阴极电解液对Cd污染红壤电动修复的影响
徐龙云, 张英杰, 董 鹏, 孙 鑫, 勾 凯, 孟 奇, 李 彬
1.昆明理工大学材料科学与工程学院, 云南 昆明 650032 2.昆明理工大学冶金与能源工程学院, 云南 昆明 650032 3.昆明理工大学环境科学与工程学院学院, 云南 昆明 650032 4.锂离子电池及材料制备技术国家地方联合工程实验室, 云南 昆明 650032
阴极电解液对Cd污染红壤电动修复的影响
徐龙云1,4, 张英杰1,2,4*, 董 鹏2,4, 孙 鑫3,4, 勾 凯1,4, 孟 奇2,4, 李 彬3,4
1.昆明理工大学材料科学与工程学院, 云南 昆明 650032 2.昆明理工大学冶金与能源工程学院, 云南 昆明 650032 3.昆明理工大学环境科学与工程学院学院, 云南 昆明 650032 4.锂离子电池及材料制备技术国家地方联合工程实验室, 云南 昆明 650032
针对土壤重金属电动修复过程中阴极电解室pH升高会对重金属的去除产生不利影响的问题,利用Fe3+Fe2+、Cu2+Cu标准电极电位较高的优势,以人工模拟Cd污染红壤为研究对象,对不同阴极电解液〔Fe(NO3)3、CuSO4、柠檬酸〕的电动修复效果进行系统分析.结果表明:分别将Fe(NO3)3、CuSO4、柠檬酸加入阴极电解室中,pH均控制在2~3,电动修复10 d后发现,将Fe(NO3)3溶液、CuSO4溶液和柠檬酸作为阴极电解液均可以有效控制阴极室的pH,CuSO4溶液、柠檬酸的加入对土壤中Cd的去除效果较差,而且Cu2+的加入增加了土壤重金属二次污染的风险.相对于CuSO4、柠檬酸试验组,Fe(NO3)3试验组土壤中Cd的去除率较高(大于87.27%),Fe(NO3)3试验组对土壤中Cd的修复效果也最为明显,土壤中w(Cd)由阴极附近的75.95 mgkg降至阳极附近的9.13 mgkg.分析电动修复后各试验组中不同形态Cd在Cd总量中所占比例的分析,结果显示,w(弱酸提取态Cd)所占比例由初始的74.57%最高可达到92.69%〔Fe(NO3)3试验组〕,表明Fe(NO3)3的加入有助于促进土壤中Cd的迁移.研究显示,相比于CuSO4溶液、柠檬酸,Fe(NO3)3溶液作为阴极电解液在控制阴极电解室pH升高的前提下,显著促进了土壤中Cd的解吸和迁移,并达到最佳修复效果.
土壤; Cd; 电动修复; 阴极电解液
Cd是土壤重金属污染的重要元素之一,含Cd污染物进入土壤会对土壤产生持久性污染,并对人类健康产生巨大危害[1- 2].我国每年受重金属污染的粮食产量达1.2×107t,直接经济损失达200×108元,受Cd污染的耕地面积超过13×104hm2[3- 5],因此土壤Cd污染问题亟待解决[6].
目前,在治理土壤Cd污染过程中,电动修复技术得到了国内外研究人员的高度重视,该技术主要通过电迁移、电渗流方式将土壤中重金属迁移出来[7- 9].土壤pH是影响电动修复效果的最主要因素[10- 11].在不控制阴极电解室pH的情况下,由于电解液中水电离产生的H+在阴极放电导致OH-浓度上升,大量的OH-形成可移动的碱性带向土壤中迁移,促使土壤pH升高[12].土壤pH升高促使Cd形成Cd(OH)2、CdCO3等重金属沉淀[13- 14],降低土壤Cd的去除效率[15- 17],而且所产生的重金属沉淀还会堵塞土壤微孔,使土壤电导率降低[18- 20].Almeira等[21]在利用电动修复技术对人工Cd污染高岭土进行电动修复时发现,发现当利用0.06 molL的HNO3对阴极电解室pH进行控制时,高岭土中98%的Cd得到去除.Lee等[22]采用微生物-电动修复技术对尾矿中重金属进行去除,通过向尾矿中加入硫氧菌来氧化尾矿中的硫,产物H2SO4能够降低尾矿中pH,使Cd的去除率达到33.9%,而没有添加硫氧菌的对照组中Cd的去除率仅为17.3%.LIU等[23]研究Cd污染废弃工业场地时,采用对换电极的方式控制土壤pH为5~7,Cd的去除率最高可达到94%.可见,在分析土壤Cd污染电动修复过程中,土壤pH升高问题是电动修复技术领域的研究热点.
Fe3+Fe2+、Cu2+Cu具有较高的标准电极电位(Eθ),在阴极惰性电极附近阳离子放电顺序为Fe3+>Cu2+>水中电离H+.该研究以云南红壤为研究对象,针对阴极电解室水电离产生的H+放电现象,向阴极电解室加入含Fe3+、Cu2+的盐溶液,研究该类溶液作为阴极电解液对红壤中重金属Cd电动修复效果的影响,以期为Cd污染土壤的电动修复提供技术参考.
1 材料与方法
1.1 试验土壤
所用土壤样取自昆明理工大学校园内的红壤(0~20 cm),经风干捣碎,去除杂质,研磨并过20目(约0.841 mm)筛.将土壤样品与Cd(NO3)2·4H2O在塑料桶中混匀后加水培养,在室温下培养2 a.根据土壤基本理化性质测试方法[24],测试结果如表1所示.
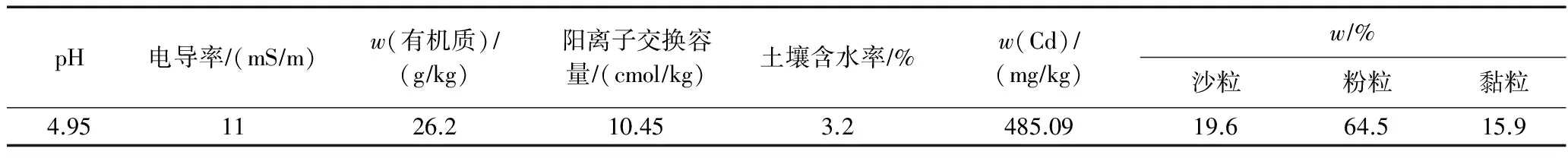
表1 土壤基本理化性质
1.2 试验装置
电动修复所用的试验装置为自行设计,利用有机玻璃加工而成,外形呈矩形,主要分为三部分,即中间的土壤室以及两边的阴阳电极室(见图1).土壤室内部尺寸(长×宽×高)为20 cm×5 cm×5 cm,阴阳电极室为6 cm×5 cm×5 cm.试验装置还包括直流稳压电源、石墨电极以及pH自动控制系统.随着试验的进行,电解液也随之消耗,将pH电极放置在阴极电解室的石墨电极旁来检测阴极室pH,并通过控制系统向阴极室添加对应的电解液来控制pH.
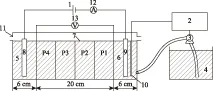
注: 1—直流稳压电源;2—pH监控器;3—蠕动泵;4—阴极电解液;5—阳极电解室;6—阴极电解室;7—土壤;8—阳极;9—阴极;10—pH检测电极;11—溢流孔;12—电流表;13—电压表. P1~P4为采样区域编号.图1 电动修复装置Fig.1 Electrokinetic remediation device
1.3 试验方法
试验共设置4组电动修复试验(见表2).试验前,将处理好的污染土壤样品装入土壤室,用去离子水饱和土壤12 h,按各试验设计方案加入电解液后连接电源,电压梯度设置为1.5 Vcm.试验过程中采用115C万用表测定电流变化.试验结束时,将土壤从阴极到阳极平分为四部分,长度均为5 cm,分别编号为P1、P2、P3、P4.将各部分土壤取出放入铝盒,在真空干燥箱中80 ℃下干燥24 h,过100目(0.147 mm)筛并充分混匀,对土壤重金属进行形态提取并测定其含量,另外测定土壤中w(Cd)、pH、电导率等指标.
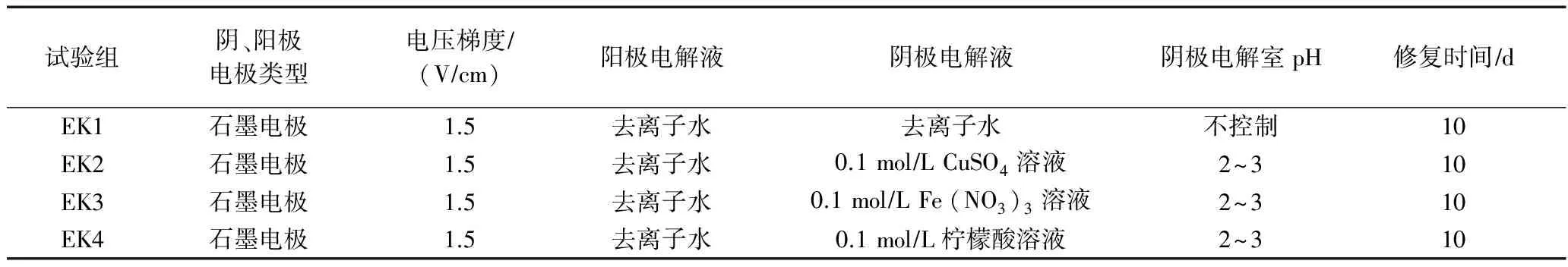
表2 电动修复试验设计方案
土壤重金属Cd形态分析参照欧洲参考交流局(European Community Bureau of Reference)提出的BCR提取法[25].操作步骤:①w(弱酸提取态Cd)测定.准确称取土壤样品1.0 g置于100 mL离心管中,加入40 mL 0.1 molL乙酸,在室温下连续振荡16 h,3 000 rmin下离心20 min,取上清液,利用ICE 3300原子吸收光谱仪(美国赛默飞世尔科技公司)测得w(弱酸提取态Cd).往残余物中加入20 mL去离子水并振荡15 min后在3 000 rmin下离心20 min,倒掉上清液完成清洗步骤.②w(可还原态Cd)测定.向第①步剩余土壤中加入40 mL 0.5 molL NH4OH·HCl,其余步骤同①.③w(可氧化态Cd)测定.向第②步剩余土壤中加入10 mL H2O2,并将pH调至2~3,在室温下静置1 h后再加入10 mL H2O2,将其转移至恒温水浴锅中85 ℃下保持1 h,加入50 mL 1 molL NH4OAc,其余步骤同①.④w(残渣态Cd)测定.将第③步剩余土壤在HF-HClO4-HNO3体系中进行消解,取消解液即可测得w(残渣态Cd).采用HF-HClO4-HNO3方法对土壤进行消解,测定消解液中的w(Cd).
土壤pH采用PHS- 29A型数字酸度计(上海大普仪器有限公司)测定;电导率采用MH-WSY土壤三参仪(北京博伦经纬科技发展有限公司)测定,水土比为2.5∶1;阳离子交换容量采用乙酸铵提取法测定;土壤粒径分布采用Mastersizer 2000激光粒度仪(英国马尔文仪器有限公司)测定;将土壤置于600 ℃马弗炉中,根据重量分析方法测定其有机质含量;土壤含水率采用环刀法测得[26].
各土壤样品均平行测定3次并取其平均值,土壤中Cd的去除率以及不同形态Cd质量分数所占比例均在平均值基础上计算得到.
2 结果与分析
2.1 电动修复过程中电流变化
电动修复过程中不同阴极电解液试验组的电流随时间的变化情况如图2所示.由图2可见,对于EK1,0~10 h内电流由最初的2 mA增至最大值(4 mA),并在128 h后稳定在2 mA左右;EK2最初电流为3 mA,修复至第23小时达到最大值(8 mA),125 h后稳定在7 mA左右;EK4最初电流为4 mA,修复至第2小时达到最大值(5 mA),128 h后稳定在2 mA左右.可见,EK1、EK2、EK4的电流变化趋势基本一致,先增后减并趋于稳定,该变化过程比较平缓,这是由于电动修复初期土壤中可移动离子较多,阳极水电解产生的H+以较快的速度在孔隙液中向阴极迁移会溶解、解吸产生更多可移动离子,使孔隙液中离子浓度增加.电流增大[27].LI等[28]研究表明,当土壤pH降低时,孔隙液中的离子浓度会通过沉淀溶解、土壤颗粒表面解吸的方式增加.对比分析显示,EK4电流的峰值稍高于EK2,这是因为柠檬酸的pH为2.4,其酸性值高于CuSO4(pH为2.7).与EK2、EK3、EK4相比,EK1阴极电解室的pH最高,其电流最低.修复一段时间后,土壤中大部分可移动离子在电迁移等作用下迁移出土壤,电流随之降低.当从土壤中迁移出的可移动离子与土壤中后期溶解、解吸出的可移动离子处于平衡状态时,电流会稳定在一定范围内.EK3修复初期电流也在缓慢增加,中后期电流迅速增至最大值(740 mA),之后开始降低,最小值(41 mA)出现在第190小时.EK3的电流始终大于其余3组,这是由于Fe3+在阴极电极附近的放电能力较强,电解液中Fe3+消耗速度会相应增加,需要加入更多Fe(NO3)3溶液来控制阴极室pH,致使电动体系中可移动离子的浓度增加.
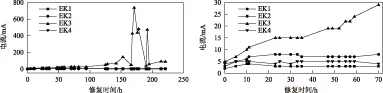
图2 电动修复过程中电流变化Fig.2 Current changes in the process of electrokinetic remediation
2.2 电动修复后土壤pH分布
电动修复后土壤不同部位pH的变化情况如图3所示.由于水的电解作用导致EK1土壤中靠近阳极室P4处的pH低于初始值,但P2、P3以及靠近阴极室P1的pH均高于初始值.有的研究[29]也发现了此类现象.EK4以柠檬酸作为阴极电解液,可以中和阴极产生的OH-[30],从而控制阴极电解室pH(2~3).由于Eθ(Fe3+Fe2+)>Eθ(Cu2+Cu)>Eθ(H2OH2),所以阴极电解液中Fe3+或Cu2+率先被还原[31],阻止了水中H+在阴极附近的放电过程,从而达到控制阴极电解室pH的目的(EK2阴极电极室石墨电极表面有Cu析出,EK3阴极电解液中含有可以使K3[Fe(CN)6]变为深蓝色沉淀的Fe2+).阴极电解室pH控制在2~3,并且阳极电解室水电解产生的H+通过土壤向阴极迁移,使得EK2、EK3土壤pH在整体上都明显低于初始值,表明Fe(NO3)3、CuSO4溶液作为阴极电解液对阴极电解室的pH控制效果显著.

注: P<0.05.图3 电动修复后土壤pH分布Fig.3 Distribution of soil pH after electrokinetic remediation
2.3 土壤中w(Cd)及Cd的去除率
由图4可见,不同处理条件下,位于阴极区附近P1处的w(Cd)均高于阳极区P4处.Sah等[32]在研究中性沙壤土Cd的电动修复时发现,由于阴极电解室附近的pH较高,修复结束后在距离阴极4 cm处的土壤中w(Cd)最高.
对于EK1,在P1、P4处w(Cd)分别为474.64、306.51 mgkg,Cd去除率由P1处的2.15%升至P4处的35.74%.
对于EK2,由于控制了阴极电解室的pH,在P4处w(Cd)较EK1低,为38.01 mgkg,Cd去除率为93.5%;在P3处w(Cd)达到1 308.62 mgkg,说明该区域发生了Cd的聚集[33].从土壤Cd的去除效果来看,EK2由于Cd聚集现象的产生,没有提高电动修复效果.
EK4中w(Cd)表现为近电极室部位较低、土壤中间部位较高,P1、P2、P3、P4处w(Cd)分别为301.79、476.99、471.06、51.02 mgkg.Cd去除率的最高值出现在P4处,为88.98%.Cd主要集聚在土壤中间部位,这是由于加入柠檬酸作为阴极电解液,并控制阴极电解室pH在2~3,这种条件下柠檬酸通常以H2L-、HL2-、H3L的形式存在并与Cd形成带有负电荷的络合物,该络合物通过电迁移向阳极进行移动,而Cd2+通过电迁移向阴极移动,由此导致Cd在土壤中间部位的聚集[34].Labanowski等[35]在柠檬酸提取农田Cd污染土壤试验时发现,柠檬酸与Cd会形成Me[citrate]-形式的络合物.
对比EK1、EK2、EK4可以发现,加入CuSO4和柠檬酸在修复试验中所用的红壤时重金属去除率较低.而对于EK3,P1、P2、P3、P4处w(Cd)分别为75.95、16.25、25.57、9.13 mgkg,Cd的去除率由P1处的87.27%升至P4处的98.29%.相对于EK1、EK2、EK4,EK3土壤 Cd平均去除率较高的可能原因是:①将Fe(NO3)3作为阴极电解液,控制阴极pH在2~3之间,促使土壤中Cd解吸进入孔隙液中;②阴极电解室Fe3+浓度较高,土壤孔隙液中Fe3+浓度较低,由此导致Fe3+从阴极电解室向土壤孔隙液中扩散,Fe3+因具有较高的价态而在土壤颗粒表面上对其他离子的吸附亲和力较强,从而使很多阳离子(如Cd2+)解吸进入孔隙液中;③由于Fe3+吸附在土壤颗粒表面,导致土壤颗粒扩散双电子层厚度减小,进而降低土壤颗粒之间的排斥力,范德华力增加,同时产生絮凝现象,絮凝结构会增加土壤颗粒孔隙,这样就增加了离子在孔隙液中的移动速度,解吸出来的重金属离子就会得到有效迁移,同时土壤中的电导率系数增加,电流随之提高,这也是图2中EK3的电流较其他3组试验偏高的另一个重要原因[36- 37].
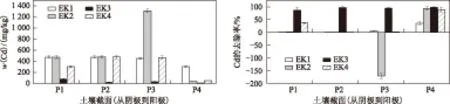
注: P<0.05.图4 土壤截面各采样区域的w(Cd)及Cd的去除率Fig.4 The concentration and removal rate of Cd in each section of soil
2.4 土壤中重金属Cd的形态变化
由图5可见,原始土壤中,弱酸提取态、可还原态、可氧化态、残渣态Cd的质量分数所占比例分别为74.57%、18.87%、6.55%、0.002%,电动修复后土壤截面各采样区域内4种形态Cd的质量分数所占比例均存在差异.Kim等[38]在研究尾矿重金属电动修复时发现,土壤中重金属形态对其电动修复效果有重要的影响.
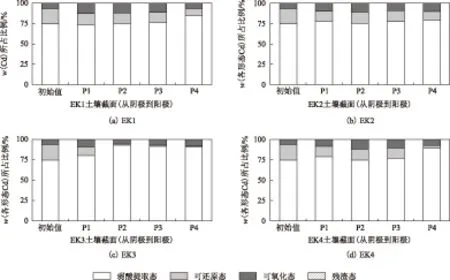
图5 土壤截面各采样区域不同形态Cd质量分数所占比例Fig.5 The proportion Cd fractions in each section of soil
对于弱酸提取态Cd,经电动修复后,4组试验中w(弱酸提取态Cd)所占比例均有所增加(除EK1中P1处为73.38%),而EK3中相应值较w(弱酸提取态Cd)初始值的增幅最大,其P1、P2、P3、P4处w(弱酸提取态Cd)分别为79.97%、92.69%、91.29%、90.60%.彭桂群等[39]在研究电动修复增强技术去除电镀污泥重金属的过程中发现,经电动修复处理后污泥中各形态重金属含量所占比例变化是由稳定态向不稳定态的弱酸提取态的转变.对于EK1,由于在试验过程中没有调节阴极电解室pH,P1处土壤pH较高,致使P1处w(弱酸提取态Cd)所占比例较w(弱酸提取态Cd)的初始值低,说明pH的升高不利于土壤中其他形态Cd向弱酸提取态Cd的转变.元素的化学形态与其生物毒性、迁移性密切相关[40].吴新民等[41]在研究土壤中Cd、Pb、Cu等元素含量及其形态特征时发现,由于弱酸提取态重金属与土壤颗粒表面结合较弱,在自然环境中更易被释放出来,因此具有可移动性强、生物危害性大的特点.
4组试验中,电动修复后土壤中w(可还原态Cd)所占比例均有所降低.Sah等[42]发现,电动修复过程可以有效降低土壤中可还原态重金属含量.由于P4位置靠近阳极电解室,该处土壤pH较低,修复后土壤中w(可还原态Cd)所占比例较其他部位更低,在EK1、EK2、EK3、EK4中P4处w(可还原态Cd)分别为8.35%、10.43%、1.09%、3.31%.说明pH降低有助于可还原态Cd在土壤颗粒表面的解吸.
电动修复后各试验组土壤中w(可氧化态Cd)所占比例均有所增加,可能是因为在电动修复过程中,土壤中部分重金属离子解吸进入孔隙液后又以络合物的形式重新吸附在土壤颗粒表面,进而形成可氧化态Cd.
2.5 能耗及阴极电解液消耗
各组试验中能量消耗与阴极电解液消耗量如表3所示.由表3可见,阴极电解液消耗越多,能耗越大.EK2的能耗为179.92 kJ(为EK1能耗的2.13倍),阴极电解液消耗量为625 mL,另外由于在P3处存在Cd的聚集现象,使得修复后土壤中平均w(Cd)大于修复前,CuSO4溶液的使用也增加了重金属对修复土壤二次污染的可能性.EK3的能耗为2 088.61 kJ(分别为EK1能耗的24.73倍、EK4能耗的13.39倍),阴极电解液消耗量2 000 mL(为EK4消耗量的3.77倍),但其修复效果最好,Cd的去除率分别为EK1的8.14倍、EK4的2.89倍.
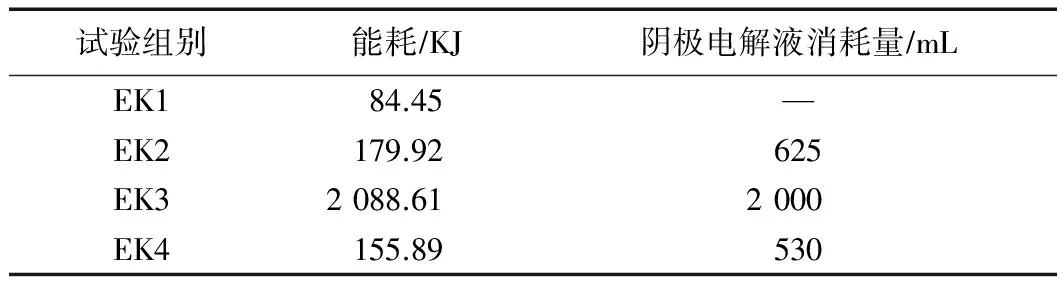
表3 电动修复试验中能耗与电解质的消耗
3 结论
a) 由于Fe3+Fe2+、Cu2+Cu具有较高的标准电极电位,Fe(NO3)3溶液、CuSO4溶液和柠檬酸作为阴极电解液时可以有效控制阴极室的pH.
b) Fe(NO3)3溶液加入阴极电解室提高了土壤中Cd的解吸和迁移,并达到最佳修复效果,土壤中w(Cd)由阴极附近的75.95 mgkg降至阳极附近的9.13 mgkg,Cd的去除率均大于87.27%;同时,w(弱酸提取态Cd)所占比例由初始值的74.57%最高可达到92.69%,从而对土壤中Cd的迁移起到促进作用.
c) CuSO4、柠檬酸溶液加入阴极电解室对土壤中Cd的去除效果较差,这是由于Cd在土壤中部会产生聚集(最高分别为1 308.62和476.99 mgkg),致使土壤中Cd的去除率较低,而且Cu2+的加入增加了土壤重金属二次污染的风险.
[1] REDDY K R,CHINTHAMREDDY S.Enhanced electrokinetic remediation of heavy metals in glacial till soils using different electrolyte solutions[J].Journal of Environmental Engineering,2004,130(4):442- 455.
[2] GANESN V.Rhizoremediation of cadmium soil using a cadmium-resistant plant growth-promoting rhizo pseudom-onad[J].Current Microbiology,2008,56(4):403- 407.
[3] ACAR Y B,GALE R J,ALSHAWABKEH A N,etal.Electrokinetic remediation:basics and technology status[J].Journal of Hazardous Materials,1995,40(2):117- 137.
[4] HUANG Deqian,XU Quan,CHENG Jiongjia,etal.Electrokinetic remediation and its combined technologies for removal of organic pollutants from contaminated soils[J].International Journal of Electrochemical Science,2012,7(5):4528- 4544.
[5] YEUNG A T.Milestone developments,myths,and future directions of electrokinetic remediation[J].Separation and Purification Technology,2011,79(2):124- 132.
[6] 陈玉娟,温琰茂,柴世伟.珠江三角洲农业土壤重金属含量特征研究[J].环境科学研究,2005,18(3):75- 77. CHEN Yujuan,WEN Yanmao,CHAI Shiwei.The heavy metal content character of agricultural soil in the Pearl River Delta[J].Research of Environmental Sciences,2005,18(3):75- 77.
[7] REN Dajun,ZHOU Sisi,LI Qian,etal.Enhanced electrokinetic remediation of quinoline-contaminated soils[J].Toxicological & Environmental Chemistry,2016,98(5):585- 600.
[8] KIM S O,MOON S H,WONG M H.Removal of heavy metals from soils using enhanced electrokinetic soil processing[J].Water, Air, and Soil Pollution,2001,125(1):259- 272.
[9] SUZUKI T,MORIBE M,OKABE Y,etal.A mechanistic study of arsenate removal from artificially contaminated clay soils by electrokinetic remediation[J].Journal of Hazardous Materials,2013, 254255:310- 317.
[10] VANE L M,ZANG G M.Effect of aqueous phase properties on clay particle zeta potential and electro-osmotic permeability:implications for electro-kinetic soil remediation processes[J].Journal of Hazardous Materials,1997,55(123):1- 22.
[11] GILL R T,HARBOTTLE M J,SMITH J W N,etal.Electrokinetic-enhanced bioremediation of organic contaminants:a review of processes and environmental applications[J].Chemosphere,2014,107:31- 42.
[12] 李欣.电动修复技术机理及去除污泥和尾砂中重金属的研究[D].长沙:湖南大学,2007:55- 100.
[13] LI Gang,GUO Shuhai,LI Shucai,etal.Comparison of approaching and fixed anodes for avoiding the ′focusing′ effect during electrokinetic remediation of chromium-contaminated soil[J].Chemical Engineering Journal,2012,203(1):231- 238.
[14] NG Y S,GUPTA B S,HASHIM M A.Remediation of PbCr co-contaminated soil using electrokinetic process and approaching electrode technique[J].Environmental Science and Pollution Research,2016,23(1):546- 555.
[15] ZHOU Dongmei,DENG Changfen,CANG Long,etal.Electrokinetic remediation of a Cu-Zn contaminated red soil by controlling the voltage and conditioning catholyte pH[J].Chemosphere,2005,61(4):519- 527.
[16] CAI Zongping,DOREN J V,FANG Zhanqiang,etal.Improvement in electrokinetic remediation of Pb-contaminated soil near lead acid battery factory[J].Transactions of Nonferrous Metals Society of China,2015,25(9):3088- 3095.
[17] AHMED O A,DERRICHE Z,KAMECHE M,etal.Electro-remediation of lead contaminated kaolinite:an electro-kinetic treatment[J].Chemical Engineering & Processing Process Intensification,2016,100:37- 48.
[18] LANNELLI R,MASI M,CECCARINI A,etal.Electrokinetic remediation of metal-polluted marine sediments:experimental investigation for plant design[J].Electrochimica Acta,2015,181(1):146- 159.
[19] MASCIA M,VACCA A,PALMAS S.Effect of surface equilibria on the electrokinetic behaviour of Pb and Cd ions in kaolinite[J].Journal of Chemical Technology and Biotechnology,2015,90(7):1290- 1298.
[20] REDDY K R,CHINTHAMREDDY S.Sequentially enhanced electrokinetic remediation of heavy metals in low buffering clayey soils[J].Journal of Geotechnical & Geoenvironmental Engineering,2003,129(3):263- 277.
[21] ALMEIRA J,PENG Changsheng,ABOU-SHADY A.Simultaneous removal of cadmium from kaolin and catholyte during soil electrokinetic remediation[J].Desalination,2012,300(17):1- 11.
[22] LEE K Y,KIM H A,LEE B T,etal.A feasibility study on bioelectrokinetics for the removal of heavy metals from tailing soil[J].Environmental Geochemistry and Health,2011,33(1):3- 11.
[23] LIU Ping,FENG Qiyan,MENG Qingjun,etal.Electrokinetic remediation of chromium-and cadmium-contaminated soil from abandoned industrial site[J].Separation and Purification Technology,2012,98(19):216- 220.
[24] 鲁如坤.土壤农业化学分析方法[M].北京:中国农业科技出版社,2000:12- 109.
[25] RAURET G,LOPEZ-SANCHEZ J F,SAHUQUILLO A,etal.Application of a modified BCR sequential extraction(three-step)procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material(CRM 483),complemented by a three-year stability study of acetic acid and EDTA extractable metal content[J].Journal of Environmental Monitoring,2000,2(3):228- 233.
[26] 史瑞和,鲍士旦,秦怀英.土壤农化分析[M].北京:农业出版社,1996:14- 373.
[27] YUAN Ching,WENG Chihuang.Remediating ethylbenzene-contaminated clayey soil by a surfactant-aided electrokinetic (SAEK) process[J].Chemosphere,2004,57(3):225- 232.
[28] LI Zhongming,YU Jiwei,NERETNIEKS I.Removal of Pb(Ⅱ),Cd(Ⅱ)and Cr(Ⅲ)from sand by electromigration[J].Journal of Hazardous Materials,1997,55(123):295- 304.
[29] NARASIMHAN B,RANJAN R S.Electrokinetic barrier to prevent subsurface contaminant migration:theoretical model development and validation[J].Journal of Contaminant Hydrology,2000,42(1):1- 17.
[30] COLETTA T F,BRUELL C J,RYAN D K,etal.Cation-enhanced removal of lead from kaolinite by electrokinetics[J].Journal of Environmental Engineering,1997,123(12):1227- 1233.
[31] ACAR Y B,ALSHAWABKEH A N.Principles of electrokinetic remediation[J].Environmental Science & Technology,1993,27(13):2638- 2647.
[32] SAH J G,CHEN J Y.Study of the electrokinetic process on Cd and Pb spiked soils[J].Journal of Hazardous Materials,1998,58(123):301- 315.
[33] 樊广萍,朱海燕,郝秀珍,等.不同的增强试剂对重金属污染场地土壤的电动修复影响[J].中国环境科学,2015,35(5):1458- 1465. FAN Guangping,ZHU Haiyan,HE Xiuzhen,etal.Electrokinetic remediation of an electroplating contaminated soil with different enhancing electrolytes[J].China Environmental Science,2015,35(5):1458- 1465.
[34] 吴婵.镉污染土壤的电动力学修复研究[D].武汉,华中科技大学,2007:32- 37.
[35] LABANOWSKI J,MONNA F,BERMOND A,etal.Kinetic extractions to assess mobilization of Zn,Pb,Cu,and Cd in a metal-contaminated soil:EDTAvs.citrate[J].Environmental Pollution,2008,152(3):693- 701.
[36] CAMESELLE C,CHIRAKKARA R A,REDDY K R.Electrokinetic-enhanced phytoremediation of soils:status and opportunities[J].Chemosphere,2013,93(4):626- 636.
[37] YEUNG A T,GU Yingying.A review on techniques to enhance electrochemical remediation of contaminated soils[J].Journal of Hazardous Materials,2011,195:11- 29.
[38] KIM S O,KIM K W,STUBEN D.Evaluation of electrokinetic removal of heavy metals from tailing soils[J].Journal of Environmental Engineering,2002,128(8):705- 715.
[39] 彭桂群,田光明.采用电动修复增强技术去除电镀污泥中重金属[J].中国环境科学,2010,30(3):349- 356. PENG Guiqun,TIAN Guangming.Removal of heavy metals from electroplating sludge by electrokinetic enhancement technology[J].China Environmental Science,2010,30(3):349- 356.
[40] 韩春梅,王林山,巩宗强,等.土壤中重金属形态分析及其环境学意义[J].生态学杂志,2005,24(12):1499- 1502. HAN Chunmei,WANG Linshan,GONG Zongqiang,etal.Chemical forms of soil heavy metals and their environmental significance[J].Chinese Journal of Ecology,2005,24(12):1499- 1502.
[41] 吴新民,李恋卿,潘根兴,等.南京市不同功能城区土壤中重金属Cu,Zn,Pb和Cd的污染特征[J].环境科学,2003,24(3):105- 111. WU Xinmin,LI Lianqing,PAN Genxing,etal.Soil pollution of Cu,Zn,Pb and Cd in different city zones of Nanjing[J].Environmental Science,2003,24(3):105- 111.
[42] SAH J G,LIN L Y.Electrokinetic study on copper contaminated soils[J].Journal of Environmental Science & Health Part A,2000,35(7):1117- 1139.
Effects of Cathode Electrolyte on Electrokinetic Remediation of Cadmium-Contaminated Red Soil
XU Longyun1,4, ZHANG Yingjie1,2,4*, DONG Peng2,4, SUN Xin3,4, GOU Kai1,4, MENG Qi2,4, LI Bin3,4
1.Faculty of Materials Science and Engineering, Kunming University of Science and Technology, Kunming 650032, China 2.Faculty of Metallurgy and Energy Engineering, Kunming University of Science and Technology, Kunming 650032, China 3.Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650032, China 4.National and Local Jocal Joint Engineering Laboratory for Lithium-Ion Batteries and Materials Preparation Technology, Kunming 650032, China
Increasing pH in the cathode electrolysis room during electrokinetic remediation has a detrimental effect on the removal of heavy metals. To resolve this problem, the effects of different cathode electrolytes (ferric nitrate, copper sulfate and citric acid) were systematically studied on the electrokinetic remediation of Cd-contaminated red soil based on the higher standard electrode potentials of Fe3+Fe2+and Cu2+Cu. The experimental results indicated that after ten days of electrokinetic remediation with ferric nitrate, copper sulfate and citric acid, whose pH were all kept at 2-3, the removal efficiency of Cd in soil was above 87.27% by adding ferric nitrate into the cathode. In addition,w(Cd) in soil decreased from 75.95 mgkg around the cathode to 9.13 mgkg around the anode. The removal efficiencies of Cd in soil decreased when adding copper sulfate and citric acid into the cathode. The addition of Cu2+increased the risk of heavy metal pollution in the soil. In addition, the proportion of weak acid extractable Cd in the soil increased to a maximum of 92.69% from the initial value of 74.57% by adding ferric nitrate, which promoted the migration of Cd in the soil. The research suggests that when adding the ferric nitrate solution as the cathode, the pH of the cathode electrolysis chamber was controlled, and the desorption and migration of Cd in soil was significantly promoted. Eventually, the removal efficiency of Cd in soil was more significant.
soil; Cd; electrokinetic remediation; cathode electrolyte
2016- 05- 22
2016- 10- 10
云南省环境保护专项资金
徐龙云(1990-),男,山东巨野人,1095703453@qq.com.
*责任作者,张英杰(1963-),女,黑龙江双鸭山人,教授,博士,博导,主要从事环境电化学研究,yingjie@kmust.edu.cn
X53
1001- 6929(2017)02- 0267- 08
A
10.13198j.issn.1001- 6929.2017.01.29
徐龙云,张英杰,董鹏,等.阴极电解液对Cd污染红壤电动修复的影响[J].环境科学研究,2017,30(2):267- 274.
XU Longyun,ZHANG Yingjie,DONG Peng,etal.Effects of cathode electrolyte on electrokinetic remediation of cadmium-contaminated red soil[J].Research of Environmental Sciences,2017,30(2):257- 274.

