富血小板血浆联合透明质酸钠关节内注射治疗膝骨关节炎的前瞻性随机对照研究
丁权威+吕帅洁+沈兴潮+童培建
摘 要 目的:评价富血小板血浆(PRP)联合透明质酸钠(HA)治疗膝骨关节炎(KOA)的临床疗效。方法:选取47例KOA患者分成PRP组和PRP+HA组,采用WOMAC、VAS和Lequesne评分评估它们在治疗前后的临床表现及比较临床疗效差异。结果: PRP组患者随访6个月时的有效率为74.1%,而PRP+HA组为80%。2组的WOMAC、VAS和Lequense评分较治疗前比较均有统计学差异(P<0.05),且PRP+HA组在3个月疗程内的VAS、WOMAC和Lequense评分均优于PRP组(P <0.05)。结论:单纯PRP或联合HA关节内注射治疗KOA均有缓解关节疼痛,改善关节功能的作用,而两者联合的疗效更加明显。
关键词 富血小板血浆 透明质酸钠 膝骨关节炎 软骨
中图分类号:R684.3 文献标识码:B 文章编号:1006-1533(2017)05-0025-04
A prospective randomized controlled study on platelet-rich plasma (PRP) combined with sodium hyaluronate (HA) intra-articular injection in the treatment of knee osteoarthritis*
DING Quanwei1**, LV Shuaijie2, SHEN Xingchao3, TONG Peijian1,2
(1. Zhejiang Chinese Medical University, Hangzhou 310053, China; 2. The First Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou 310053, China; 3. Shaoxing Traditional Chinese Medical Hospital, Shaoxing 312000, China)
ABSTRACT Objective: To evaluate clinical efficacy of platelet-rich plasma (PRP) combined with sodium hyaluronate(HA) in the treatment of knee osteoarthritis (KOA). Methods: Forty-seven cases of patients with KOA were selected and divided into a PRP group and a PRP+HA group and their manifestation in clinic was evaluated before and after treatment and clinical efficacy was compared based on WOMAC, VAS and Lequesne scores. Results: The effective rate was 74.1% in the PRP group and 80% in the PRP+HA group during six months of follow-up. The WOMAC, VAS and Lequense scores of both groups showed significant differences before and after treatment (P<0.05), and the VAS, WOMAC and Lequense scores were better in the PRP+HA group than in the PRP group during 3 months of treatment (P<0.05). Conclusion: PRP or PRP+HA intra-articular injection in the treatment of KOA can relieve joint pain and improve joint function, and furthermore the efficacy of PRP and HA in association is more significant.
KEY WORDS platelet-rich plasma; sodium hyaluronate; knee osteoarthritis; cartilage
膝關节骨关节炎(knee osteoarthritis, KOA)是一种由多因素导致的慢性进行性骨关节病,65岁以上人群的患病率达68%,表现为关节肿痛和功能障碍等,具有患病率高、晚期功能障碍程度高等特点[1]。目前为止并没有明确的药物和方法能够阻止软骨的退化进程,其治疗的首要目的是缓解膝关节疼痛和改善膝关节功能,包括药物疗法、关节内注射和手术等方法[2-3]。透明质酸钠(sodium hyaluronate , HA)作为一种关节润滑剂已被广泛应用于临床[4],具有润滑关节、保护软骨、抑制炎症和缓解疼痛等作用[5]。此外,富血小板血浆(plateletrich plasma,PRP)疗法作为具有修复受损软骨可能的自体生长因子注射疗法正逐渐引起重视。目前,有关PRP与HA联合使用的研究,未见报道。我院自2014年7月至2014年11月期间,对PRP联合HA关节内注射治疗KOA进行了前瞻性随机对照研究,报告如下。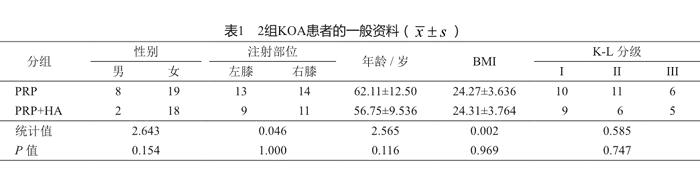
1 资料与方法
1.1 一般资料
本项目是一项临床前瞻性随机对照研究,已通过我院医学伦理委员会伦理审查(2013-X-063),参与本项目的所有患者在治疗前均签署了相关的知情同意书和诊疗同意书。
病例纳入标准:①年龄35至85岁;②符合1995年美国风湿病协会修订的《膝关节骨关节炎分类标准》[6];③Kellgren-Lawrence(KL)分级I~III级[7];④血小板数量等血液指标正常;⑤患者同意本治疗方法,并签署知情同意书。排除标准:①不符合KOA临床诊断标准者;②患有糖尿病、血液病等全身性疾病,或局部感染;③血红蛋白<11 g/L、血小板<1.5×105/L;④治疗前5 d内有非甾体类抗炎药使用史;⑤3个月内有服用抗凝血剂和免疫抑制剂者。
本研究共纳入47例,男10例,女37例;KL分级I级19例,II级17例,III级11例;平均年龄59.8岁。通过随机数字表法,将47例KOA患者分成PRP组和PRP+HA组。2组患者术前资料差异无统计学意义(P>0.05,表1),具有可比性。
1.2 手术方法
1.2.1 术前准备
所有患者术前均摄膝关节正侧位X线,并作出KL分级。术前进行膝关节WOMAC、VAS[8]和Lequesne评分[9],以了解患者的生活质量和患膝功能状况。术前检查患者血常规+CRP、生化类和凝血功能,以达到治疗前要求。
1.2.2 PRP制備
用一次性采血针及采血管,经患者肘前静脉取全血40 ml,用枸橼酸钠抗凝。采用二次离心法:第1次用1 450 r/min离心(离心机型号:TAS10-TLL700A)10 min。用吸管吸取全部上清液至液固交界面下3 mm。将取出的上清液再次离心。第2次3 370 r/min离心10 min。吸取约3/4上清液弃掉,剩余摇匀,即为PRP,共5 ml。取1 ml PRP 行血小板计数。剩余4 ml在2 h内完成注射。注射前在PRP中加入1 ml氯化钙(上海信谊金朱药业有限公司)以激活血小板。
1.2.3 注射治疗
1)PRP组 患者取仰卧位,伸直患膝。局部消毒,选取髌骨下方的髌韧带内侧或外侧关节间隙为进针点,用一次性注射器经皮穿刺入关节腔。如关节腔内有较多积液时可先抽出部分积液。将4 ml PRP注射入膝关节病变部位。1次/周,3次/疗程,每个疗程间隔1周,共3疗程。
2)PRP+HA组 将激活的PRP与HA(25 mg/2.5 ml;Hyalgan, Fidia, Abano Terme, Italy)在一次性注射器内混合后,采用相同体位和方法将6.5 ml混合物注射入膝关节。疗程同PRP组。
1.3 术后处理及疗效判定
术后局部无菌包扎,并嘱患者屈伸膝关节数次,常规口服头孢地尼分散片0.1 g tid 2 d以防感染。嘱患侧关节避免剧烈运动,告知患者术后疼痛及肿胀等相关可能性。采用WOMAC、VAS和Lequesne评分标准进行疗效判定。随访时以WOMAC评分下降至少36%[10]及以上定义为治疗有效。
1.4 统计学处理
2 结果
所有患者均获得至少6个月随访。2组患者在治疗过程中均未出现膝关节红肿、发热等局部感染和其他不适症状。随访显示,2组的WOMAC、VAS和Lequense评分较治疗前比较均有统计学差异(P1 <0.05,表2),且PRP+HA组在治疗开始后的最初3个月,VAS、WOMAC和Lequense评分均优于PRP组,差异有统计学意义(P<0.05,表2)。但是3个月疗程结束后随访发现,2组在VAS、WOMAC和Lequense评分方面均无统计学差异(P>0.05,表2)。根据WOMAC评分至少下降36%为治疗有效计算,PRP组患者随访6个月的有效率为74.1%,略低于PRP+HA组的80%。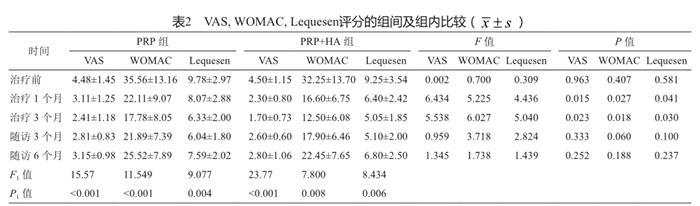
3 讨论
膝关节骨关节炎作为一种慢性进行性的关节疾病,是造成工作能力丧失的第二大主要原因[11],随之而来的是沉重的经济与社会负担[12]。关于KOA 的病因和发病机制等至今尚不十分明确[13],关节软骨退行性改变是KOA的基本病理变化和病理基础,同时受累关节滑膜所分泌的滑液成分也发生改变[14]。由于KOA患者关节腔内HA合成受损、自由基降解[15]和积液稀释[16]等原因,导致内生HA的分子量和浓度下降,引起关节黏弹性降低,关节软骨抗机械应力的能力下降而关节受损[17]。
作为滑液中最主要的成分,HA是一种由N-乙酰-D-氨基葡萄糖和D-氨基葡萄糖组成的无支链的聚阴离子聚合物,在关节内起营养与保护作用[18]。虽然最新的美国KOA诊疗指南不推荐使用HA[19],但是动物实验发现,HA在抗炎、抗细胞凋亡、抗血管生成和抗纤维化方面具有重要作用[20]。而大量临床研究也表明,HA具有缓解关节疼痛和改善关节功能等作用[21-23]。但是有学者认为,HA对于KOA病情严重的患者疗效不佳[24],而且随着时间延长,HA的疗效呈下降趋势[25],这在高龄患者中尤为明显[26],同时HA也无法使受损软骨再生[27]。
PRP作为大量生长因子的载体[28],具有促进组织修复的功能[29],正越来越多地被应用于KOA的治疗。PRP的制备目前多采用二次离心法[30],通过此法我们将血小板浓缩至原始数值的4.6倍。有学者认为,PRP可能使滑膜组织、脂肪垫或者软骨下骨中的前体细胞迁移、增殖和分化,从而促进受损软骨的修复[31-32],同时达到减轻疼痛、减少炎症反应的作用[33]。本研究显示,PRP组在VAS、WOMAC和Lequesen评分方面均优于治疗前(差异有统计学意义),说明PRP确实具有缓解膝部疼痛和改善关节功能的作用。但临床疗效随着时间的延长呈逐渐下降趋势。
有研究发现,HA和PRP可以在不改变两者最初特性的情况下发挥作用[34]。Anitua等[35]通过比较肌腱细胞和滑膜成纤维细胞在单纯PRP溶液和PRP+HA溶液中的迁移能力发现,PRP与HA混合后能显著提高细胞的运动能力。Marmotti等[36]则发现PRP中加入HA能有效促进软骨细胞的增殖,提高软骨修复能力。因此,我们将PRP与HA联合注射治疗,3个月随访示,联合组疗效优于單纯PRP组,这可能与HA协同润滑关节、改善关节功能有关。随访6个月时,虽然2组在VAS、WOMAC和Lequesen评分方面的差异无统计学意义。但是PRP+HA组的评分结果优于PRP组,说明HA作为载体,或许有延缓生长因子释放、延长PRP作用时间的功效,这仍有待于进一步研究证实。
治疗期间,部分患者出现局部肿胀、酸楚感,大多数患者在1 d后症状消失,2组患者中均无感染等不良事件发生。本研究属于临床短期疗效评价,因此没有进行关节软骨变化的观察,而长期疗效则需更大样本量及更长随访时间的观察。此外,PRP与HA混合后的协同作用及机制,仍需我们进一步的深入探讨。
参考文献
[1] Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25, 124 knee arthroscopies[J]. Knee, 2007, 14(3): 177-182.
[2] Filardo G, Kon E, Buda R, et al. Platelet-rich plasma intraarticular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis[J]. Knee Surg Sports Traumatol Arthrosc, 2011, 19 (4): 528-535.
[3] Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intraarticular knee injections produced favorable results on degenerative cartilage lesions[J]. Knee Surg Sports Traumatol Arthrosc, 2010, 18(4): 472-479.
[4] Peyron JG, Balazs EA. Preliminary clinical assessment of Na hyaluronate injection into human arthritic joints[J]. Pathol Biol(Paris), 1977, 22(8): 731-736.
[5] 侯德才. 膝关节骨性关节炎的分期治疗[J]. 中医正骨, 2014, 26(1): 3-5.
[6] Hochberg MC, Altman RD, Brandt KD, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee. American College of Rheumatology[J]. Arthritis Rheum, 1995, 38(11): 1541-1546.
[7] Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis[J]. Ann Rheum Dis, 1957, 16(4): 494-502.
[8] Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinical important patient relevant outcomes to antirheumatic drug therapy in patients with OA of the hip or knee[J]. J Rheumatol, 1988, 15(12): 1833-1840.
[9] Lequesne MG. The algofunctional indices for hip and knee osteoarthritis[J]. J Rheumatol, 1997, 24(4): 779-781.
[10] Goldsmith CH, Boers M, Bombardier C, et al. Criteria for clinically important changes in outcomes; development, scoring and evaluation of rheumatoid arthritis patient and trial profiles. OMERACT Committee[J]. J Rheumatol, 1993, 20(3): 516-527.
[11] Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States Part II[J]. Arthritis Rheum, 2008, 58(1): 26-35.
[12] Stewart WF, Ricci JA, Chee E, et al. Lost productive time and cost due to common pain conditions in the US workforce[J]. JAMA, 2003, 290(18): 2443-2454.
[13] Gobbi A, Lad D, Karnatzikos G. The effects of repeated intra-articular PRP injections on clinical outcomes of early osteoarthritis of the knee[J]. Knee Surg Sports Traumatol Arthrosc, 2015, 23(8): 2170-2177.
[14] Aigner T, Kim HA. Apoptosis and cellular vitality: issues in osteoarthritic cartilage degeneration[J]. Arthritis Rheum, 2002, 46(8): 1986-1996.
[15] Myint P, Deeble DJ, Beaumont PC, et al. The reactivity of various free radicals with hyaluronic acid: steady-state and pulse radiolysis studies[J]. Biochim Biophys Acta, 1987, 925(2): 194-202.
[16] Ono Y, Sakai T, Hiraiwa H, et al. Chondrogenic capacity and alterations in hyaluronan synthesis of cultured human osteoarthritic chondrocytes[J]. Biochem Biophys Res Commun, 2013, 435(4): 733-739.
[17] Campo GM, Avenoso A, DAscola A, et al. 4-mer hyaluronan oligosaccharides stimulate inflammation response in synovial fibroblasts in part via TAK-1 and in part via p38-MAPK[J]. Curr Med Chem, 2013, 20(9): 1162-1172.
[18] Reitinger S, Lepperdinger G. Hyaluronan, a ready choice to fuel regeneration: a mini review[J]. Gerontology, 2013, 59(1): 71-76.
[19] Jevsevar DS, Brown GA, Jones DL, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition[J]. J Bone Joint Surg Am, 2013, 95(20): 1885-1886.
[20] Abate M, Pulcini D, Di Iorio A, et al. Viscosupplementation with intra-articular hyaluronic acid for treatment of osteoarthritis in the elderly[J]. Curr Pharm Des, 2010, 16(6): 631-640.
[21] Hunter DJ, Lo GH. The management of osteoarthritis: an overview and call to appropriate conservative treatment[J]. Rheum Dis Clin North Am, 2008, 34(3): 689-712.
[22] Campbell J, Bellamy N, Gee T. Differences between systematic reviews/meta-analyses of hyaluronic acid/ hyaluronan/hylan in osteoarthritis of the knee[J]. Osteoarthritis Cartilage, 2007, 15(12): 1424-1436.
[23] Miller LE, Block JE. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline controlled trials[J]. Clin Med Insights Arthritis Musculoskelet Disord, 2013, 6: 57-63.
[24] Dagenais S. Intra-articular hyaluronic acid(viscosupplementation) for knee osteoarthritis[J]. Issues Emerg Health Technol, 2006(94): 1-4.
[25] Bannuru RR, Natov NS, Dasi UR, et al. Therapeutic trajectory following intraarticular hyaluronic acid injection in knee osteoarthritis-meta-analysis[J]. Osteoarthritis Cartilage, 2011, 19(6): 611-619.
[26] U?ar D, D?ra?o?lu D, Süleyman T, et al. Intra-articular hyaluronic Acid as treatment in elderly and middle-aged patients with knee osteoarthritis[J]. Open Rheumatol J, 2013, 7: 38-41.
[27] Andia I, Abate M. Knee osteoarthritis: hyaluronic acid, platelet-rich plasma or both in association? [J]. Expert Opin Biol Ther, 2014, 14(5): 635-649.
[28] Cole BJ, Seroyer ST, Filardo G, et al. Platelet-rich plasma: where are we now and where are we going? [J]. Sports Health, 2010, 2(3): 203-210.
[29] Patel S, Dhillon MS, Aggarwal S, et al. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial[J]. Am J Sports Med, 2013, 41(2): 356-364.
[30] Karystinou A, DellAccio F, Kurth TB, et al. Distinct mesenchymal progenitor cell subsets in the adult human synovium[J]. Rheumatology (Oxford), 2009, 48(9): 1057-1064.
[31] Manferdini C, Maumus M, Gabusi E, et al. Adipose-derived mesenchymal stem cells exert anti-inflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2[J]. Arthritis Rheum, 2013, 65(5): 1271-1281.
[32] de Vries-van Melle ML, Narcisi R, Kops N, et al. Chondrogenesis of mesenchymal stem cells in an osteochondral environment is mediated by the subchondral bone[J]. Tissue Eng Part A, 2013, 20(1-2): 23-33.
[33] 呂帅洁, 厉驹, 何斌, 等. 富血小板血浆关节内注射治疗膝骨关节炎的前瞻性随机对照研究[J]. 中华创伤杂志, 2016, 32(7): 626-631.
[34] Sundman EA, Cole BJ, Karas V, et al. The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis[J]. Am J Sports Med, 2013, 42(1): 35-41.
[35] Anitua E, Sanchez M, De la Fuente M, et al. Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid[J]. Knee Surg Sports Traumatol Arthrosc, 2012, 20(9): 1657-1665.
[36] Marmotti A, Bruzzone M, Bonasia DE, et al. One-step osteochondral repair with cartilage fragments in a composite scaffold[J]. Knee Surg Sports Traumatol Arthrosc, 2012, 20(12): 2590-2601.

