero,S.;Cárdenas-Lizana,F.;Keane,M.A.Ind.Eng. Chem.Res.2008,47,6841.doi:10.1021/ie0716565
(54)Aytam,H.P.;Akula,V.;Janmanchi,K.;Kamaraju,S.R.R.; Panja,K.R.;Gurram,K.;Niemantsverdriet,J.W.J.Phys. Chem.B 2002,106,1024.doi:10.1021/jp012357a
(55)Panpranot,J.;Kontapakdee,K.;Praserthdam,P.J.Phys.Chem. B 2006,110,8019.doi:10.1021/jp057395z
(56)Xu,J.;Sun,K.;Zhang,L.;Ren,Y.;Xu,X.Catal.Commun. 2005,6,462.doi:10.1016/j.catcom.2005.04.006
(57)Liu,Y.N.;Feng,J.T.;He,Y.F.;Sun,J.H.;Li,D.Q.Catal.Sci. Techno l.2015,5,1231.doi:10.1039/c4cy01160k
(58)Kim,E.;Shin,E.W.;Bark,C.W.;Chang,I.;Yoon,W.J.;Kim, W.J.Appl.Catal.A 2014,471,80.doi:10.1016/j. apcata.2013.11.036
(59)Zhang,S.;Chen,C.Y.;Jang,B.W.L.;Zhu,A.M.Catal.Today 2015,256,161.doi:10.1016/j.cattod.2015.04.002
(60)Pachulski,A.;Schödel,R.;Claus,P.Appl.Catal.A 2011,400, 14.doi:10.1016/j.apcata.2011.03.019
(61)Lopez,E.;Ordonez,S.;Diez,F.V.Appl.Catal.B 2006,62,57. doi:10.1016/j.apcatb.2005.06.014
(62)Azizi,Y.;Petit,C.;Pitchon,V.J.Catal.2008,256,338. doi:10.1016/j.jcat.2008.04.003
Cata lytic Perform ance and Charac terization of Anatase TiO2Suppo rted Pd Catalysts for the Selec tive Hyd rogenation of Acety lene
GAO Xiao-Ping1,2GUO Zhang-Long1,2ZHOU Ya-Nan1,2JING Fang-Li1CHUWei1,2,*
(1Schoo l ofChem ical Engineering,Sichuan University,Chengdu 610065,P.R.China;
2Institute ofNew Energy and Low-Carbon Technology,Sichuan University,Chengdu 610207,P.R.China)
Anatase TiO2nanospindles containing 89%exposed{101}facets(TiO2-101)and nanosheets with 77%exposed{001}facets(TiO2-001)were hydrotherma lly synthesized and used as supports for Pd catalysts. The effec ts of the TiO2m aterials on the cata lytic performance of Pd/TiO2-101 and Pd/TiO2-001 catalysts we re investigated in the selective hyd rogenation ofacetylene to po lymer-grade ethylene.The Pd/TiO2-101 catalyst exhibited enhanced performance in terms ofacetylene conversion and ethylene yield.To understand these effects,the cata lystswere characterized by H2temperature-programmed desorption(H2-TPD),H2temperatureprogrammed reduction(H2-TPR),transm ission electronm icroscopy(TEM),pulse CO chem isorption,X-ray photoelectron spectroscopy(XPS),and thermog ravim etric analysis(TGA).The TEM and CO chem isorption results confirmed thatPd nanoparticles(NPs)on the TiO2-101 supporthad a smalleraverage particle size(1.53 nm)and a higher dispersion(15.95%)than those on the TiO2-001 support(average particle size of4.36 nm and dispersion of9.06%).The smaller particle size and higher dispersion of Pd on the Pd/TiO2-101 catalystprovided more reaction active sites,which contributed to the im proved catalytic activity of this supported catalyst.
Pd/TiO2catalyst;Acetylene selective hydrogenation;Anatase TiO2;{101}p lane;Structure characterization
.Email:chuwei1965@scu.edu.cn;Tel:+86-28-85403836.
Theprojectwas supported by theNationalNatural Science Foundation of China(21476145).
国家自然科学基金(21476145)资助项目©Editorialoffice of Acta Physico-Chim ica Sinica
O643
im,W.J.;Moon,S.H.Catal.Today2012,185,2.
10.1016/j.cattod.2011.09.037
doi:10.3866/PKU.WHXB201611251
www.whxb.pku.edu.cn
Received:August26,2016;Revised:November25,2016;Published online:November25,2016.*

