养殖污水中蛋白核小球藻的分离鉴定及其污水处理效果
陆洪省,刘亚樵,刘文君,孔凡民,谭好臣
养殖污水中蛋白核小球藻的分离鉴定及其污水处理效果
陆洪省,刘亚樵,刘文君,孔凡民,谭好臣
(山东科技大学化学与环境工程学院,青岛 266590)
利用BG-11培养基从养殖污水中分离和筛选到小球藻,采用相差显微镜对其外部形态进行观察、傅里叶红外光谱分析球藻组成、X-ray方法分析其晶体组成、凯氏定氮以及有机溶剂萃取方法分别测定其蛋白质以及脂质含量、丙酮萃取法测定其叶绿素a以及国标法测定其处理污水效果。结果表明:根据相差显微镜对球藻外部观察,初步判断该小球藻为蛋白核小球藻;红外光谱测定结果表明,在波数1 080和1 240 cm-1处均有明显的吸收峰,证明了该研究分离的小球藻含有大量蛋白质以及糖类和脂类成分;X-衍射分析结果显示在32°和20°两处均有明显的吸收峰,证明了本研究分离的小球藻含有蛋白质和脂类成分;定量成分测定表明该蛋白核小球藻蛋白质量分数为45.6%,脂质质量分数为8.1%;污水处理结果为化学需氧量去除率为70.9%;总氮、总磷去除率分别为23%和34.7%。该研究对蛋白核小球藻蛋白含量、脂肪含量的测定以及提取进行了分析,证明了蛋白核小球藻对养殖场污水的处理具有很好的效果,为生物新资源的开发以及水环境治理提供了一定的参考。
污水;净化;氮;磷;蛋白核小球藻;筛选鉴定;成分分析;处理效果
0 引 言
藻类具有生长速度快、容易获得、代谢迅速、吸附能力强、对营养物质需求低,主要利用光、水和CO2进行生长等特点[1],越来越多的引起了人们的重视。对藻类通过吸附作用处理污水的研究,国外从20世纪80年代开始,国内近年来才陆续开始这方面的研究[2-9]。
养殖污水中富含N、P等化合物,如果直接排放到环境中很容易造成水体富营养化,如何去除养殖污水中N、P化合物越来越引起人们的关注。微藻有较强的适应环境的能力,并且能在异养条件下吸收污水中大量的有机物,因而被广泛应用到生活污水或者有机废水的处理中[10-13]。
影响微球藻生长的因素包括营养条件、光照和温度等。有研究报道,在异养条件下,碳、氮、磷是影响微藻生长的最重要的营养元素[14],尤其是碳、氮2种元素对微藻生长的影响更加明显一些[15]。利用球藻对水体中氮磷的去除研究有很多,如周培疆等[16]报道了普通小球藻对水体中不同形态磷的利用;另外,利用螺旋藻对造纸和酿酒工业污水、养殖废水、城市垃圾处理液以及城市生活废水(domestic wastewater, DW)等进行生物净化和修复的报道也很多[17-21]。
到目前为止,对藻类的研究大多集中在对藻类的加工利用方面,尤其是利用藻类生产生物柴油方面,利用藻类处理污水的研究较少,特别是利用藻类处理养殖污水的报道非常少。微藻包括蓝藻门、绿藻门、金藻门和红藻门,蛋白核小球藻属于绿藻门中的一种,为球形单细胞淡水藻,富含叶绿素和蛋白质等成分。本研究首先对蛋白核小球藻()进行富集、筛选、纯化以及其营养组成的分析,在此基础上分析蛋白核小球藻()对养殖污水的处理效果,包括化学需氧量(chemical oxygen demand,COD)、总氮(total nitrogen,T-N)和总磷(total phosphorus,T-P)的去除,为蛋白核小球藻()在污水处理以及生物资源的开发利用方面提供理论基础。
1 材料与方法
1.1 球藻的分离与富集
球藻分离:球藻分离培养基组成(g/L):KNO31.25,KH2PO41.25,MgSO40.49,乙二胺四乙酸二钠(EDTA二钠)0.5,H3BO30.1,质量体积比3%的FeCl3溶液0.21 ml,琼脂15(配制平板固体培养基),pH值调整为7.1,121 ℃灭菌20 min。采用平板法分离藻,具体步骤:用消毒过的小型喷雾器将含有藻的水样(来自某养鸭场)喷射到固体平板培养基上,盖好培养皿盖,在光照(4.8±0.2)×103lx(日光灯源)条件下培养,培养温度为28 ℃,待长出藻菌落后,借助镜检,用消毒过的接种环挑选球藻,将挑选到的球藻再分别多次划线平板培养,通过外形观察,确定分离藻为纯藻为止。
球藻的富集:将上述分离到的小球藻在BG-11培养基中富集培养。BG-11培养基组成(g/L):NaNO31.5,K2HPO40.04,MgSO4.7H2O 0.075,CaCl2.7H2O 0.036,Na2CO30.02,柠檬酸0.006,柠檬酸铁0.006,氨苄青霉素0.05,微量元素溶液1 mL,固体平板培养基配制时加琼脂15.0,蒸馏水1 000 mL。微量元素溶液组成(g/L):H3BO42.86,MnCl2·4H2O 1.81,ZnSO40.222,Na2MoO40.39,CuSO4·5H2O 0.079,Co(NO3)2·6H2O 49.4,用1 000 mL水溶解,培养条件同球藻分离过程。
1.2 球藻外部形态以及基本成分定量测定
将上述分离到的球藻在BG-11培养基中富集培养,用相差显微镜(型号:BM-PH;上海光学仪器厂)观察藻形态,放大倍数为400。小球藻细胞水分含量测定:取富集培养中的藻液,进行高速离心(12 000 r/min)10 min,用万分之一电子天平称量湿质量,然后在105 ℃烘箱中烘干至恒质量[22],用万分之一电子天平称量干质量,根据公式:水分含量=(湿质量-干质量)/湿质量。灰分采用灼烧法[23],粗脂含量测定采用超声波提取法[24-25]:称取0.2 g(记作)藻粉置于离心管中,加4 mL 1∶1正己烷-异丙醇(体积比)溶液,摇匀,放入超声仪中萃取25 min,萃取温度为30 ℃,然后将萃取液于10 000 r/min 离心10 min,取上层清液加入到质量为1的离心管中,105 ℃烘箱烘干2 h,称质量为2,重复上述试验3次,则粗脂含量通过以下公式求出:粗质含量=(2-1)/× 100%,蛋白质含量测定采用凯氏定氮方法。
1.3 球藻叶绿素a的变化与废水CODcr、总氮(T-N)以及总磷(T-P)的关系
将10 mL密度为170 cells/mL的藻培养液接种到200 mL灭菌过的养鸭厂污水中,灭菌条件为121 ℃ 20 min,进行光照培养,光照强度为(4.8±0.2)×103lx (日光灯源),培养温度为28 ℃,每隔12 h取20 mL藻液测定发酵液中藻叶绿素a的浓度、CODcr、总氮(T-N)和总磷(T-P)。叶绿素a测定方法采用丙酮提取法[26],CODcr测定采用国标GB11914-1989中规定的重铬酸钾氧化法,总氮(T-N)用过硫酸钾氧化-紫外分光光度法测定(HJ/T 199-2005),总磷(T-P)测定采用钼酸铵分光光度法(国标GB/T 11893-1989 )。藻叶绿素a、废水CODcr、总氮(T-N)和总磷(T-P)测定时均采用3个平行试验,求平均值。
1.4 球藻傅里叶红外(FT-IR)分析
利用藻培养基BG-11对藻富集过程中,取对数增长期的球藻进行离心,转速为6 000 r/min,用超纯水洗涤藻泥3次,所得干净藻泥,然后在50 ℃条件下烘干上述藻泥,用玛瑙研钵磨细,用KBr固定,利用傅里叶红外光谱仪(型号为FTIR-8900;天津市拓普仪器有限公司)进行测定,样品扫描时间为2 min,背景扫描时间为2 min,波数扫描范围为400~4 000 cm。
1.5 球藻X-衍射分析
将1.4中制备的球藻粉于X-衍射仪(型号:Saturn 724)进行分析,确定其晶体组成,测定方法按仪器说明书。
1.6 球藻生长与水体中溶解氧(Dissolved oxygen,DO)的相关性分析
球藻能利用水体中氮磷等物质进行代谢,同时能利用光能和CO2进行光合作用,释放出氧气(O2),引起污水中溶解氧(DO)升高,本研究利用可见分光光度计测定球藻的生长变化,具体过程:将富集培养2d的球藻液接种到灭菌后的BG-11液体培养基中,接种方法以及培养条件同1.3,每隔24 h取藻液用可见分光光度计(型号为722)测定其吸光度,吸收波长采用680 nm[27],便携式溶氧仪(型号为Thermo Orion 3 star)测定水体中溶解氧DO的变化。
2 结果与分析
2.1 藻外部形态
将小球藻接种到养殖场污水中,生长繁殖3 d后利用相差显微镜进行观察,小球藻形状如图1所示。从图1可以看出,分离到的小球藻为绿藻门,小球藻科,小球藻属(),球状,直径在3~8 mm,且在藻中心处有一明显核存在,因此,分离到球藻为小球藻属中的蛋白核小球藻,该蛋白核小球藻在水体中呈单细胞存在,分裂速度快。
2.2 蛋白核球藻基本成分测定结果
蛋白核球藻经28 ℃条件下培养、富集、离心、洗涤收集后通过烘干、灼烧、萃取和凯氏定氮方法对其灰分、蛋白质以及粗脂肪进行定量测定,结果为:灰分及其他(29.3%),蛋白质(46.7%)和粗脂(24%),以上数值均为干质量百分数。不同的环境条件,如不同pH值、氮源、温度等都会影响到蛋白核小球藻组成成分。
2.3 球藻生长与溶解氧相关性
小球藻在污水中生长过程中,每隔1 d取藻液测定其吸光度(OD680),同时测定藻液中溶解氧(DO),做出吸光度-时间、溶解氧(DO)-时间曲线(图2)。从图2可以看出,小球藻在培养到2 d时开始出现快速生长,到第7天生长量最大。溶解氧(DO)总体上随时间也呈现上升趋势,但与吸光度(OD680)上升趋势并非完全一致,在藻生长到2 d前,藻液中溶解氧(DO)并没有出现像吸光度(OD680)类似的快速升高的现象,这可能与空气中氧气对藻液的复氧有关。
2.4 藻叶绿素a的变化与废水CODcr、T-N以及T-P的关系
将蛋白核小球藻(3×108)藻液按体积比1:20接种到养殖污水中,在光照以及通入CO2条件下下培养,每隔1 d取培养液测定叶绿素a、水体总氮、水体总磷以及水体CODcr,结果如图3所示。从图3可以看出,总氮、总磷去除率分别为23%和34.7%,随着叶绿素a量升高,水体中总氮、总磷均呈下降趋势,这说明随着藻类的大量繁殖,藻类吸收养殖污水中氮、磷化合物,从而导致水体中氮、磷含量降低,CODcr去除率高达70.9%,且随着藻类的生长,水体CODcr也呈现出下降趋势。
2.5 藻FT-IR测定结果
红外光谱是一种分析有机化合物官能团有效手段[28]。本研究对养鸭废水中分离到的球藻进行红外光谱分析,如图4所示,官能团与波峰分析结果如表1中所示。
参考邢波[29]的苯系列傅里叶变换红外(FT-IR)光谱的研究,结合图4可以看出,3 320 cm-1的吸收归属为O-H键的振动吸收峰,2 930 cm-1的吸收归属为C-H键的振动吸收峰,1 650 cm-1的吸收归属为C=O双键的振动吸收峰,1 080 cm-1的吸收代表C-N键的存在,归属为蛋白质,1 240 cm-1处的振动峰,代表C-O键存在,与脂类和糖类的存在相关。
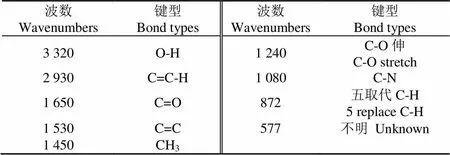
表1 球藻官能团的红外分析结果
2.6 蛋白核小球藻的X-衍射测定结果
对蛋白核小球藻进行离心、超纯水洗涤后,进行烘干,对获得的干藻通过X-ray进行测定,测定条件是2从10°到80°区间,结果如图5所示。从图5可以看出,在32°处有一明显峰,在20°左右也有明显的峰出现,参考高华娜等[30]有关螺旋藻的XRD分析报道以及X-衍射图谱的分析手册,认为本研究中分离到的蛋白核小球藻含有大量的蛋白类和脂类物质。
3 结 论
利用球藻分离培养基从养鸭场污水中分离到小球藻,通过外形观察(相差显微镜)初步判定该球藻为蛋白核小球藻。在此基础上,对蛋白核小球藻的组成包括蛋白质和脂肪物质进行了定性(FT-IR和X-衍射)和定量分析(凯氏定氮)。将此蛋白核小球藻应用到养殖场污水处理过程中,通过比较处理前与处理后水中CODcr、总氮(T-N)以及总磷(T-P)的变化,结果表明该蛋白核小球藻对CODcr的去除率为70.9%,对总氮、总磷去除率分别为23%和34.7%,从而证明了该蛋白核小球藻()具有很好的养殖场污水处理能力。
本研究中分离到的蛋白核小球藻在污水处理中的其他应用,如对水体中铅、铬和汞等重金属的吸附去除也是未来的研究方向之一,对拓展蛋白核小球藻在污水处理中的应用具有积极的意义。此外,该研究中分离到的蛋白核小球藻()富含蛋白质、脂肪酸以及一定量的促生长因子,对其中蛋白质的分离、纯化,脂肪酸的分离和提取、促生长因子的提取以及其对活细胞代谢的影响等研究也在进行中。本研究中分离到的蛋白核小球藻不仅对污水的处理具有很好的效果,而且富含蛋白质和脂肪酸,具有良好的深加工前景,包括制备生物柴油。在以后的研究中,在利用藻类处理污水的同时,将大量生长的蛋白核小球藻()进行收集并深加工,开发出新的生物新资源也是未来研究的方向之一。
[1] 莫健伟,姚兴东,张谷兰,等. 海藻去除水中双偶氮染料机理及重金属离子研究[J]. 中国环境科学,1997,17(3):241-243.
Mo Jianwei, Yao Xingdong, Zhang Gulan, et al. Study on the removal mechanism of azo dyes and heavy metal ions in water with algae ()[J]. China Environmental Science, 1997, 17(3): 241-243. (in Chinese with English abstract)
[2] Leusch A, Holan Z R, Volesky B. Biosorption of heavy metals (Cd, Cu, Ni, Pb, Zn) by chemically reinforced biomassof marine algae[J]. J Chem Tech Biotechnol, 1995, 62(3): 279-288.
[3] Wilke A, Bunke G, Gotz P, et al. Removal of lead, cadmium, zinc andnickel by aadsorption on microalgae[J]. Prog Min Oilfield Chem, 1999, 4(1): 337-344.
[4] Volesky B, Holan Z R. Biosorption of heavy metals[J]. Biotechnol Prog, 1995, 11: 236-241.
[5] Bakkaloglu I, Butter T J, Evison L M, et al. The kinetics of metal uptake by microbial biomass:Implications for the design of a biosorption reactor[J]. Wat Sci Tech, 1998, 38: 269-275.
[6] Matsunaga T, Takeyama H, Nakao T, et al. Screening of marine microalgae for bioremediation of cadmium-polluted seawater[J]. Journal of Biotechnology, 1999, 70: 33-40.
[7] 林荣根,黄朋林,周俊良. 两种褐藻对铜和镉的吸着及洗脱研究[J]. 海洋环境科学,1999,18(4):8-13.
Lin Ronggen, Huang Penglin, Zhou Junliang. Study on the adsorption and desorption of copper and cadmium in water on two species of brown algae[J]. Marine Environmental Science, 1999, 18(4): 8-13. (in Chinese with English abstract)
[8] 李英敏,杨海波,吕福荣,等. 小球藻对Pb2+的吸附及生物吸附机理初探[J]. 农业环境科学学报,2004,23(4):696-699.Li Yingmin, Yang Haibo, Lu Furong, et al. Sorption of Pb2+by Chlorella Vulgaris and biosorption mechanism[J]. Journal of Agro-Environment Science, 2004, 23(4): 696-699. (in Chinese with English abstract)
[9] 尹平河,赵玲,于平,等. 海藻生物吸附废水中铅、铜和镉的研究[J]. 海洋环境科学, 2000,19(3):11-15.
Yin Pinghe, Zhao Ling, Yu Ping, et al. Biosorption of lead, copper and cadmium by marine macro algae[J]. Marine Environmental Science, 2000, 19(3): 11-15. (in Chinese with English abstract)
[10] 高正权,孟春晓. 微藻与水环境修复[J]. 环境科学与技术,2008,25(3):21-25.
Gao Zhengquan, Meng Chunxiao. Microalgae and rehabilitation of water environment[J]. Environmental Science and Technology, 2008, 25(3): 21-25.
[11] 董芳芳,程凯,许敏. 藻类对造纸废水中COD 的去除效率研究[J]. 环境科学技术,2010,33(1):166-169.
Dong Fangfang, Cheng Kai, Xu Min. Removal of COD in paper-making wastewater by algae[J]. Environmental Science and Technology, 2010, 33(1): 166-169. (in Chinese with English abstract)
[12] 李岩,周文广,张晓东,等. 微藻培养技术处理猪粪厌氧发酵废水效果[J]. 农业工程学报,2011,27(增刊1):101-104.
Li Yan, Zhou Wenguang, Zhang Xiaodong, et al. Effect of microalgae culture on treatment of wastewater from anaerobic digested swine manure[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2011, 27(Supp.1): 101-104. (in Chinese with English abstract)
[13] 张聪. 利用城市污水厂污泥培养海洋微藻技术研究[D]. 青岛:中国海洋大学,2007.
Zhang Cong. A Study on the Technique for Culturing Marine Microalgae with Sewage Sludge from Municipal Wastewater Treatment Plants[D]. Qingdao: Ocean University of China, 2007. (in Chinese with English abstract)
[14] Chen G Q, Chen F. Growing phototrophic cells without light[J]. Biotechnology Letters, 2006, 28(9): 607-616.
[15] Li Z S, Yuan H L, Yang J S, et al. Optimization of the biomass production of oil algaeUTEX2341[J]. Bioresource Technology, 2011, 102(19): 9128-9134.
[16] 周培疆,郑振华,余振坤,等.普通小球藻生长与武汉东湖水体磷形态的相关研究[J]. 水生生物学报,2001,25(6):571-576.
Zhou Peijiang, Zheng Zhenhua, Yu Zhenkun, et al. Studies on the relationship between the growth ofand phosphorus fractions in water of lake Donghu, Wu Han[J]. Act Hydrobiologica Sinica, 2001, 25(6): 571-576. (in Chinese with English abstract)
[17] Olguin E J, Galicia S, Mercardo G, et al. Annual productivity of() and nutrient removal in a pig wastewater recycling process under tropical conditions[J]. J Appl Phyco, 2003, 15(2/3): 249-257.
[18] Phang S M, Miahm S, Yeoh B G, et al. Spirulinacultivation in digested sago starch factory wastewater[J]. J Appl Phyco, 2000, 12(3/4/5): 395-400.
[19] Kim M H, Chungw T, Leem K, et al. Kinetics ofremoving nitrogenous and phosphorus compounds from swine waste by growth of microalga,[J]. J Microbiol Biotechn, 2000, 10(4): 455-461.
[20] 黄昆,黄峙,吕颂辉. 螺旋藻()对垃圾填埋渗滤液污染物的净化作用[J]. 生态环境,2006,15(3):509-512.
Huang Kun, Huang Zhi, Lü Songhui. Removal effects of microalga () on landfill leachate purification[J]. Ecology and Environment, 2006, 15(3): 509-512. (in Chinese with English abstract)
[21] 黄昆,张逸波,黄峙,等. 生活废水培养螺旋藻()中的营养物质构成[J]. 暨南大学学报:自然科学版,2009,30(5):576-580.
Huang Kun, Zhang Yibo, Huang Zhi, et al. Nutrient composition ofgrown on digested domestic wastewater[J]. Journal of Ji Nan University: Natural Science, 2009, 30(5): 576-580. (in Chinese with English abstract)
[22] 牛向丽. 杜氏盐藻生物学特征及表达载体pGEM-D18S-BAR的构建[D]. 郑州:郑州大学,2003.Niu Xiangli. Biological Characteristics ofand Construction of Expression Vector pGEM-D18S-BAR[D]. Zhengzhou: Zhengzhou University, 2003. (in Chinese with English abstract)
[23] 何珣,魏晓惠,郭浩,等. 马弗炉程序控温测定茶叶灰分含量[J]. 福建茶叶,2008,30(3):34-35.
He Xun, Wei Xiaohui, Guo Hao, et al. The measurement of ash in tea with muffle program controlling temperature[J]. Fujian Tea, 30(3): 34-35. (in Chinese with English abstract)
[24] 冯棋琴,胡爱军,胡小华. 超声波技术在提取保健油脂中应用[J]. 粮食与油脂,2009(8):4-6.
Feng Qiqin, Hu Aijun, Hu Xiaohua. Application of ultrasonic techniques in health oil extraction[J].Foods and Oil, 2009(8): 4-6. (in Chinese with English abstract)
[25] 贾元超,徐琅,陈重,等. 不同提取方法对续随子种子中不饱和脂肪酸得率的影响[J]. 安徽农业科学,2008,36(18):7509-7511,7513.
Jia Yuanchao, Xu Liang, Chen Zhong, et al. Effect of different extraction methods on Yield of unsaturated fatty acids from. Seeds[J]. Journal of Anhui Agriculture Science, 2008, 36(18): 7509-7511, 7513. (in Chinese with English abstract)
[26] 张丽君,杨汝德,肖恒. 小球藻的异养生长及培养条件优化[J]. 广西植物,2001,21(4):353-357.
Zhang Lijun, Yang Rude, Xiao Heng. The hetertrophic culture of Chlorella and optimization of growth condition[J]. Guangxi Plant, 2001, 21(4): 353-357. (in Chinese with English abstract)
[27] 吕旭阳,张雯,杨阳,等.分光光度法测定小球藻数量的方法研究[J]. 安徽农业科学,2009,37(23):11104-11105.
Lü Xuyang, Zhang Wen, Yang Yang, et al. Methodological research on measuring Chlorella quantity by spectrophotometry[J]. Journal of Anhui Agricultural Science, 2009, 37(23): 11104-11105. (in Chinese with English abstract)
[28] Zou S, Wu Y, Yang M, et al. Bio-oil production from sub-and supercritical water liquefaction of microalgaeand related properties[J]. Energy & Environmental Science, 2010, 3(8): 1073-1078.
[29] 邢波. 苯系列傅里叶变换红外(FT-IR)光谱的研究[J]. 现代科学仪器,2002(4):43-45.
Xing Bo. The study of FT-IR spectra of benzene series[J]. Modern Scientific Instruments, 2002(4): 43-45. (in Chinese with English abstract)
[30] 高华娜,赵海英,王志宙,等. 螺旋藻的PXRD和XRF分析[J]. 食品科技,2008,33(12):270-272.
Gao Huana, Zhao Haiying, Wang Zhizhou, et al. Analysis on theby PXRD and XRF[J]. Food Science and Technology, 2008, 33(12): 270-272. (in Chinese with English abstract)
Isolation, identification offrom aquaculture wastewater and its purification of wastewater
Lu Hongsheng, Liu Yaqiao, Liu Wenjun, Kong Fanmin, Tan Haochen
(,,266590,)
Algaehas been studied for many years about its characteristics of rapid breed, easily obtained, high adsorption capacity and low nutrition demands and so on.The emission of aquaculture wastewater will caused severe damage on environment.Thereforehow to remove the compound of nitrogen and phosphorus from aquaculture wastewater becomes a serious problem around the world.Based above, many researchers have been focusing on this field and a lot of achievements have been found. Many environmental factors could influence on the growth of algae, including temperature, illumination and nutrition. For heterotrophism, carbon, nitrogen and phosphorus are the three most important factors influencing on the growth ofMicroalgaeconsist of several varieties, including.is belonged toUntil now, studies ofhave mainly focused on the processing, especially in the field of producing biodiesel, but few studies focused on the wastewater treatment byThis study carried out the following experiments: the enrichment, isolation, purification and nutritional ingredient analysis for. Then, the effect of treating with wastewater bywas carried out, including the removal of total nitrogen, total phosphorus and reducing COD.was isolated from aquaculture wastewater with BG-11medium. The morphology and components ofwere observed by phase contrast microscope and Fourier transform infrared spectrometer (FT-IR), respectivelyThe crystal structure ofwas determined with X-ray. Protein and fat contents were measured by Kjeldahl determination and organic solvent extract method, respectively. The effect of treating with wastewater bywas tested with national standard methods. Chlorophyll a was extracted and analyzed with acetone extract method. The morphology observation with phase contrast microscope showed thatwas belonged toFT-IR analysis showed that two peaks appeared at the wave numbers of 1 080 and 1 240 cm-1, which proved high contents of protein, carbohydrate and fat contained in. X-ray diffraction spectrogram showed two main peaks appeared at about 32° and 20°, which proved both protein and fat were contained in. Quantitative analysis indicated that contents of protein and fat were 45.6% and 8.1%, respectively. Correlational study indicated that the negative correlation of the increase of Chlorophyll a inwith the decrease of total nitrogen (T-N) and total phosphorus (T-P) remained in the wastewater. The removals of COD, total nitrogen (T-N) and total phosphorus (T-P) bywere 70.9%, 23% and 34.7%, respectively. Based on above results, the capability of treating with wastewater bywasproved in this study. Therefore, this study could provide a reference for the exploitation of a new bioresource and the water environment treatment.
wastewaters; purification; nitrogen; phosphorus;; screening and identification; component analysis; treatment effect
10.11975/j.issn.1002-6819.2017.04.037
Q938
A
1002-6819(2017)-04-0273-05
2016-05-05
2017-01-24
山东省自然科学基金面上项目(No. ZR2014EMM005);山东省人力资源与社会保障厅资助项目(No. 20101008);潍坊市峡山水库管理局资助项目(No. 201303201603).
陆洪省,男,汉族,副教授,博士(后),硕士生导师,从事水污染控制方面的研究。青岛 山东科技大学化学与环境工程学院,266590。Email:hslu628@163.com.
陆洪省,刘亚樵,刘文君,孔凡民,谭好臣. 养殖污水中蛋白核小球藻的分离鉴定及其污水处理效果[J]. 农业工程学报,2017,33(4):273-277. doi:10.11975/j.issn.1002-6819.2017.04.037 http://www.tcsae.org
Lu Hongsheng, Liu Yaqiao, Liu Wenjun, Kong Fanmin, Tan Haochen. Isolation, identification offrom aquaculture wastewater and its purification of wastewater[J]. Transactions of the Chinese Society of Agricultural Engineering (Transactions of the CSAE), 2017, 33(4): 273-277. (in Chinese with English abstract) doi:10.11975/j.issn.1002-6819.2017.04.037 http://www.tcsae.org

