Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis
Hangyu Zhang, Jianhai Guo, Song Gao, Pengjun Zhang, Hui Chen, Xiaodong Wang, Xiaoting Li, Xu Zhu
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),1Department of Interventional Therapy;2Department of Radiology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis
Hangyu Zhang1*, Jianhai Guo1*, Song Gao1, Pengjun Zhang1, Hui Chen1, Xiaodong Wang1, Xiaoting Li2, Xu Zhu1
Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),1Department of Interventional Therapy;2Department of Radiology, Peking University Cancer Hospital & Institute, Beijing 100142, China
Objective:To investigate the prognostic factors in chemorefractory colorectal cancer liver metastasis (CRCLM) patients treated by transarterial chemoembolization (TACE) and sustained hepatic arterial infusion chemotherapy (HAIC).
Colorectal cancer; transarterial chemoembolization; hepatic artery infusion chemotherapy
View this article at: http://dx.doi.org/10.21147/j.issn.1000-9604.2017.01.05
Introduction
Liver metastasis occurs frequently in colorectal cancer and develops in about 50% of patients (1). Hepatic resection is still the only potentially therapeutic treatment for colorectal cancer liver metastasis (CRCLM), which can be available for no more than 20% of patients (2,3). Patients who were involved in inoperable liver metastases or contraindications to surgical resection are routinely treated with systemic chemotherapy. Standard first-linechemotherapy can achieve 7.0–12.3 months of median progression-free survival (PFS) and 15.0–29.8 months of median overall survival (OS) (4-6), but the median PFS and OS would be only 4.8–6.8 months and 11–15 months even with molecular target drugs in second and subsequent treatment (7,8).
Without other treatment, the median OS of patients who failed from primary chemotherapy could be only 3.5 months (9). Alternative treatment is in great need. Compared with systemic chemotherapy and surgery, minimally invasive interventional therapy such as radiofrequency ablation (RFA), transarterial chemoembolization (TACE) and hepatic artery infusion chemotherapy (HAIC) has the advantages of repeatability and minimal invasion. Minimally invasive interventional therapy in the multi-disciplinary treatment (MDT) has gained more acceptance.
TACE and HAIC are the most typical treatments of interventional therapies via the vessels. TACE has been proved to have a higher response rate than systemic chemotherapy (10-14), and HAIC with oxaliplatin (OXA), calcium folinate (CF) and 5-fluorouracil (5-Fu) in pretreated patients with CRCLM had also proved to be a feasible and low-toxicity treatment (15,16). Liver metastasis of colorectal cancer is considered lack of blood supply, so the clinical outcome of TACE for patients with CRCLM is expected to be improved by HAIC; however, there has so far been no evidence for this expectation. Previous studies have described prognostic indicators for CRCLM, including the primary colorectal cancer stage, tumor differentiation, the size and number of metastases, carcinoembryonic antigen (CEA) level, time to liver metastasis, and extrahepatic disease (17,18). However, no consensus exists regarding the indications for combined TACE and HAIC. A new strategy to improve the prognoses of patients undergoing TACE/HAIC is needed. The aim of the present study was to investigate the prognostic factors in chemorefractory CRCLM patients treated by TACE/HAIC.
Materials and methods
Patients

Figure 1 Flowchart detailing patient selection.
Between 2006 and 2015, 214 CRCLM patients underwent TACE and sustained HAIC in Peking University Cancer Hospital & Institute (Figure 1). The indications for performing the TACE were defined as follows: 1) pathologically diagnosed as adenocarcinoma of the colon or rectum; 2) inoperable liver metastases or contraindications to liver resection; 3) failed from previous systemic chemotherapy (experience at least one line of chemotherapy) or could not suffer its side effects; and 4) the Eastern Cooperative Oncology Group (ECOG) performance status score was less than 2. Patients (56%, n=91) who had extrahepatic metastases were included, considering their main lesion still remained in the liver. Excluded criteria of this retrospective study were conditions as followed: 1) inadequate medical records (n=30); 2) previously received TACE or other interventional treatment (n=3); 3) acquired further resection of liver metastasis after TACE (n=5); or 4) infused chemotherapy agents were not based on OXA (n=14). Patients with poor performance status (ECOG ≥2), tumor involvement of more than 70% of liver volume and liver or renal dysfunction (total bilirubin serum levels >3 mg/dL, serum albumin level <20 g/L, serum creatinine level >2 mg/dL) would not consider a TACE/HAIC. Finally, 162 CRCLM patients who underwent 763 TACE/HAIC in total were enrolled in this retrospective study, including 110 males and 52 females, with a median age of 60 (range, 26–83) years.
The retrospective study was in accordance with the ethical standards of the Ethic Committee of Peking University Cancer Hospital and received Institutional Review Board approval. The informed consent was waived.
TACE
The Seldinger technique was used to access the femoralartery after rejection of local anesthesia. Then arteriography was performed routinely before the chemoembolization to gather information about abdominal aortic, celiac trunk and portal venous system was evaluated indirectly. Then a coaxial catheter (Renegade Hi Flo, Boston Scientific, USA; Stride ASAHI INTECC, Japan) was inserted into the hepatic artery and subsegmental arteries. Another arteriography was performed to find the feeding arteries to the tumor. According to tumor stain, Spongostan particles (Jinling, Nanjing, China), and iodized oil (Lipiodol; Laboratoire Andre Guerbet, Aulnaysous-Bois, France), which was mixed with 20–40 mg epirubicin hydrochloride (Main Luck Pharmaceutical, Shenzhen, China) were injected. Tumor stain under arteriography was artificially classified as “poor” when tumor feeding vessels could not be found, there is no stain or only light stain in the tumor area, and the boundary of normal liver tissue is not clear; “moderate” when tumor vessels were rare and slender, tumor stain was stronger than normal liver tissue, and the boundary of normal liver tissue could be found; and“well” when tumor vessels were clear and definite, contrast stain was significantly, and the boundary between tumor and normal liver tissue is clear. Lipiodol deposit after TACE was artificially classified as “poor” when the lesion outline was incomplete and the internal iodine oil deposits were not obvious; “moderate” when the lesion outline was relatively complete and the internal iodine oil deposits were weak; and “well” when the shape of the lesion was complete and the internal iodine oil deposits were compact. Two experienced professional doctors made the judgement together.
HAIC
The temporary indwelling catheter would be kept into the hepatic artery after TACE until the end of HAIC. HAIC was carried out via the catheter with OXA (Jiangsu Hengrui Medicine Co, Ltd., China) 85 mg/m2in 4 h, 5-Fu (Jinyao Aminoacid Co, Ltd., Tianjing, China) 2,000 mg/m2in about 44 h and CF (Jiangsu Hengrui Medicine Co, Ltd., China) 200 mg/m2in 2–4 h versus peripheral vein. A small part of patients (n=22) received raltitrexed instead of 5-Fu, which was given 4 mg per patient in 1 h.
Treatment was repeated every 3–4 weeks by experienced physicians, until patient died, complete response (CR) was obtained, liver function turned out to Child-pugh C, disease progressed, or adverse effects became intolerable to the patients.
Follow-up care
All the patients were regularly followed up. The laboratory examinations were obtained every week, and enhanced computed tomography (CT) or magnetic resonance imaging (MRI) was periodically performed to evaluate therapeutic efficiency every 6–8 weeks after first TACE/HAIC. Objective response rate (ORR) and disease control rate (DCR) were evaluated by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 (before 2009) and RECIST version 1.1, and adverse reaction was recorded. The decision of another TACE was depending on the results of the examinations and patients’ general state.
Survival analysis
To identify the prognostic factors for the long-term outcome, we analyzed factors related to the primary lesion and liver metastases and factors related to the treatment. The endpoint evaluated was patient survival from the date of first TACE. PFS is defined as the time from first TACE to the date of “progresses” judged by RECIST or the date on which the patient died. Data of patients lost to followup were censored at the date of the last observation. The survival durations after first TACE and HAIC were calculated using the Kaplan-Meier method and were analyzed by the Log-rank test to compare the cumulative survival durations. The Cox proportional hazards model was used to determine the univariate and multivariate hazards ratios for the study parameters. Pearson productmoment correlation analysis was used to measure the relationship between the two variables. For all tests, P<0.05 was defined as statistically significant. The IBM SPSS Statistics (Version 19.0; IBM Corp., New York, USA) was used for the analyses. The GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) was used for chart making.
Results
Characteristics of patients
All the patients were heavily pre-treated by systemic chemotherapy. The characteristics of patients (Table 1) showed that the primary tumor was located in the right hemicolon in 33 (20.4%) patients and in the left hemicolon in 129 (79.6%) patients. Most of the patients (81.5%, n=132) had primary tumor resected. As the time of liver metastases, 125 (77.2%) patients had synchronous livermetastasis and 37 (22.8%) were metachronous. In all patients, only 9 patients had single liver metastasis. A small part of (n=28) patients received epirubicin only combined sustained HAIC during the procedure in accordance with the poor blood supply. OXA, CF and 5-Fu infusion were administered in 140 patients, and OXA and raltitrexed infusion was carried out in 22 patients.
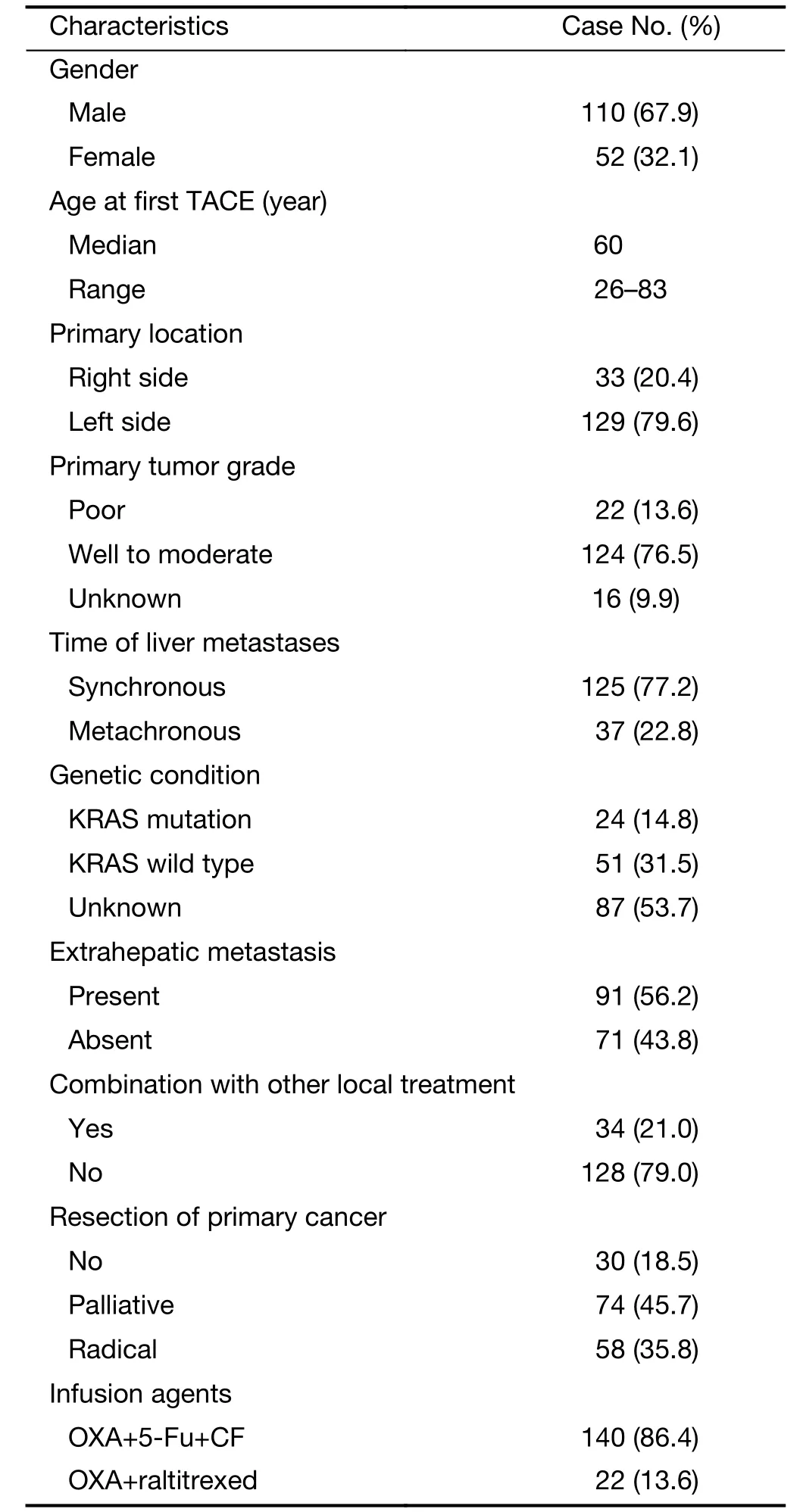
Table 1 Clinical characteristics of 162 patients
Survival data and response rate
During the follow-up time, 134 out of 162 patients died, 11 patients lost follow-up and 17 patients remained alive. The median survival time (MST) was 29.5 months from diagnosis of colorectal cancer and was 15.6 months from the start of TACE/HAIC treatment (Figure 2). The median PFS was 5.5 months after first TACE and HAIC. The actuarial survival rate after TACE and HAIC was 63% [95% confidence interval (95% CI), 56%–70%], 26% (95% CI, 19%–33%), and 10% (95% CI, 5%–15%) after 1, 2 and 3 years.
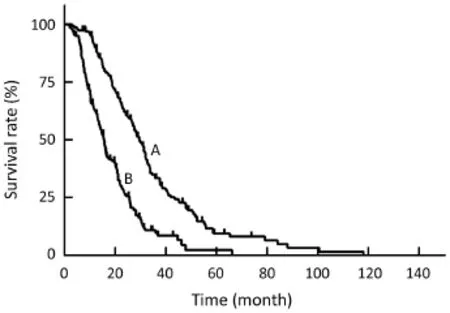
Figure 2 Survival data of patients received transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) (n=162). The median survival time (MST) after diagnosis of colorectal cancer was 29.5 months (curve A). The MST after first TACE was 15.6 months (curve B).
There were 2–20 (mean 4.7) repeated TACE performed per patient. There was only one patient identified as CR. Forty-seven (29.0%) patients achieved partial response (PR), 74 (45.7%) achieved stable disease (SD) and 40 (24.7%) achieved progressive disease (PD). The DCR was 75%. Patients achieving CR benefited the most while those with PD benefited little in survival. During the treatment, 35 patients progressed due to extrahepatic disease, but analysis showed no significance (P=0.474) between these two groups in OS after first TACE and HAIC. Most patients (86.4%, n=140) were infused OXA, CF and 5-Fu after embolization and other patients (13.6%, n=22) received raltirexed plus OXA. There was no significant (P=0.994) difference in survival between these two different chemotherapy regimens. Analysis showed that poorer blood supply and lower lipiodol deposit may result in better prognostic, but there was no significant difference (P=0.079for tumor stain, P=0.162 for lipiodol deposit).
The hospital mortality rate and 30 d treatment-related mortality were 0% for all 162 patients analyzed. The most common complications were anorexia, nausea, transient fever, abdominal pain, neuropathy, and increased alanine aminotransferase levels, which were controlled with symptomatic treatments. Grade 3–4 bone marrow toxicity occurred in 13 patients and there were two patients died of grade 4 bone marrow suppression. One patient developed liver abscess after treatment and recovered by effective drainage.
Prognostic factors
Prognostic factors including age, gender, primary tumor characteristic, liver metastasis characteristic, extrahepatic metastasis, different treatment and serum tumor marker were examined. Among the factors related to survival time after TACE/HAIC, combination with other local treatment (P=0.034), response to TACE (P<0.001), and normal serum CA19-9 (P<0.001) were significant predictors (Table 2). Factors including gender, age, primary tumor site, size of liver metastasis, number of liver metastasis, infusion agents, tumor stain and lipidol deposit had no significant differences.
The multivariate analysis was conducted to identify the predictive indicators for a good prognosis using the parameters which were identified to have P value less than 0.15 by the univariate analysis. Among these parameters, normal serum CA19-9 (P<0.001), response to TACE (P<0.001) and combination with other local treatment (P=0.001) were independent factors for OS after TACE/HAIC (Table 3,Figure 3–5). Pearson productmoment correlation analysis showed that serum CEA (P=0.029) and CA-724 (P=0.024) had significant correlation with survival time after first TACE/HAIC.
Discussion
Local treatments are increasingly accepted as alternative selections for CRCLM patients. Meta-analyses (19-21) about hepatic arterial infusion (HAI) for chemotherapy refractory patients show higher local response rate but give controversial views of its advantage in OS. Since TACE could reduce the blood supply and sustained HAIC could reach high level of chemotherapeutic agent in tumor area, the combination of these two treatments is worth further exploration. Our study found some prognostic factors, and discussed response rate and survival benefit of this treatment.
Patients received TACE/HAIC in our study achieved 75% DCR, the median PFS reached 5.5 months and OS reached 15.6 months in chemotherapy refractory patients, which were longer than results in other similar researches (9,22-25). As reported in patients treated by TACE only (14), local response turned out to be a significant prognostic factor of this combined therapy. Previous studies (26-28) revealed that elevated CEA and CA19-9 could be poor prognostic factors for CRCLM patients who underwent liver resection. But the meanings of tumor markers in TACE and HAIC were not clear. The tumor marker CA19-9 was found to be an effective prognostic factor in our study. Patients with elevated serum CA19-9 seemed to have a poorer prognosis, which was supported by several other studies (29,30). While elevated serum CEA did not achieve great significance when classified as normal group or elevated group. But Pearson product-moment correlation analysis showed significant correlation between this tumor marker and survival. That may be attributed to the large number (140/162) of elevated serum CEA patients. There is interaction between serum CA72-4 and CA19-9, so multivariate analysis found no significant difference of CA72-4. The patients received other local treatment such RFA and liver radiotherapy significantly reduce the risk in survival rate either from diagnosis of the disease or from first TACE and HAIC, demonstrating that non-vascular minimally invasive treatment could be a necessary complement to the comprehensive treatment ofcolorectal liver metastases. Wienerset al. (31) had also reported that combination of two regional treatment approaches may prolong OS. That means CRCLM patients could get more chance to be treated. RAS mutation was proved to be a prognostic biomarker for CRCLM patients (32). The test rate of gene expression analysis (75/162) is relatively low in our study and we did not get significant difference in these groups. There were no significant difference observed in size and number of liver metastasis, which was also controversial in previous reports (28,33). This could mainly attribute to that all the patients were heavily treated before, and there was only 9 patients had single liver metastases. Patients received embolization had a tendency of longer survival than that of counterpart, but there was no significant difference either. Survival benefit tendency was also discovered in the patients who had poorer tumor straining under arteriography or lower Lipiodol deposit after TACE. Thisreveals that sufficient blood supply of CRCLM may result in poor prognosis and this trend remains even under efficient treatment.
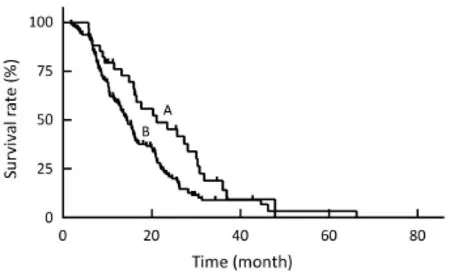
Figure 3 The Kaplan-Meier curves show the survival data after transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) combined with or without other local treatment. The median survival time (MST) after TACE, HAIC and other local treatment was 21.1 months (curve A) and that of TACE and HAIC only was 14.4 months (curve B).
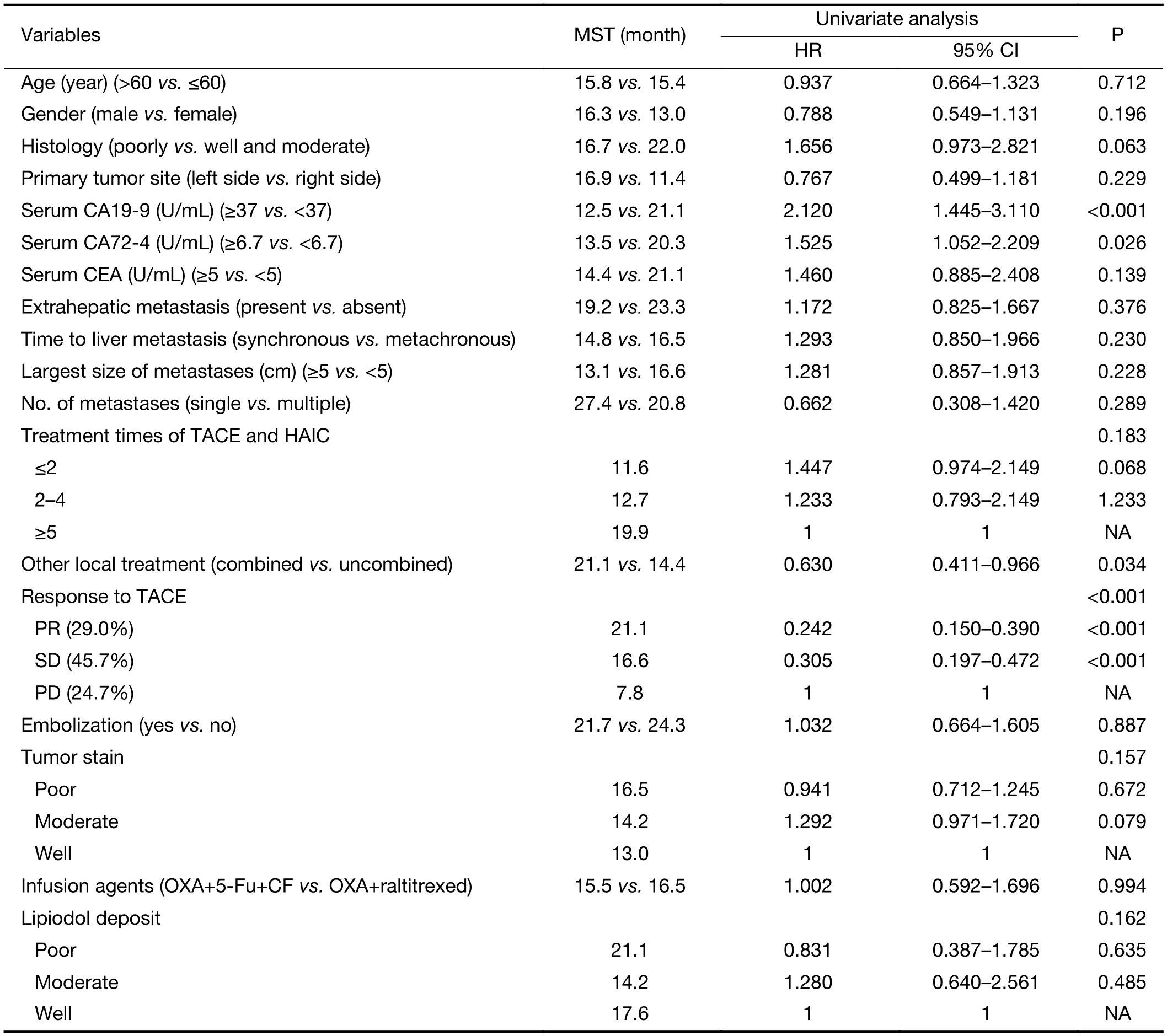
Table 2 Univariate analysis of survival after first TACE and HAIC
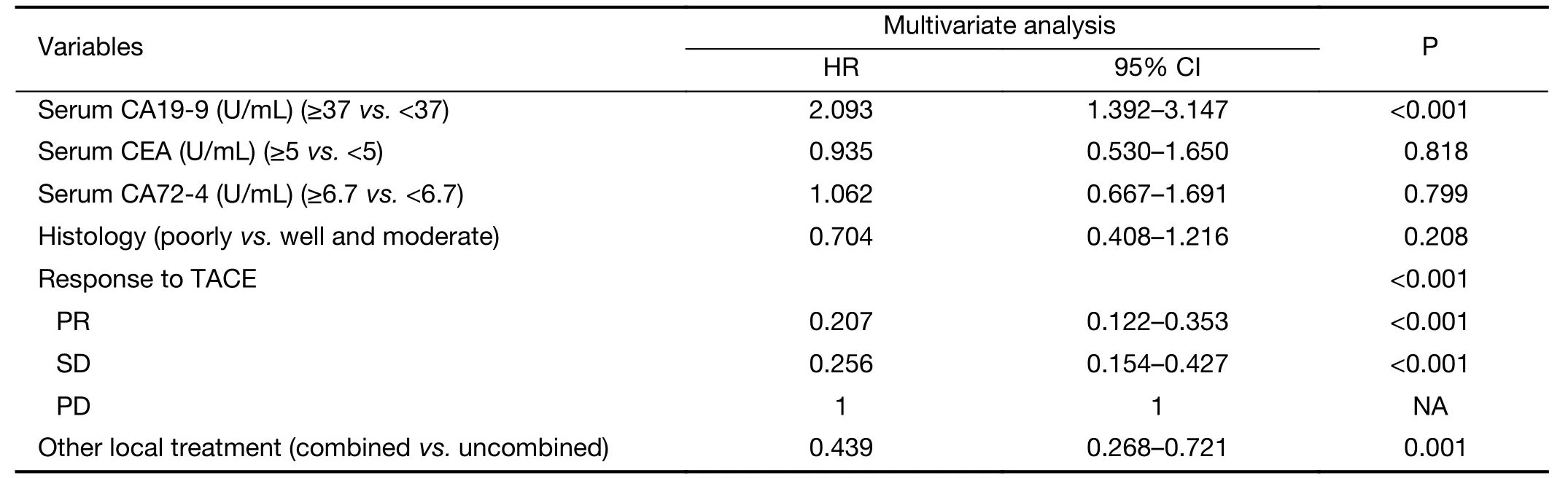
Table 3 Multiple Cox regression analysis of survival after first TACE and HAIC
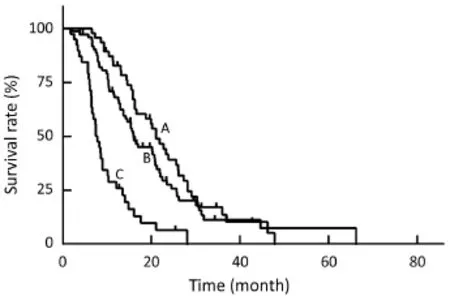
Figure 4 The Kaplan-Meier curves show the survival data after transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) according to the results of tumor response. The median survival time (MST) of patients achieved partial response (PR) was 21.1 months (curve A), for patients achieved stable disease (SD) was 16.6 months (curve B), and for patients achieved progressive disease (PD) was 7.8 months (curve C).
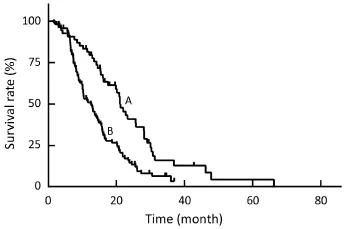
Figure 5 The Kaplan-Meier curves show the survival data after transarterial chemoembolization (TACE) and hepatic arterial infusion chemotherapy (HAIC) according to serum carbohydrate antigen 19-9 (CA19-9). Patients with normal serum CA19-9 had a median survival time (MST) of 21.1 months (curve A), and patients with elevated CA19-9 was 12.5 months (curve B).
As our study is a single-center retrospective research, we could not avoid some biases for the evaluation of clinical outcome and the incomplete patient data. The number of patient was unbalanced in different groups, which result in no significant difference of serum elevated CEA and size and number of liver metastases. A larger study may have demonstrated a statistical difference. Another limitation of our study is the subjectivity in image interpretation of tumor stain and lipidol deposit, but all the doctors joined in this study were experienced and professional doctors and worked in the same department and obeyed the same criteria. But our results provide some new directions for clinical practice and ideas. The relationship of serum tumor markers and survival was analyzed and we found that CA19-9 was a significant prognostic factor. Response to TACE/HAIC was proved to be an excellent predictive factor for OS. We also tried to explore the relation between tumor blood supply and survival, and pointed out that poorer blood supply may lead to better prognosis. But further randomized control clinical tries are needed to confirm it.
Conclusions
TACE combined with OXA based HAIC could be a safe, feasible and effective choice for liver-dominant refractory disease for whom there are limited treatment options. Normal serum CA19-9 and different response to TACE are independent risk factors for prognosis.
Acknowledgements
The authors thank the patients who participated in this study.
Funding: This study was supported by Capital Medical Development and Scientific Research Fund, China (No. 2014-2-2154); and National Science Foundation of China (No. 81571781).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
1.Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 2012;17:1225-39.
2.Nordlinger B, Van Cutsem E, Rougier P, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer 2007;43:2037-45.
3.Li Y, Bi X, Zhao J, et al. Simultaneous hepatic resection benefits patients with synchronous colorectal cancer liver metastases. Chin J Cancer Res 2016;28:528-35.
4.Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol 2005;23:4866-75.
5.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol 2004;22:229-37.
6.Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306-15.
7.Masi G, Salvatore L, Boni L, et al. Continuation or reintroduction of bevacizumab beyond progression to first-line therapy in metastatic colorectal cancer: final results of the randomized BEBYP trial. Ann Oncol 2015;26:724-30.
8.Pantelic A, Markovic M, Pavlovic M, et al. Cetuximab in third-line therapy of patients with metastatic colorectal cancer: A single institution experience. J BUON 2016;21:70-9.
9.Tellez C, Benson AB 3rd, Lyster MT, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 1998;82:1250-9.
10.Wasser K, Giebel F, Fischbach R, et al. Transarterial chemoembolization of liver metastases of colorectal carcinoma using degradable starch microspheres (Spherex): personal investigations and review of the literature. Radiologe (in German) 2005;45:633-43.
11.Gruber-Rouh T, Naguib NN, Eichler K, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer 2014;134:1225-31.
12.Massmann A, Rodt T, Marquardt S, et al. Transarterial chemoembolization (TACE) for colorectal liver metastases – current status and critical review. Langenbecks Arch Surg 2015;400:641-59.
13.Gruber-Rouh T, Marko C, Thalhammer A, et al. Current strategies in interventional oncology of colorectal liver metastases. Br J Radiol 2016 May 26:20151060. [Epub ahead of print]
14.Vogl TJ, Gruber T, Balzer JO, et al. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology 2009;250:281-9.
15.Del Freo A, Fiorentini G, Sanguinetti F, et al. Hepatic arterial chemotherapy with oxaliplatin, folinic acid and 5-fluorouracil in pre-treated patients with liver metastases from colorectal cancer. In Vivo 2006;20:743-6.
16.Boige V, Malka D, Elias D, et al. Hepatic arterial infusion of oxaliplatin and intravenous LV5FU2 inunresectable liver metastases from colorectal cancer after systemic chemotherapy failure. Ann Surg Oncol 2008;15:219-26.
17.Nozoe T, Kohno M, Iguchi T, et al. The prognostic nutritional index can be a prognostic indicator in colorectal carcinoma. Surg Today 2012;42:532-5.
18.Yoshida D, Ikeda Y, Waki K, et al. Different incidence of synchronous liver metastasis between proximal and distal colon cancer. Surg Today 2012;42:426-30.
19.Meta-Analysis Group in Cancer, Piedbois P, Buyse M, et al. Reappraisal of hepatic arterial infusion in the treatment of nonresectable liver metastases from colorectal cancer. J Natl Cancer Inst 1996;88:252-8.
20.Mocellin S, Pilati P, Lise M, et al. Meta-analysis of hepatic arterial infusion for unresectable liver metastases from colorectal cancer: the end of an era? J Clin Oncol 2007;25:5649-54.
21.Zacharias AJ, Jayakrishnan TT, Rajeev R, et al. Comparative effectiveness of hepatic artery based therapies for unresectable colorectal liver metastases: a meta-analysis. PloS one 2015;10:e0139940.
22.Leichman CG, Jacobson JR, Modiano M, et al. Hepatic chemoembolization combined with systemic infusion of 5-fluorouracil and bolus leucovorin for patients with metastatic colorectal carcinoma: A Southwest Oncology Group pilot trial. Cancer 1999;86:775-81.
23.Müller H, Nakchbandi W, Chatzissavvidis I, et al. Intra-arterial infusion of 5-fluorouracil plus granulocyte-macrophage colony-stimulating factor (GM-CSF) and chemoembolization with melphalan in the treatment of disseminated colorectal liver metastases. Eur J Surg Oncol 2001;27:652-61.
24.Aliberti C, Tilli M, Benea G, et al. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res 2006;26:3793-5.
25.Tsuchiya M, Watanabe M, Otsuka Y, et al. Transarterial chemoembolization with irinotecan (CPT-11) and degradable starch microspheres (DSM) in patients with liver metastases from colorectal cancer. Gan To Kagaku Ryoho (in Japanese) 2007;34:2038-40.
26.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997;15:938-46.
27.Sakamoto Y, Miyamoto Y, Beppu T, et al. Postchemotherapeutic CEA and CA19-9 are prognostic factors in patients with colorectal liver metastases treated with hepatic resection after oxaliplatin-based chemotherapy. Anticancer Res 2015;35:2359-68.
28.Qian Y, Zeng ZC, Ji Y, et al. Microinvasion of liver metastases from colorectal cancer: predictive factors and application for determining clinical target volume. Radiat Oncol 2015;10:125.
29.Yang Q, Liao F, Huang Y, et al. Longterm effects of palliative local treatment of incurable metastatic lesions in colorectal cancer patients. Oncotarget 2016;7:21034-45.
30.Chen L, Jiang B, Di J, et al. Predictive value of preoperative detection of CEA and CA199 for prognosis in patients with stage II-III colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi (in Chinese) 2015;18:914-9.
31.Wieners G, Pech M, Hildebrandt B, et al. Phase II feasibility study on the combination of two different regional treatment approaches in patients with colorectal “liver-only” metastases: hepatic interstitial brachytherapy plus regional chemotherapy. Cardiovasc Intervent Radiol 2009;32:937-45.
32.Osumi H, Shinozaki E, Suenaga M, et al. RAS mutation is a prognostic biomarker in colorectal cancer patients with metastasectomy. Int J Cancer 2016;139:803-11.
33.Xu JM, Qin XY, Zhong YS, et al. Survival of patients with liver metastasis from colorectal cancer by different modes of therapy: a report of 363 cases. Zhonghua Zhong Liu Za Zhi (in Chinese) 2007;29:54-7.
Cite this article as: Zhang H, Guo J, Gao S, Zhang P, Chen H, Wang X, Li X, Zhu X. Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatinbased hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis. Chin J Cancer Res 2017;29(1):36-44. doi: 10.21147/j.issn.1000-9604.2017.01.05
10.21147/j.issn.1000-9604.2017.01.05
*These authors contributed equally to this work.
Xu Zhu. Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Department of Interventional Therapy, Peking University Cancer Hospital & Institute, No. 52 Fucheng Road, Haidian District, Beijing 100142, China. Email: drzhuxu@163.com.
Methods:Between 2006 and 2015, 162 patients who underwent 763 TACE and HAIC in total were enrolled in this retrospective study, including 110 males and 52 females, with a median age of 60 (range, 26–83) years. Prognostic factors were assessed with Log-rank test, Cox univariate and multivariate analyses.
Results:The median survival time (MST) and median progression-free survival (PFS) of the 162 patients from first TACE/HAIC were 15.6 months and 5.5 months respectively. Normal serum carbohydrate antigen 19-9 (CA19-9, <37 U/mL) (P<0.001) and carbohydrate antigen 72-4 (CA72-4, <6.7 U/mL) (P=0.026), combination with other local treatment (liver radiotherapy or liver radiofrequency ablation) (P=0.034) and response to TACE/HAIC (P<0.001) were significant factors related to survival after TACE/HAIC in univariate analysis. A multivariate analysis revealed that normal serum CA19-9 (P<0.001), response to TACE/HAIC (P<0.001) and combination with other local treatment (P=0.001) were independent factors among them.
Conclusions:Our findings indicate that serum CA19-9 <37 U/mL and response to TACE/HAIC are significant prognostic indicators for this combined treatment, and treated with other local treatment could reach a considerable survival benefit for CRCLM. This could be useful for making decisions regarding the treatment of CRCLM.
Submitted Nov 03, 2016. Accepted for publication Jan 12, 2017.
 Chinese Journal of Cancer Research2017年1期
Chinese Journal of Cancer Research2017年1期
- Chinese Journal of Cancer Research的其它文章
- Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in treatment of gastric cancer with peritoneal carcinomatosis
- PEG-asparaginase in BFM-90 regimen improves outcomes in adults with newly diagnosed lymphoblastic lymphoma
- Extranodal involvement in young patients with diffuse large B-cell lymphoma: distribution, prognostic value and treatment options
- Upregulation of kazrin F by miR-186 suppresses apoptosis but promotes epithelial-mesenchymal transition to contribute to malignancy in human cervical cancer cells
- Clinical characteristics and response to tyrosine kinase inhibitors of patients with non-small cell lung cancer harboring uncommon epidermal growth factor receptor mutations
- Influencing factors of inpatient expenditure pattern for cancer in China, 2015
