生物可降解的聚合物自组装的纳米药物的结构和特征
赵 明 彭师奇
(首都医科大学药学院,北京 100069)
· 综 述 ·
生物可降解的聚合物自组装的纳米药物的结构和特征
赵 明 彭师奇*
(首都医科大学药学院,北京 100069)
由生物可降解的聚合物为载体的纳米药物(例如脂质体、微球和微囊)可能有较好的药动学和药效学性质。如果生物可降解聚合物能携带药物穿越生物屏障,显示疾病部位靶向性,生物可降解的聚合物为载体的纳米药物既可监测药物实时分布,又可帮助理解疾病发病和病理。特别在药物化学领域,生物可降解聚合物受到许多研究者关注。本文重点综述了生物可降解聚合物的纳米药物的组装过程、形貌和特征。为了帮助相关领域的读者理解生物可降解聚合物的组装过程和纳米体系的形貌,本文提供了29幅图。这些图使生物可降解聚合物的纳米体系的特征可视化。
可生物降解的聚合物;纳米药物;纳米结构;组装过程;组装机制

Dr.ZhaoMing,ProfessorofSchoolofPharmaceuticalSciences,PekingUniversity(2001-2006),ProfessorofCollegeofPharmaceuticalSciences,CapitalMedicalUniversity(2006-2014),InvitedProfessoratthePhar⁃macySchool,UniversityofNorthCarolinaatChapelHill(2007),Pro⁃fessorandDeanofCollegeofChemi⁃calBiologyandPharmaceuticalSci⁃ences,CapitalMedicalUniversity(2014tillnow),receivedherBSinPekingUniversity,andPh.D.fromBeijingMedicalUniversity.Dr.Zhaohaswritten6books,published134articlesininternationalscientificjour⁃nals,andholds156patents.Dr.PengShiqi,ProfessorandpastDeanofCollegeofPharmaceuticalSciences,BeijingMedicalUniversity(1999-2000),ProfessorandpastDeanofCollegeofPharmaceuticalSciences,PekingUniversity(2000-2004),ProfessorandpastDeanofCollegeofChemicalBiologyandPharmaceuticalSciences,CapitalMedicalUniversity(2004-2014),re⁃ceivedhisBS,MSandPh.D.fromBeijingMedicalUniversity.HewasHumboldtFellow(1987-1990,1992and1998).Hehaswritten10books,published154papersininternationalscientificjournals,andholds186pa⁃tents.
1 Introduction
Recently, major progress in the understanding of the onset and pathology of a series of diseases, such as cancer, thrombosis, Alzheimer’s disease, Parkinson’s disease, osteoporosis, allergic disease, inflammation, rheumatoid arthritis, epidural analgesia, diabetes, hemophilia and oxidative stress injuries, highlights the rationality of drug design and deepens the recognition of the benefits of biodegradable polymers based nano-medicine to water solubility and bioavailability of the drugs, the imaging agents and the diagnostic agents, in particular. Of various nanomedicines, the nanoparticles, nanocomplexes, nanofilms, nanoshells, nanosponge and micelles are the desirable nano-forms for loading or conjugating drugs, imaging agents and diagnostic agents. The advantages of nano-medicines include having desirable pharmaceutical and pharmacological property, capable of targeting issues or cells, carrying drugs to cross biological barriers, releasing guests in targeting manner, exhibiting multiple functions, and monitoring guests in real-time manner. These advantages lead the nano-medicine attracting a great deal of research interests, and lead the biodegradable polymers to be deeply emphasized by numerous reviews. To have a clear perspective, the polymers, the nano-medicines and the topics/aims mentioned in the major reviews published in the past 5 years are compared and summarized in Tab 1. Besides, the architecture, the feature and the assembly process of the nanomedicine assembled by biodegradable copolymers bearing hydrophobic and hydrophilic blocks in typical representatives are graphically proposed (Fig.1-Fig.4).

Tab.1 Biodegradable polymers formed nano-species and aims thereof

Continued Table 1
Continued Table 1
Tab.1 and Fig.1-Fig.4 show that the descriptor polymer therapeutics is an umbrella term for polymeric drugs, polymer-drug conjugates, polymer-protein conjugates, micelles and multicomponent polyplexes that are being developed as non-viral vectors, onto which drug is covalently bound. All subclasses use specific water-soluble polymers, either as bioactive agent itself or as an inert functional part of the multifaceted construct of the improved delivery for drug or protein or gene. There is a considerable hope that such bionanotechnologies, designed with an appreciation of the pathophysiology of both normal and diseased tissues by using advanced polymer chemistry and precision engineering at a molecular level, will help realize the full therapeutic potential of the post-genomic era. From the industrial standpoint, these nanomedicines are more like new chemical entities than conventional drug-delivery systems or formulations that simply entrap, solubilize or control drug release without resorting to chemical conjugation. Conceptually, polymer therapeutics share some features with other macromolecular drugs (proteins, antibodies, oligonucleotides) and macromolecular prodrugs including immunoconjugates. A bonus, however, is the versatility of synthetic chemistry that allows tailoring of molecular weight, addition of biomimetic features and even the possibility of bioresponsive elements to man-made construct.

Fig. 1 Proposed architecture, feature and assembly process of the nanoparticles of water-soluble polymer drugs and therapeutics[17]
A: proposed architecture and assembly process of the nano-particles of water-soluble polymeric drug in water; B: proposed architecture and assembly process of the nanoparticles of water-soluble polymeric drug with target pendant in water; C: proposed architecture and assembly process of the nanoparticles of water-soluble polymer-protein conjugate in water; D: proposed architecture and assembly process of the nanoparticles of water-soluble polymer drug conjugate in water; E: proposed architecture and assembly process of the nanoparticles of hydrophobic drug loaded PEG in water.

Fig.2 Proposed architecture, feature and assembly process of the micelles of biodegradable block copolymers in water[28]
A: proposed architecture and assembly process of the micelles of biodegradable copolymers bearing hydrophobic and hydrophilic blocks, and the effect of the length of hydrophobic block vis-α-vis the length of hydrophilic block on the architecture of the micelles, for instance when core radius exceeds the length of the hydrophobic block the copolymers could form lamellar or cylindrical micelles; B: proposed architecture and assembly process of unimolecular micelles of asymmetric biodegradable copolymer; C: proposed architecture and assembly process of flower like micelles of biodegradable triblock copolymers with looped long hydrophilic middle block and short hydrophobic end blocks; D: proposed architecture and assembly process of pompon like micelles of biodegradable triblock copolymers with looped long hydrophobic middle block and short hydrophilic end blocks.

Fig. 3 Proposed architecture, feature and assembly process of hydrophobic drug loaded micelles of biodegradable amphiphilic copolymers, in which monoclonal antibody, pH sensitive segment and TAT peptide offer the micelles targeting ability[29]
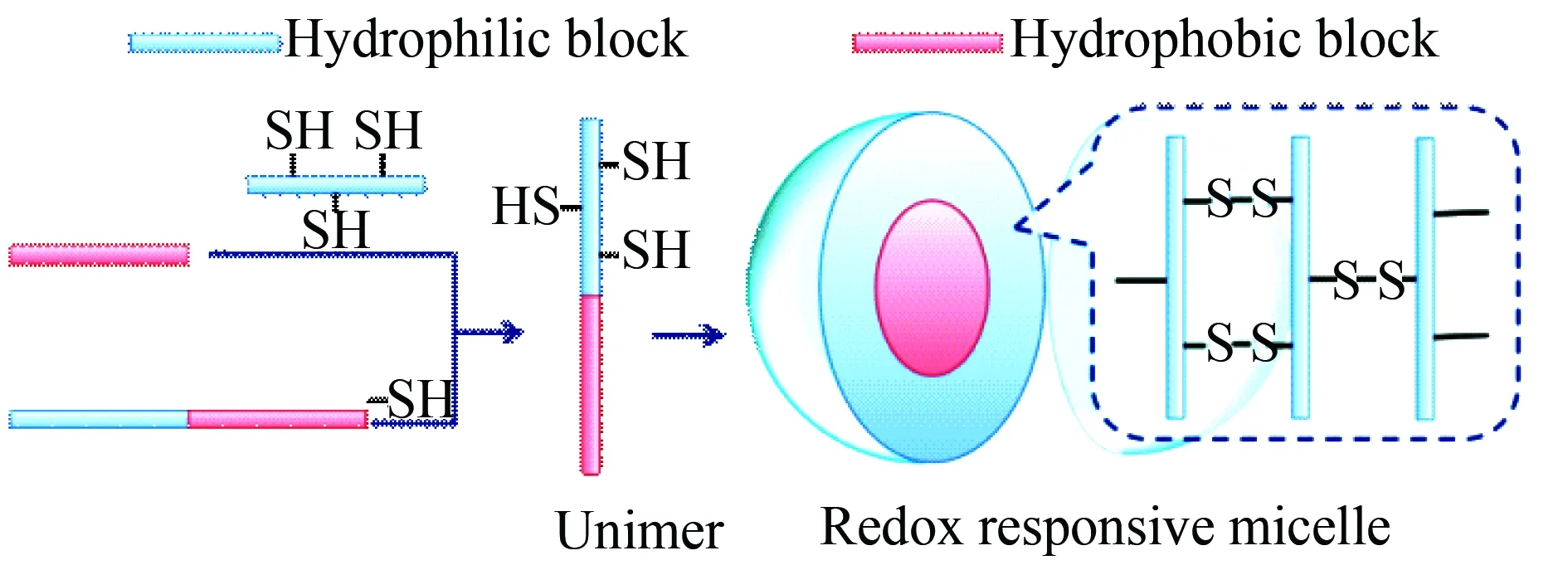
Fig. 4 Formation and feature of redox responsive micelles of mercapto group modified biodegradable amphiphilic copolymers, the redox potential difference between the extracellular and the intracellular space (i.e. the glutathione concentration difference between the extracellular and the intracellular space) leads to oxidation in extracellular space while reduction in the intracellular space[29]
Tab.1 and Fig.1-Fig.4 also indicate that the most common blocks in biodegradable polymers are PEG, chitosan, polyamino acids, polyesters and lipids. By means of nanomedicines the biodegradable polymers should contribute to clinical treatment, imaging and/or diagnostics. In pharmaceutical opinion the nanomedicine formed by biodegradable polymers provides a platform for drug combination and synergistic action. Thus the architecture, the feature and the assembly process of the nano-medicine formed by biodegradable copolymers bearing hydrophobic and hydrophilic blocks are of essential importance. In this context, this review analyzes, summarizes and proposes the architecture, feature and assembly process of the nanoparticles, the micelles and the liposomes formed by biodegradable polymers.
2 Biodegradable polymer or copolymer formed nanoparticles
The first one of the important forms of nanomedicines should be the nanoparticles assembled by biodegradable lymers or copolymers. In this review the classification of the nanoparticles follows the therapeutic effectiveness, such as anti-cancer, reversal of multidrug resistance, anti-thrombosis and removing Pb(II), and the nanoparticles that rely on internal environment sensitivity to release drugs is assigned to another type.
Cancer seriously threatens the public health worldwide. It is well known that in the chemotherapy the routine administration and usual drug combination lack targeting efficacy, while dose dependently induce side effect, thereby limits the therapeutic efficacy of cancer patients. In this case nanoparticles based drug combination emerges as a promising strategy. The hydrophilic and hydrophobic interactions lead to the self-assembly of the biodegradable polymers or copolymers forming nanoparticles capable of loading anti-cancer drugs or siRNA, such as doxorubicin (Dox)[41], docetaxel plus tamxifen[42], leuprolide acetate[43], 3-methyladenine, chloroquine[44], and AR siRNA (ARHP8)[45]. To clarify the principle, the structure and the preparation method of the nanoparticles assembled by the biodegradable polymers or copolymers are proposed and visualized with five figures (Fig.5-Fig.9), hopefully to remedy the relative lack of the images of transmission electron microscope (TEM), scan electron microscope (SEM) and atomic force microscopy (AFM) in the original texts[41-45]. The preparation methods of the nanoparticles include double emulsion (DE), nanoprecipitation (NP), double emulsion-solvent evaporation (DESE), emulsification-solvent diffusion (ESD) and solvent diffusion methods.
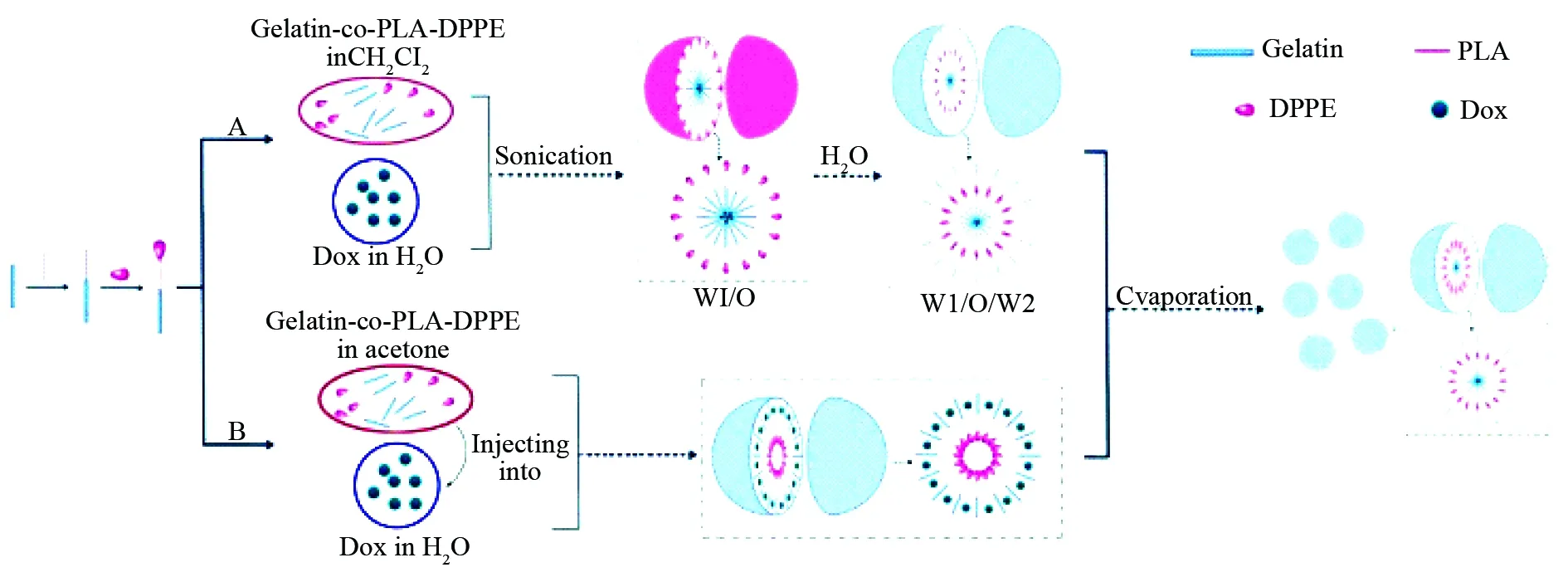
Fig. 5 Dox loaded nanoparticles of gelatin-co-PLA-DPPE, which were prepared with both double emulsion (DE) and nanoprecipitation (NP) methods, wherein DPPE represents 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine, PLA represents polylactic acid[41]
A: DE method consists of sonicating a solution of gelatin-co-PLA-DPPE in CH2Cl2and a solution of Dox in water to form the emulsion (W1/O), sonicating W1/O in an aqueous phase to form emulsion (W1/O/W2), and evaporating the solvent to precipitate the nanoparticles; B: NP method consists of injecting a solution of gelatin-co-PLA-DPPE in acetone into aqueous Dox, and evaporating the solvent to precipitate the nanoparticles.

Fig.6 Nano-precipitation scheme of preparing docetaxel and tamoxifen loaded nanoparticles of PLA-TPGS, wherein PLA-TPGS represents polylactide-D-α-tocopheryl polyethylene glycol succinate[42]
In principle, in THF the surface of the nanoparticles formed by PLA-TPGS contains numerous pores capable of simultaneously encapsulating the nanoparticles formed by docetaxel and the nanoparticles formed by tamoxifen. This leads to the reducing of the antagonism between docetaxel and tamoxifen. Nano-precipitation practice includes that at 4 ℃ into 6 mL ultrapure water a solution of 10 mg PLA-TPGS, 1 mg docetaxel and 1 mg tamoxifen in 1 mL THF was added drop-wise by using a syringe with 21G needle, this mixture was centrifuged at 15 000 r/min for 20 min to precipitate pellets which were re-suspended in 6 mL ultrapure water by vortex, the suspension was sonicated and centrifuged to precipitate docetaxel and tamoxifen loaded nanoparticles of PLA-TPGS.
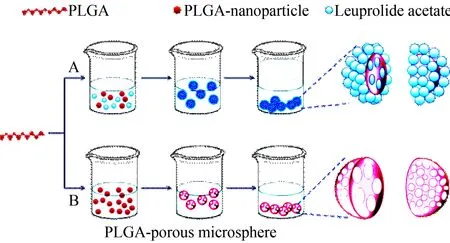
Fig.7 Using double emulsion-solvent evaporation method (DESEM) and emulsification-solvent diffusion method (ESDM) to prepare porous microspheres and nanoparticles of PLGA, wherein PLGA represents poly-D,L-lactide-co-glycolide[43]
A: To practice ESDM, ethyl acetate and distilled water was manually mixed in a separatory funnel for 20 min to achieve thermodynamic equilibrium for separating water saturated ethyl acetate and ethyl acetate saturated water; 400 mg PLGA were dissolved in 20 mL ethyl acetate saturated by water to form organic phase (O); 2 mL PVAL were dissolved in 40 mL water saturated by ethyl acetate to form water phase (W); 20 mL organic phase and 40 mL water phase were centrifuged at 11 000 r/min for 10 min to form O/W emulsion; into this emulsion 160 mL distilled water were added to induce the diffusion of ethyl acetate towards distilled water and to induce the conversion of polymer aggregation in the suspension towards the nanoparticles; at 90 r/min and 30 ℃ ethyl acetate was removed under reduced pressure; at 20 000 r/min the residue was centrifuged for 20 min to recover nanoparticles; the nanoparticles were washed with distilled water (3 mL×3) and finally lyophilized. B: To practice DESEM, 500 mg PLGA were dissolved in 8 mL CH2Cl2to prepare organic phase (O); 25 mg NH4HCO3were dissolved in 2.5 mL distilled water to prepare water phase (W1) and to help the microspheres forming interconnected pores through gas generation; 2.5 mL W1 phase and 8 mL O phase were at 11 000 r/min centrifuged for 1 min to form the emulsion (W1/O); into a solution of 1.5 mL PVAL in 300 mL distilled water W1/O emulsion was poured dropwise, emulsified with a variable-speed agitator at 250 r/min for 4 h, and the solvent was evaporated to recover the porous polymeric microspheres.

Fig.8 Nano-precipitation scheme of preparing coumarin-6 or docetaxel loaded nanoparticles of PLGA, CA-PLGA, PLGA-b-PEG, CA-PLGA-b-PEG, PLGA-b-TPGS and CA-PLGA-b-TPGS, wherein PLGA represents poly-D,L-lactide-co-glycolide, PEG represents polyethylene glycol, CA represents cholic acid and TPGS represents D-α-tocopheryl polyethylene glycol 1 000 succinate[44]
In principle, in acetone the surface of the nanoparticles formed by PLGA, CA-PLGA, PLGA-b-PEG, CA-PLGA-b-PEG, PLGA-b-TPGS and CA-PLGA-b-TPGS contains numerous pores capable of encapsulating the nanoparticles of coumarin-6 or the nanoparticles of docetaxel. To practice nano-precipitation, at 4 ℃ into 8 mL acetone containing 100 mg PLGA, CA-PLGA, PLGA-b-PEG, CA-PLGA-b-PEG, PLGA-b-TPGS or CA-PLGA-b-TPGS and 10 mg coumarin-6 or docetaxel were dissolved by vortexing and sonication, the formed solution was added into 100 mL aqueous TPGS (0.03%) drop-wise by using a syringe with 21G needle, this mixture was stirred and uncovered overnight to remove acetone completely, the suspension was centrifuged at 20 000 r/min for 30 min to precipitate the nanoparticles.

Fig.9 Formation of ARHP8 loaded nanoparticles of PSMA-A10 conjugated PLGA-PEG, wherein copolymer PLGA-PEG represents poly-D,L-lactide-co-glycolide, PEG represents polyethylene glycol, ARHP8 represents AR shRNA and PSMA-A10 represents the recognizer of human PSMA extracellular domain[45]
The nanoparticles were prepared with two-step procedure. The first step was preparing ARHP8 loaded nanoparticles of PLGA-PEG with solvent diffusion method, i.e. 18 mg PLGA-PEG were dissolved in 0.8 mL F3CCH2OH, this solution was added into 0.2 mL solution of 200-500 μg ARHP8 in TE buffer (pH 8.0), the formed mixture was added into 10 mL modified Pluronic©F127-CO2H in TE buffer (pH 8.0, 0.1%) with a syringe pump (17.5 mL/h) under 250 r/min stirring, which was used as a surfactant to activate the carboxyl groups on the particle surface. The second step was PSMA-A10 modifying the surface of ARHP8 loaded nanoparticles, i.e. the suspension of ARHP8 loaded nanoparticles of PLGA-PEG buffered by 2-N-morpholinoethane-sulfonic acid (pH 6.5) was treated with 100 mmol/L 1-ethyl-3,3-dimethylaminopropylcarbodiimide hydrochloride and 50 mmol/L N-hydroxysulfosuccinimide for 15 min, and then at room temperature conjugated by PSMA-A10 (15 mmol/L) for at least 6 h[45].
The clinical efficacies of a series of chemotherapeutic agents are undermined by the occurrence of multiple drug resistance (MDR). One of the well known mechanisms of MDR is the over-expression of efflux pump, ABC-transporter family P-glycoprotein, on the surfaces of the carcinoma cells, which transport chemotherapeutic agents out of the cells and prevent their intracellular accumulation. To overcome MDR the nano-particles assembled by the biodegradable polymers or copolymers are used to deliver chemotherapeutic agents, such as Dox and tetrandrine[46], Dox and m-THMPP (photo-sensitizer)[47], TPGS and docetaxel[48]. To clarify the principle, the structure and the preparation method of the nanoparticles assembled by the biodegradable polymers or copolymers are proposed and visualized with three figures (Fig.10-Fig.12), again hopefully to remedy the relative lack of the images of TEM, SEM and AFM in the original texts[46-48]. The preparation methods of the nanoparticles include DESE, NP and solvent evaporation methods.

Fig.10 Scheme of preparing NIR797, Dox and Tf loaded nanoparticles of PEG-PLL-PLGA by using DESEM, wherein PLGA represents poly-D,L-lactide-co-glycolide, PEG represents poly-ethylene glycol, PLL represents poly-L-lysine, Tf represents transferring, Dox represents doxorubicin, Tet represents tetrandrine and NIR797 represents near-infrared fluorescent dye[46]
To practice DESDM, Dox, Tet, and NIR797 loaded nanoparticles of PEG-PLL-PLGA were directly prepared and were re-suspended in phosphate-buffered saline and sonicated for 30 s; into the suspension tetrandrine was added (molar ratio of tetrandrine:PEG-PLL-PLGA, 8:1) and stirred at room temperature for 3 h to load tetrandrine onto the surface of Dox, Tet, and NIR797 loaded nanoparticles of PEG-PLL-PLGA; at 4 ℃ the nanoparticles were in the disperse system centrifuged (1 500 r/min) for 30 min.

Fig.11 Formation and feature of Dox loaded nanoparticles of PPLA with solvent evaporation method, wherein m-THMPP represents meso-tetra-(p-hydroxymethylphenyl)-porphyrin, a photo-sensitizer, PPLA represents 4-armed porphyrin-PLA, PLA represents polylactic acid, and TPGS represents D-α-tocopheryl-polyethylene glycol 1 000 succinate[47]
To practice solvent evaporation method, a solution of 0.2 mg Dox and 2 mg PPLA in 1 mL acetone was added to 5 mL solution of TPGS in water (0.03%), this mixture was stirred at 450 r/min, acetone was evaporated at room temperature by stirring overnight, the nanoparticles were collected by centrifugation.

Fig.12 Formation and feature of docetaxel loaded nanoparticles of PLGA with nanoprecipitation method, wherein PLGA represents poly-D,L-lactide-co-glycolide, TPGS represents D-α-tocophe-ryl-polyethylene glycol 1 000 succinate[48]
In docetaxel loaded nanoparticles of PLGA, TPGS has dual functions, i.e. pore-forming function in forming nanoparticles and inhibiting P-glycoprotein induced MDR. The pore-forming function gives the nanoparticles higher drug encapsulation and faster drug release profile. To practice nanoprecipitation method, 90 mg PLGA were mixed with TPGS (in various percentage) and 10 mg docetaxel in 8 mL acetone to form a solution which was injected into aqueous solution under stirring to form homogenous emulsion, after evaporation the residue was centrifuged and washed to obtain nanoparticle pellets, the pellets were resuspended and frozen-dry for 48 h to generate nanoparticle powders.
Thrombosis, a pathological condition, has been one of the most prominent causes of morbidity and mortality worldwide. In the design of the anti-thrombotic agent and the thrombolytic agent biodegradable poly-α,β-aspartic acid (PD) was coupled with L-arginine or Arg-Gly-Asp-Phe to form anti-thrombotic nanoparticles[49-50], and with L-alanine to form thrombolytic nanoparticles[51]. The exposure of Pb(II) is implicated in the onset of hypertension, hyperthyroidism, osteoporosis, skeletal disorders, kidney cancer, and developmental, neuropsychological as well as behavioral deficits. Chelation therapy is the treatment of choice for Pb(II) poisoning. Unfortunately, following removal of Pb(II) chelation therapy also remove essential elementals and induces the redistribution of Pb(II) towards the brain. In the discovery of safe chelation agents PD was coupled with L-cysteine or L-methionine to form Pb(II) chelating nanoparticles[52-53]. Here Fig.13 is given to proposed the formation of the nanoparticles and to simply visualize the inner architecture of TEM and SEM images in the original texts[51-53].
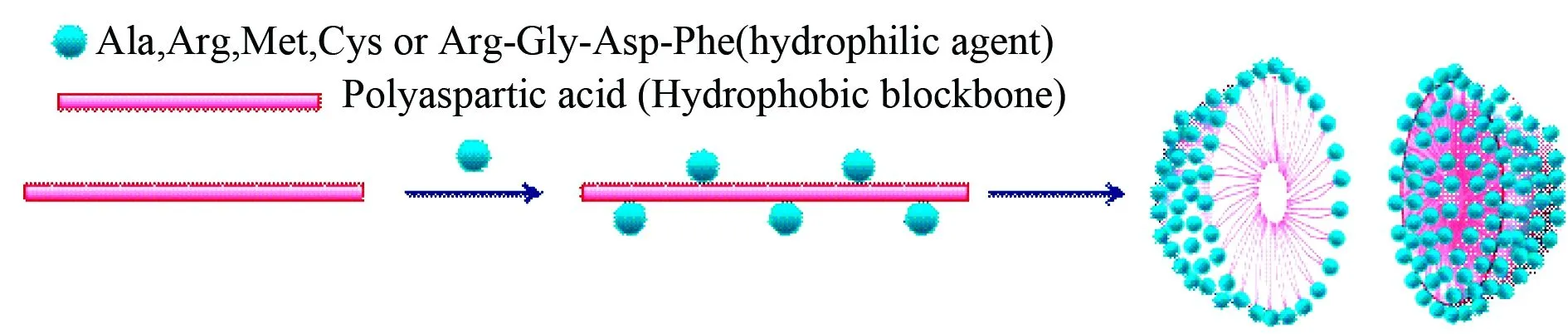
Fig.13 Formation and inner architecture of the nanoparticles of poly-α,β-aspartic acid conjugated L-arginine or L-alanine or L-cysteine or L-methionineor or Arg-Gly-Asp-Phe as anti-thrombotic nanoparticles, thrombolytic nanoparticles, and Pb(II) chelating nanoparticles, respectively, which could simply visualize the inner architecture of TEM and SEM images in the original texts[49-53]
The effect of internal environment on the sensitivity of the nanoparticles formed by the biodegradable copolymers is correlated with specific release of conjugated and/or loaded drugs. For instance the nanoconjugate of poly-β-L-malic acid (PMLA), methoxypolyethylene glycol-5000 (mPEG5000-NH2) and Dox is correlated with pH sensitive release of Dox to inhibit the proliferation of the invasive breast carcinoma cell lines (Fig. 14)[54], N-vinyl pyrrolidone (N-VP) modified polymethacrylic acid (PMAA) and polyvinyl pyrrolidone (PVP) assemble to form the nanoparticles for loading and pH controlled release of rifampicin (RFP) or Dox (Fig. 15)[55]. Acetal-PEG-b-PRAS, a conjugate of acetal-PEG and poly-nitroxylradicalaminomethyl-styrene, assembles to form the nanoparticles for pH controlled exposing of acetal-PEG-b-PRAS, thereby scavenging free radicals and treating cerebral ischemia-reperfusion injury (Fig. 16)[39].
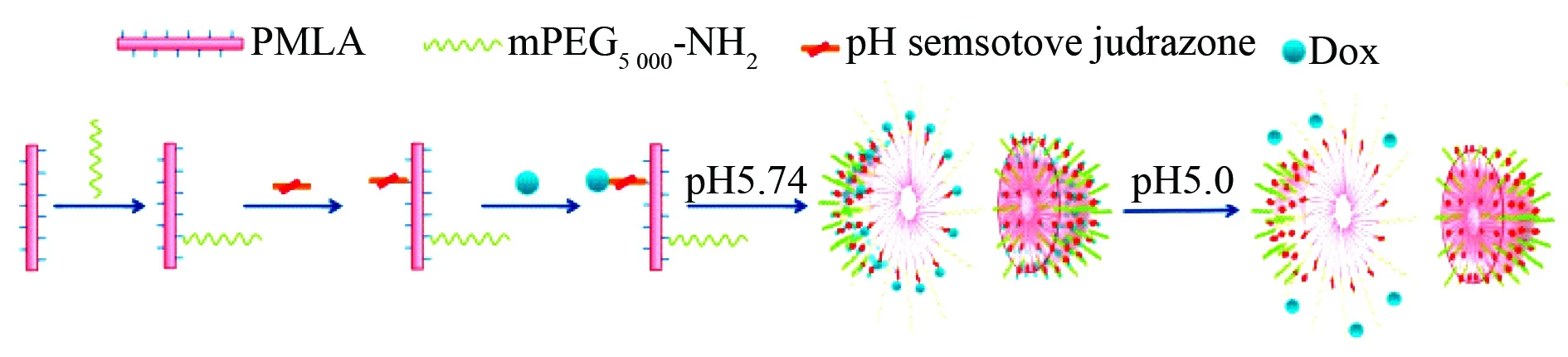
Fig.14 Formation and inner architecture of anticancer nanoparticles of PMLA, mPEG5000-NH2and Dox conjugate capable releasing Dox at pH 5, but not pH 7, wherein PMLA represents poly-β-L-malic acid, mPEG5000-NH2represents glycine hydrazide modified methoxypolyethylene glycol-5000[54]
In physiological condition (pH 7) these anti-cancer nanoparticles very slowly release Dox and exhibit no anti-proliferation activity, while in mature endosome/lysosome of the invasive breast carcinoma cell lines (pH 5) these anticancer nanoparticles release Dox in a burst-manner.
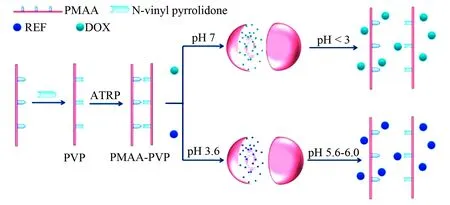
Fig. 15 Formation and inner architecture of anticancer or antituberculotic nanoparticles of PMAA-PVP and Dox or RFP, wherein PMAA represents polymethacrylic acid, PVP represents N-vinyl pyrrolidone modified PMAA, PMAA-PVP represents water insoluble copolymer of water soluble PMAA and PVP, which was formed via atom transfer radical polymerization (ATRP) in aqueous solution, and RFP represents rifampicin[55]
At pH 3.6 RFP loaded nanoparticles of PMAA-PVP are stable, while at pH 5.6-6.0 they collapse and release RFP. At pH 7.0 Dox loaded nanoparticles of PMAA-PVP are stable, while at a pH less than 3.0 they collapse and release Dox.
Fig. 16 Formation and inner architecture of acetal-PEG-b-PRAS’s nanoparticles, wherein PCMS represents polychloromethylstyrene, acetal-PEG represents acetalized PEG, TEMPO represents 2,2,6,6-tetramethylpiperidinyloxyl, and PRAS represents TEMPO containing polyaminomethyl-styrene[39]
At pH 7.4 the nanoparticles are stable and have no biological activity, while at pH 5.9 they collapse and thereby expose acetal-PEG-b-PRAS to show biological activity.
The formation and inner architecture of anti-inflammatory Lys-Ala-Phe-Ala-Lys (KAFAK) loaded thermosensitive nanoparticles assembled by the biodegradable copolymer (NGPEGSS), PEGylated poly-N-isopropylacrylamide-2-acrylamido-2-methyl-1-propanesulfonate and N,N′-bis(acryloyl)cystamine, for treating inflammatory disorders are proposed with Fig. 17. Dithiothreitol or glutathione reduces the disulfide bonds in the nanoparticles to release KAFAK, an antiinflammatory peptide that can suppress the levels of TNF-α and IL-6 in macrophages[56].
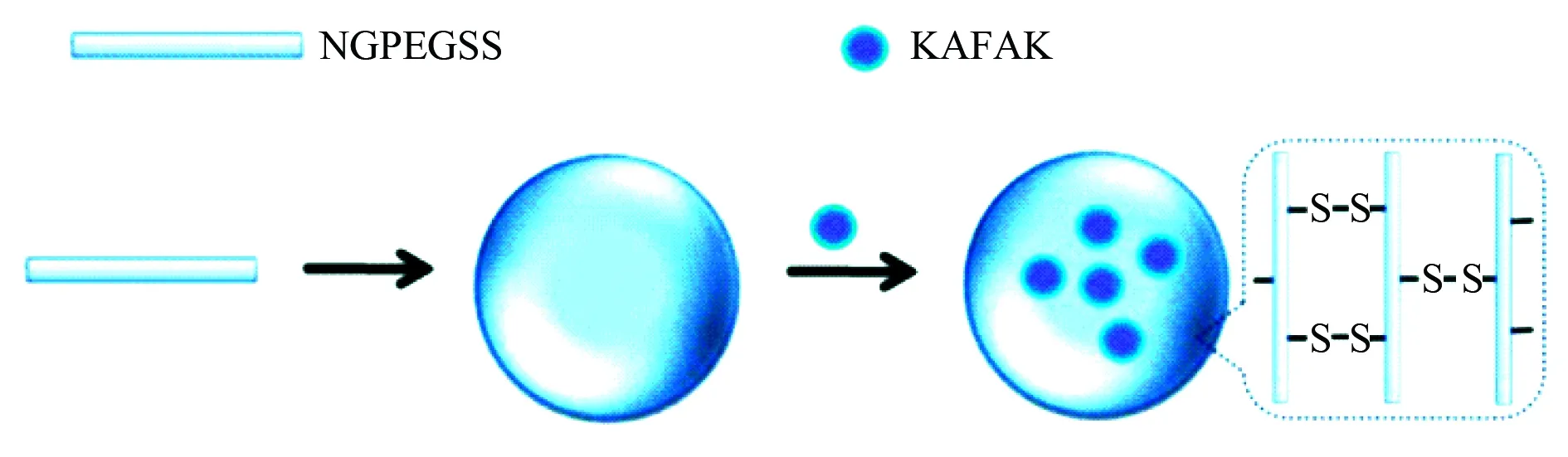
Fig. 17 Formation and inner architecture of KAFAK loaded nanoparticles of NGPEGSS, wherein NGPEGSS represents PEGylated poly-N-isopropylacrylamide-2-acrylamido-2-methyl-1-propane-sulfonate cross-linked with disulfide [N,N′-bis(acryloyl)cystamine][56]
To form the nanoparticles, pure KAFAK was dissolved in ultrapure water to form a loading solution (1 mg/mL), this was added to 1 mg of lyophilized nanoparticles of NGPEGSS, at 4 °C incubated for 24 h, at 35 000 r/min (105 000g) centrifuged for 1 h, the pellets were briefly resuspended in 1 mL ultrapure water and the suspension was lyophilized. In the presence of dithiothreitol (DTT) or in pH 4 environment the nanoparticles collapse and release KAFAK.
To encapsulate water soluble peptides and proteins the hydrophobic ion-pairing complexation based nanoparticles were designed. In the preparation of nanoparticles sodium dodecyl sulfate (SDS) and lysozyme were formulated by using solid-in-oil-in water (s/o/w) emulsion solvent evaporation method (ESEM). The formation and inner architecture of lipid-polymer hybrid nanoparticles are proposed and visualized with Fig. 18[57].

Fig.18 Formation and inner architecture of lipid-polymer hybrid nanoparticles, wherein HIP represents hydrophobic ion-pairing, SDS represents sodium dodecyl sulfate, o phase represent organic phase, w phase represents water phase[57]
To form the nanoparticles ESEM was used, i.e. 200 mg poly-ε-caprolactone (PCL) and 45 mg lipids of phosphatidylcholine (PC) plus glyceryl tripalmitate were dissolved in 8 mL organic solvent of dichloromethane plus acetone to form organic phase. Lyophilised hydrophobic ion pairing (HIP) complex of lysozyme and SDS were dispersed in the organic phase with 2-min sonication in an ice bath, which was poured into 10 mL Pluronic F-127 solution (1%) and sonicated for 5 min in an ice bath. The emulsion was at room temperature stirred overnight to evaporate dichloromethane and acetone, the lipid-polymer hybrid nanoparticles were collected by centrifugation, washed with ultrapure water and lyophilized to obtain the nanoparticle powders.
The typical protein loaded nanoparticles could be exampled with insulin loaded nanoparticles of TPGS-emulsified and PEG-capped PLGA. Insulin is the first-line treatment drug for patients with insulin-dependent diabetes mellitus. To address oral insulin a number of deliveries were made in past decades, but oral administration of insulin still encounters with some difficulties, such as rapid degradation and poor intestinal absorption. To overcome these difficulties, D-α-tocopherol polyethylene-glycol1000succinate (TPGS) emulsified polyethyleneglycol (PEG) capped polylactic-co-glycolic acid (PLGA) nanoparticles were prepared for sustained delivery of insulin. The formation and inner architecture of insulin loaded nanoparticles are proposed and visualized with Fig. 19[58].

Fig. 19 Formation and inner architecture of insulin loaded nanoparticles of copolymer PLG4-PLGA emulsified by TPGS and capped by PEG, wherein PEG represents polyethyleneglycol, PLGA represents polylactic-co-glycolic acid, TPGS represents tocopherol polyethyleneglycol1000succinate[58]
To form the nanoparticles, at 4 ℃ -100 mg copolymer PLG4-PLGA (80/20) and -50 mg TPGS were sonicated in 5 mL acetone for 60 s and continuously stirred to form a solution, into which 2 mL human insulin (100 IU/mL) was added for 60 s sonication, this solution was kept in an ice bath to form a water-in-oil (W/O) emulsion, this was with 10 mL aqueous PEG-2000 (1%) sonicated for 180 s to form (W/O/W) double emulsion, continuously stirred for 6 h to remove organic solvent, at 4 ℃ centrifuged (12 000 r/min) for 15 min to form insulin loaded nanoparticles of PLG4-PLGA copolymer emulsified by TPGS and capped by PEG.
3 Biodegradable copolymers formed micelles
The second one of the important forms of nanomedicines should be the micelles assembled by biodegradable copolymers. Similarly the classification of the micelles follows the therapeutic effectiveness, such as inhibiting the formation of atherosclerotic plaque, anticancer, reversal of MRD, antiinflammation and removing Pb(II), and the micelles that rely on internal environment sensitivity to release drugs is assigned to another type. Atherosclerosis, one of the leading causes of cardiovascular disease, forms plaques in the arterial wall, which occlude the vascular lumen and block blood flow through the vessel. To inhibit the formation of atherosclerotic plaques multifunctional micelles were prepared. In brief, the individual lipopeptide monomer with a polar head group, such as Cys-Arg-Glu-Lys-Ala (CREKA) or carboxyfluorescein or hirulog-2, and a 1,2-di-stearoyl-sn-glycero-3-phosphoethanolamine (DSPE) lipid tail. Between the polar head and the lipid tail PEG-2000 was inserted as a spacer to form lipopeptide monomers. In aqueous solution the lipopeptide monomers assemble and form targeting micelles of -17 nm in diameter. In vivo the targeting micelles bind onto the entire surface of atherosclerotic plaque to inhibit atherosclerotic aortas forming thrombus. The formation and inner architecture of multi-functional micelles formed by lipopeptide monomer having a variable head group, a PEG-2000 spacer and a 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE) tail are proposed and visualized with Fig. 20[59].
Mithramycin (MTM) is an anticancer product derived naturally from Streptomyces genus, and displays severe side effects and toxicities. Modifying the 3-side-chain of MTM with (3R,4S)-3-hydroxy-4-methoxybutan-2-one-4-yl and (4S)-4-methoxybutane-2,3-dione-4-yl provide analogs SK or SDK may increase the anticancer activity, but still show low bioavailability, short plasma retention time and low tumor accumu-lation. To overcome these shortcomings, copolymer PEG-p(Asp-Hyd) is conjugated with SK or SDK to form self-assembled and cross-linked micelles. The formation and inner architecture of the micelles of SK or SDK conjugated copolymer PEG-p(Asp-Hyd) are proposed and visualized with Fig.21[60]. Polyethyleneglycol-block-poly-D,L-lactic acid (PEG-b-PLA) micelles and polyethyleneglycol-block-poly-ε-caprolactone (PEG-b-PCL) micelles carrying 1,1’-dioctadecyltetramethylindotricarbocyanine iodide (DIR) are used to load 3 poorly water-soluble drugs (paclitaxel, 17-allylamino-17-demethoxygeldana mycin, and rapamycin) for treating cancer, while DIR allows near infrared imaging for intraoperative surgical guidance in oncology. The formation and inner architecture of poorly soluble drugs loaded micelles of PEG-b-PLA and DIR loaded micelles of PEG-b-PCL are proposed and visualized with Fig.22[61].
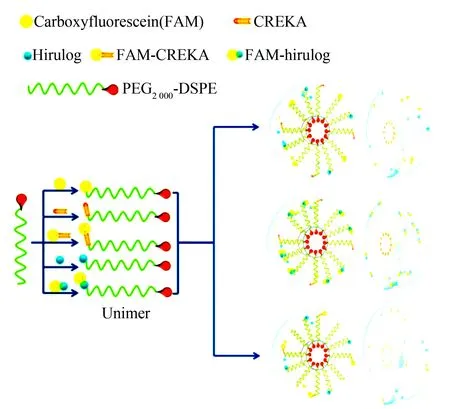
Fig. 20 Formation and inner architecture of multi-functional micelles of lipopeptide monomers having a variable head group, a PEG-2000 spacer and a DSPE tail, which combines the targeting, tracking and therapeutic modalities, wherein FAM represents carboxyfluorescein, CREKA is Cys-Arg-Glu-Lys-Ala, DSPE represents 1,2-distearoyl-sn-glycero-3-phosphoethanolamine. In aqueous solution the diameter of the micelles is -17 nm[59]
This micelle system consists of the polymeric unimers containing DSPE-PEG-2000 and variable head groups, such as blood clot binding peptide CREKA, fluorophore and anticoagulant peptide hirulog. Hirulog is able to directly inhibit the clotting protein thrombin, even after binding fibrin, while fluorophore is able to trace the micelles localizing the plaques, thereby reflecting the ability of CREKA targeting atherosclerosis sites.

Fig. 21 Formation and inner architecture of SK or SDK loaded self-assembled micelles and cross-linked micelles, wherein MTM represents mithramycin, SK represents 3-side-chain of MTM been modified with (3R,4S)-3-hydroxy-4-methoxybutan-2-one-4-yl, SDK represents represents 3-side-chain of MTM been modified with (4S)-4-methoxybutane-2,3-dione-4-yl, p(Asp-Hyd) represents poly(aspartate hydrazide)[60].
These micelles are capable of improving the bioavailability, the plasma retention time and the tumor accumulation of 3-side-chain modified mithramycin (MTM) analogs. In deionized water these micelles selfassemble and form micelles.
In the preparation of Dox loaded star-shaped micelles of copolymer monomethoxy-polyethyleneglycol-poly-ε-caprolactone (MPEG-PCL) was firstly acrylated to form acryloyl MPEG-PCL (AMPEG-PCL), secondly AMPEG-PCL self-assembled into micelles, thirdly AMPEG-PCL micelles polymerized into SSMPEG-PCL micelles, and finally Dox was loaded into the micelles to form the anti-tumor nanomedicine. The formation and inner architecture of Dox loaded micelles of AMPEG-PCL are proposed and visualized with Fig.23[62].

Fig. 22 Formation and inner architecture of PTX, 17-AAG and RAPA loaded PEG-b-PLA micelles, and DIR loaded PEG-b-PCL micelles, wherein PEG-b-PLA represents polyethyleneglycol-block-poly-D,L-lactic acid, PEG-b-PCL represents polyethyleneglycol-block-poly-ε-caprolactone, DIR represents 1,1’-dioctadecyltetramethylindotricarbocyanineiodide, PTX represents paclitaxel, 17-AAG represents 17-allylamino-17-demethoxygeldanamycin, RAPA represents rapamycin[61]
Thus a tandem of 3 (poorly water-soluble PTX, 17-AAG and RAPA)-in-1 (PEG-b-PLA and PEG-b-PCL micelles) could potentially be used for neoadjuvant cancer therapy and tumor-primed NIR optical imaging for intraoperative surgical guidance in oncology, offering a promising multimodal strategy for cancer therapy and imaging.
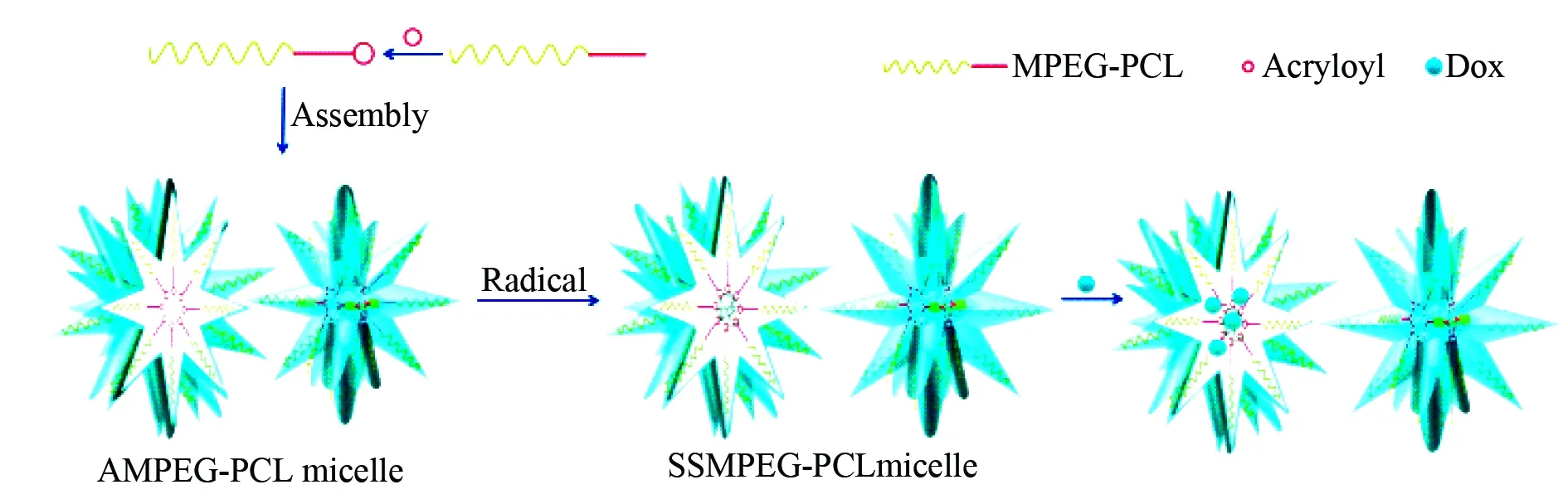
Fig. 23 Formation and inner architecture of Dox loaded star-shaped micelles of SSMPEG-PCL, wherein PCL represents poly-ε-caprolactone, MPEG represents monomethoxy polyethyleneglycol, AMPEG represents acryloyl monomethoxy poly-ethyleneglycol, SSMPEG represents self-assembled star-shaped MPEG[62]
Dox loaded SSMPEG-PCL micelles were prepared in two steps. First, at 60 °C 1.2 g SSMPEG-PCL copolymer were dissolved in 10 mL distilled water, into this solution 48 mg K2S2O8(4 % of SSMPEG-PCL copolymer) were added with a constant stirring (600 r/min) to prepare SSMPEG-PCL micelles by self-assembly. Then, 1.4 mL SSMPEG-PCL micelles were placed into a tube, into which 0.2 mL phosphate buffered saline (a pH 7.4, 10 times usual concentration) were added under stirring, and 0.4 mL aqueous Dox (5 mg/mL) was dropped into this solution in 20 min. Due to its low solubility in PBS of pH 7.4 Dox self-assembled into the hydrophobic core of SSMPEG-PCL micelles to form Dox loaded SSMPEG-PCL micelles.

Fig. 24 Formation and inner architecture of docetaxel loaded and cetuximab conjugated micelles of TPGS, wherein TPGS represents D-α-tocopheryl polyethylene glycol succinate, TPGS-NH2represents amine terminated TPGS, cetuximab is an anti-tumor monoclon antibody and can targets EGRF[63]
To prepare docetaxel loaded TPGS micelles, 2 mg docetaxel was added into a chloroform solution of 50 mg TPGS, chloroform was removed, the formed thin film was dried in vacuum overnight and hydrated in PBS buffer, this mixture was incubated at 37 ℃ with constant agitation and a few min sonication. Into 5 mL micelles (3 mg/mL) 8 mg NHS and 40 mg EDC were added, the pH of reaction mixture was adjusted to 8.4 by sodium borate buffer (pH 8.4), at room temperature with gentle end-to-end mixing cetuximab was added at a concentration range of 0.01-0.20 mg/mL for 4 h reaction, and the micelles were collected by centrifugation.
Quercetin (3,3,4,5,7-pentahydroxyflavone) is a flavonoid from plants and can treat acute cystitis. To improve water solubility quercetin was loaded into the micelles of monomethoxy polyethylene glycol-poly-ε-caprolactone (mPEG-PCL). In the preparation of quercetin loaded mPEG-PCL micelles monomethoxy polyethylene glycol (mPEG) was firstly polymerized with poly-ε-caprolactone (PCL) to form diblock copolymer (mPEG-PCL), and then quercetin loaded micelles of mPEG-PCL formed by self-assembly. The formation and inner architecture of quercetin loaded micelles of mPEG-PCL are proposed and visualized with Fig.25[64].

Fig. 25 Formation and inner architecture of quercetin loaded micelles of mPEG-PCL, wherein mPEG represents monomethoxy polyethyleneglycol, PCL represents poly-ε-caprolactone[64]
To prepare quercetin loaded micelles of MPEG-PCL 7 mg quercetin and 93 mg copolymer mPEG-PCL were dissolved in 6 mL mixture of dichloromethane and methanol (2:1), at 55 ℃ this mixture was evaporated under reduced pressure, into the residue 5 mL normal saline was added to induce the self-assembly of MPEG-PCL and to create core-shell structured MPEG-PCL micelles encapsulating quercetin.
4 Biodegradable polymers formed liposomes and nanogels
Lipsomes and nanogel are the other two types of nanoforms and commonly used for constructing nanomedicines. Here the formation and the application of liposomes are represented with two types. The first type is multimodal thermosensitive polymer modified liposomes encapsulating Dox, MnSO4and rhdamine. In the lipsomes Dox, MnSO4and rhdamine are used as the anticancer drug, the magnetic resonance contrast agent and the fluorescent dye, respectively. When this type of lipsomes been heated at lower critical solution temperature, Dox and MnSO4will flow out from the liposomes, and only rhodamine still remains in the lipid membrane. This leads to the application of the liposomes in chemotherapy, magnetic resonance imaging and fluorescent trace. The formation, the inner architecture and drug release of Dox, MnSO4and rhdamine loaded multimodal thermosensitive polymer modified liposomes are proposed and visualized with Fig.26[65].
The second type of liposomes is formed by 1-palmitoyl-2-oleoylphosphatidyl-choline, distearoyl-sn-glycero-3-phosphatidylethanolamine-N-methoxy-polyethylene-glycol-2000 and the recognition sequences of coagulation factor VIII or activated factor VII for treating hemophilia. Due to PEG is able to limit the reticoloendothelial system swallowing the liposomes, in blood circulation this type of the liposomes has an extended half-life. The formation and the inner architecture of the recognition sequences of coagulation factor VIII or activated factor VII loaded liposomes are proposed and visualized with Fig.27[38].
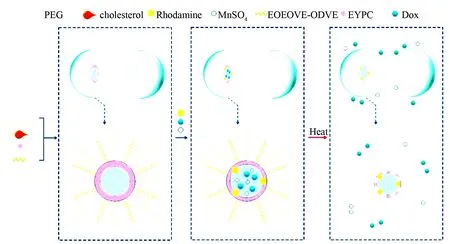
Fig. 26 Formation, inner architecture and drug release of Dox, MnSO4and rhodamine loaded multimodal thermosensitive polymer modified liposome (MTPL), wherein EYPC represents egg-yolk phosphatidylcholine, DOPE represents dioleoylphosphatidylethanolamine, ODVE represents octadecylvinyl ether, EOEOVE represents 2,2-diethoxyethylvinyl ether. The membrane of MTPL consists of EYPC, DOPE, PEG-2000, cholesterol, block copolymer EOEOVE-ODVE and rhodamine-PE[65]
EOEOVE-ODVE is a thermosensitive copolymer, and at lower critical solution temperature it changes from hydrophilic copolymer to hydrophobic copolymer. Thus upon heating MnSO4and Dox are released by MTPL, while rhodamine remains in the lipid membrane.
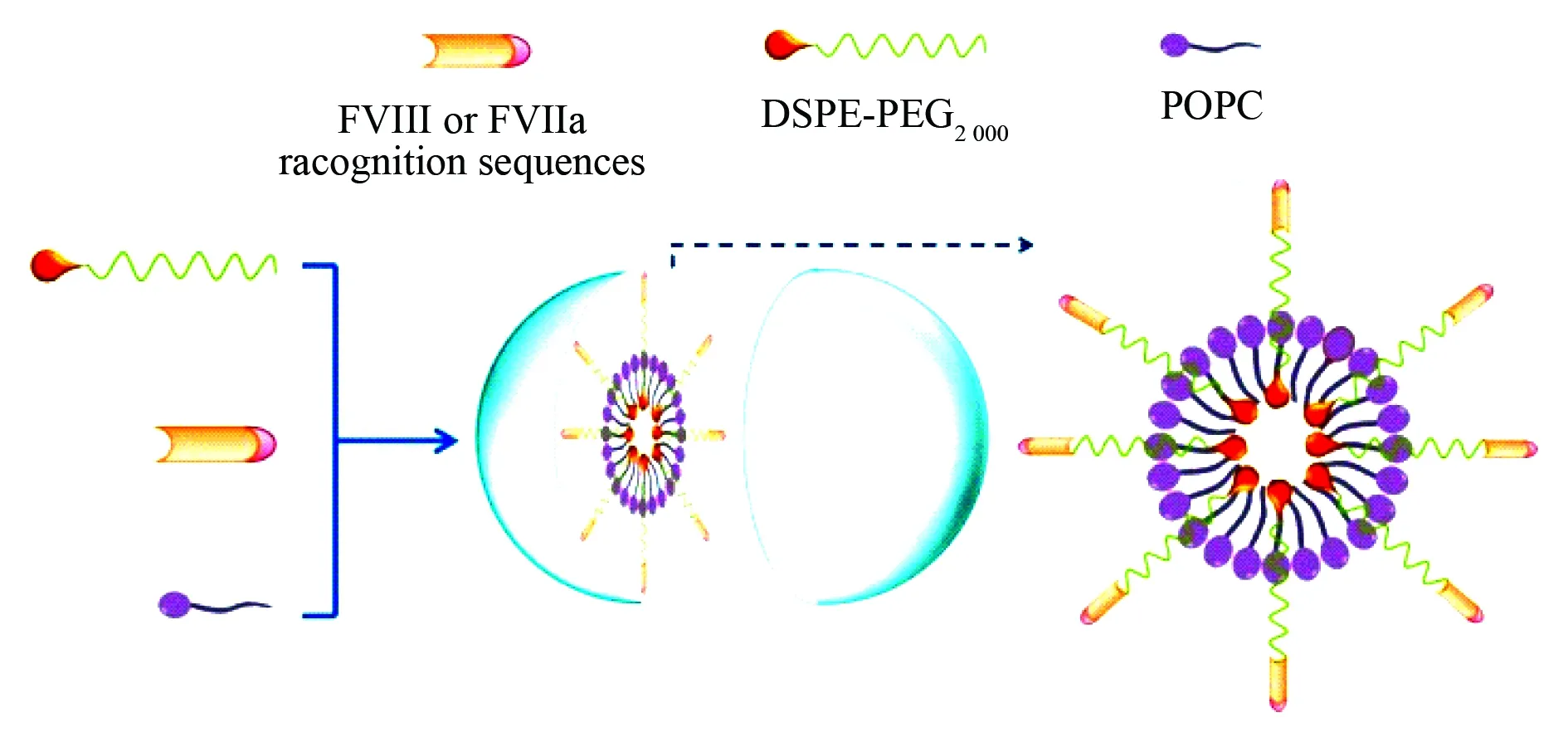
Fig. 27 Formation and inner architecture of PEGylated liposomes, wherein FVIII represents coagulation factor VIII, FVIIa represents activated factor VII, POPC represents 1-palmitoyl-2-oleoylphosphatidylcholine, DSPE-PEG-2000 represents distearoyl-sn-glycero-3-phosphatidyletha nolamine-N-methoxypolyethyleneglycol-2000[38]
To prepare PEGylated liposomes the lipids, PEG and FVIII or FVIIa recognition sequences are dissolved in t-butanol and lyophilized, the dry lipid powders are re-suspended with 110 mmol/L lipid in 50 mmol/L sodium citrate (pH 6.7) to form liposomes. The liposomes are down-sized by extrusion through sequentially smaller polycarbonate filters until reaching a final diameter of 80-100 nm. PEG molecules extend outward liposome surface non-covalently bound the recognition sequences and mediate the binding of them towards FVIII or FVIIa. Besides, PEG also limits the uptake of liposomes by reticulo-endothelial system (RES), thereby extends the half-life of liposomes in the circulation.
Similarly, the formation and application of nanogels are represented with two types. The first type of nanogels of less than 100 nm in diameter is formed by cholesteryl, hyaluronic acid and etoposide or salinomycin or curcumin. In aqueous media the amphiphilic cholesteryl-hyaluronic acid covalently coupled with etoposide or salino-mycin or curcumin, assembled and formed the nanogels. Due to drug-resistant tumors and cancer stem cells have elevated CD44 receptor capable of binding hyaluronic acid, the nanogels consist of cholesteryl, hyaluronic acid and etoposide or salinomycin or curcumin are able to effectively bind cancer cells, penetrate cancer cell membrane, to enter cytoplasma, to have its ester bonds been hydrolyzed, to release etoposide or salinomycin or curcumin and to effectively exhibit the proliferation of cancer cells. The formation and the inner architecture of the nanogels are proposed and visualized with Fig.28[66].
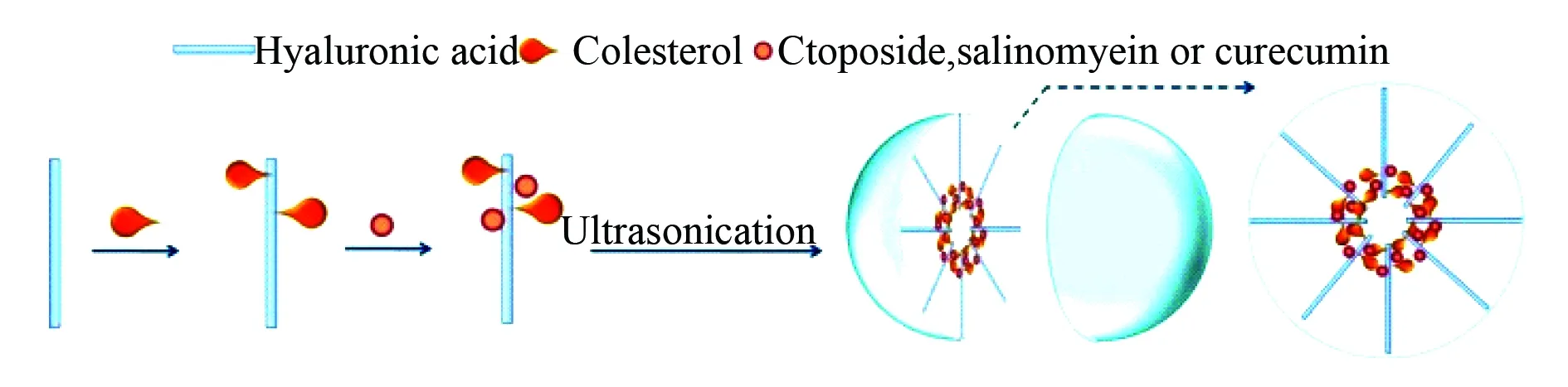
Fig. 28 Formation and inner architecture of nanogel of CHA-etoposide, CHA-salinomycin or CHA-curcumin in aqueous media, wherein CHA represents copolymer cholesteryl-hyaluronic acid[66]
In aqueous media ultrasonication readily induces CHA-etoposide, CHA-salinomycin or CHA-curcumin to form compact nanogels. Usually, 30-60 min ultrasonication is sufficient to form uniform nanogel having a spherical morphology and 29-40 nm of diameter.
The second type of nanogels is related to curcumin.The interactions of curcumin with cancer cells, serum proteins and human red blood cells are involved in five formulations prepared with curcumin and polylactic-co-glycolic acid (PLGA) or β-cyclodextrin or cellulose or poly-N-isopropylacrylamide or dendrimers, of which only the formulation of curcumin and poly-N-isopropylacrylamide belongs to the nanogels. According to the interactions of curcumin with cancer cells, serum proteins and human red blood cells, the nanogels have low serum protein binding characteristics and are suitable for treating malignancy. The formation and the inner architecture of curcumin loaded nanoparticles of PLGA, β-cyclodextrin and cellulose, of curcumin loaded nanogel of poly-N-isopropylacrlamide and curcumin loaded dendrimer of polyamidoamine are proposed and visualized with Fig.29[67].
In the preparation of the formulations PLGA, β-cyclodextrin, cellulose, dendrimer and poly-N-isopropylacrlamide through self-assembly form nanoparticles or dendrimer or nanogels and encapsulate curcumin.
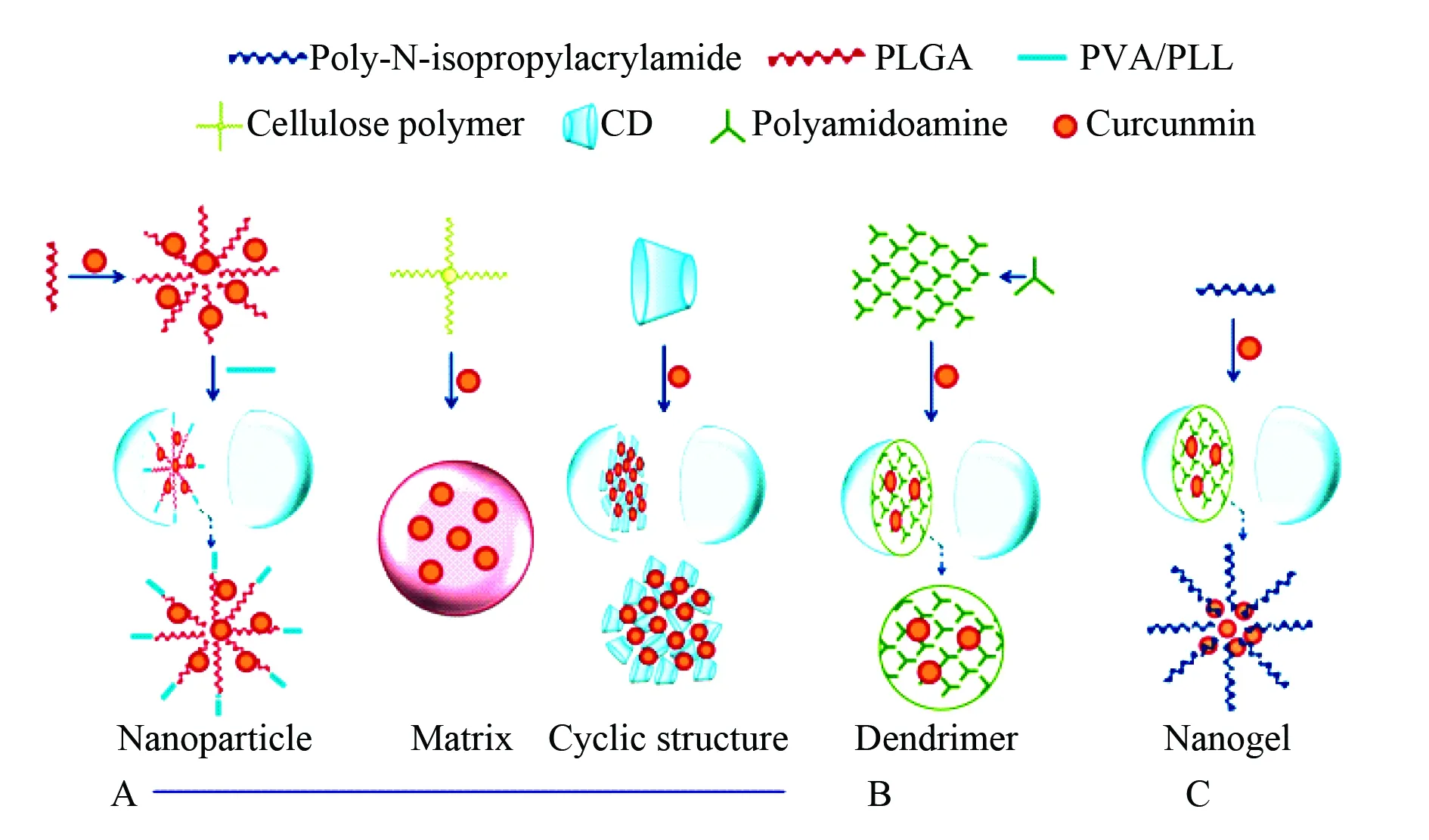
Fig. 29 Formation and inner architecture of curcumin loaded nanoparticles of PLGA, β-cyclodextrin and cellulose[67]
A:curcumin loaded dendrimer of polyamidoamine B:curcumin loaded nanogel of poly-N-isopropylacrlamide C: wherein PVA represents polyvinyl alcohol, PLL represents poly-L-lysine, CD represents β-cyclodextrin.
5 Conclusions and future perspectives
The improvements of water solubility, bioavailability, pharmacokinetics and distribution of the drugs or the lead compounds have been the critical issues of medicinal chemical research. In this regard, one of the most promising approaches should be designing biodegradable polymeric conjugates containing therapeutic, targeting, diagnostic and imaging blocks. Both hydrophobic and hydrophilic interactions could drive the self-assembly of biodegradable polymeric conjugates to form nanoparticles, micelles, lipsomes or nanogels, the so called nanomedicine. Due to biodegradable polymeric nanomedicines at least combine the knowledge of biology, chemistry and imaging so far the known nanoformulations of therapeutic, targeting, diagnostic and imaging agents loaded nanoparticles, micelles, lipsomes and nanogels of the biodegradable polymers occasionally give no exact composition and architecture, lack TEM, SEM and AFM images and confuse the methods of preparing the polymers and formulations in particular.
In contrast to nanoformulation,the nanoparticles, micelles, lipsomes and nanogels of biodegradable polymeric conjugates or biodegradable polymers have exact composition and structure, especially the nanoparticles, micelles, lipsomes and nanogels of stimuli-responsive biodegradable copolymers, such as redox responsive micelles[29 ], pH sensitive nanoparticles of acetal-PEG-b-PRAS for treating cerebral ischemia-reperfusion injury[39], pH sensitive anti-cancer nanoparticles of PMLA, mPEG5000-NH2and Dox conjugate[54], and pH sensitive Dox or RFP loaded anticancer or antituberculotic nanoparticles of PMAA-PVP[55], KAFAK loaded thermo, dithiothreitol sensitive and pH sensitive antiinflammatory nanoparticles of NGPEGSS[56], could optionally release therapeutics and open an important field. Whereas the liposomes of the conjugate DSPE-PEG-2000 and the recognition sequences of coagulation factor FVIII or FVIIa may act on platelets[38 ], ARHP8 loaded nanoparticles of PSMA-A10 conjugated PLGA-PEG may recognize human PSMA extracellular domain[45], and anti-thrombotic nanoparticles of poly-α,β-aspartyl-Arg-Gly-Asp-Phe may recognize GPIIb/IIIa[50]could provide targeting therapy for hemophilia, cancer and thrombosis, respectively.
The advantages[68-70], such as having desirable pharmaceutical and pharmacological properties, targeting disease site, crossing biological barriers, releasing drug in a specific manner, exhibiting multiple functions and monitoring drug-distribution in a real-time manner, opens a window for practical use of the biodegradable polymeric conjugate formed nanomedicines in the future. In easy synthetic opinion, the biodegradable polymeric conjugate constructed with therapeutic moiety and targeting moiety or diagnostic moiety or imaging moiety, i.e. the biodegradable polymeric conjugate having dual functions, should be the most useful monomer for preparing the nanomedicine.
6 References
[1] Ensign L M, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers[J]. Adv Drug Deliv Rev, 2012, 64(6): 557-570.
[2] Koo O M, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review[J]. Nanomedicine, 2005, 1(3): 193-212.
[3] Mathur A B, Gupta V. Silk fibroin-derived nanoparticles for biomedical applications[J]. Nanomedicine, 2010, 5(5): 807-820.
[4] Kaminskas L M, Boyd J, Porter C J. Dendrimer pharmacokinetics: the effect of size, structure and surface characteristics on ADME properties[J]. Nanomedicine, 2014, 6(6): 1063-1084.
[5] Gao W, Xiao Z, Radovic-Moreno A, et al. Progress in siRNA delivery using multifunctional nanoparticles[J]. Methods Mol Biol, 2010, 629: 53-67.
[6] Trotta F, Zanetti M, Cavalli R. Cyclodextrin-based nanosponges as drug carriers[J]. Beilstein J Org Chem, 2012, 8(23): 2091-2099.
[7] Tian L, Bae Y H. Cancer nanomedicines targeting tumor extracellular pH[J]. Colloids Surf B Biointerfaces, 2012, 99: 116-126.
[8] Duncan R, Vicent M J. Polymer therapeutics-prospects for 21st century: The end of the beginning[J]. Adv Drug Deliv Rev, 2013, 65(1): 60-70.
[9] Marin E, Briceno M I, Caballero-George C. Critical evaluation of biodegradable polymers used in nanodrugs[J]. Int J Nanomed, 2013, 8: 3071-3090.
[10] Vakili M, Rafatullah M, Salamatinia B, et al. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review[J]. Carbohydr Polym, 2014, 113: 115-130.
[11] Yang Y, Wang S, Wang Y, et al. Advances in self-assembled chitosan nanomaterials for drug delivery[J]. Biotechnol Adv, 2014, 32(7): 1301-1316.
[12]Elgadir M A, Uddin M S, Ferdosh S, et al. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review[J]. J Food Drug Anal, 2015, 23(4): 619-629.
[13]Prabaharan M. Chitosan-based nanoparticles for tumor-targeted drug delivery[J]. Int J Biol Macromol, 2015, 72(5): 1313-1322.
[14]Wang H, Xu M, Xiong M, et al. Reduction-responsive dithiomaleimide-based nanomedicine with high drug loading and FRET-indicated drug release[J]. Chem Commun:Camb, 2015, 51(23): 4807-4810.
[15] Mei L, Zhang Z, Zhao L,et al. Pharmaceutical nanotechnology for oral delivery of anticancer drugs[J]. Adv Drug Deliv Rev, 2013, 65(6): 880-890.
[16]Huang P, Cui C Y, Wang Y J, et al. Preparation and evaluation of a liposome of PEG and epirubicin [J]. Journal of Capital Medical University, 2015, 36(2): 166-172.
[17] Azzopardi E A, Conlan R S, Whitaker I S. Polymer therapeutics in surgery: the next frontier[J]. J Interdiscip Nanomed, 2016, 1(2): 19-29.
[18] Mura S, Bui D T, Couvreur P, et al. Lipid prodrug nanocarriers in cancer therapy[J]. J Control Release, 2015, 208: 25-41.
[19]Fay F, Scott C J. Antibody-targeted nanoparticles for cancer therapy[J]. Immunotherapy, 2011, 3(3): 381-394.
[20]Cheng Y, Zhao L, Li Y, et al. Design of biocompatible dendrimers for cancer diagnosis and therapy: current status and future perspectives[J]. Chem Soc Rev, 2011, 40(5): 2673-2703.
[21] Macewan S R, Callahan D J, Chilkoti A. Stimulus-responsive macromolecules and nanoparticles for cancer drug delivery[J]. Nanomedicine, 2010, 5(5): 793-806.
[22]Kedar U, Phutane P, Shidhaye S, et al. Advances in polymeric micelles for drug delivery and tumor targeting[J]. Nanomed, 2010, 6(6): 714-729.
[23]Prabhu R H, Patravale V B, Joshi M D. Polymeric nanoparticles for targeted treatment in oncology: current insights[J]. Int J Nanomed, 2015, 10(1): 1001-1018.
[24]Friberg S, Nystrom A M. Nanomedicine: will it offer possibilities to overcome multiple drug resistance in cancer?[J]. J Biotechnol, 2016, 14(1): 1-17.
[25]Agyare E, Kandimalla K. Delivery of Polymeric Nanoparticles to Target Vascular Diseases[J]. J Biomol Res Ther , 2014, 3(1):1-24.
[26]Rubinstein I, Weinberg G L. Nanomedicines for chronic non-infectious arthritis: the clinician 3s perspective[J]. Nanomedicine, 2012, 8 (Suppl 1): 77-82.
[27] Low S A, Kopecek J. Targeting polymer therapeutics to bone[J]. Adv Drug Deliv Rev, 2012, 64, 1189-1204.
[28] Topete A, Barbosa S, Taboada P. Intelligent micellar polymeric nanocarriers for therapeutics and diagnosis[J]. J Appl Polym Sci, 2015, 132(41): 1-18.
[29]Movassaghian S, Merkel O M, Torchilin V P. Applications of Polymer Micelles for Imaging and Drug Delivery[J]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2015, 7(5): 691-707.
[30]Ryvolova M, Chomoucka J, Drbohlavova J, et al. Modern Micro and Nanoparticle-Based Imaging Techniques[J]. Sensors Basel, 2012, 12: 14792-14820.
[31]Georgieva J V, Hoekstra D, Zuhorn I S. Smuggling Drugs into the Brain: An Overview of Ligands Targeting Transcytosis for Drug Delivery across the Blood-Brain Barrier[J]. Pharmaceutics, 2014, 6(4): 557-583.
[32]van O, Tellingen, B. Yetkin-Arik, M C de Gooijer,et al. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment[J]. Drug Resist Updat, 2015, 19(10): 1-12.
[33]Forde E, Devocelle M. Pro-Moieties of Antimicrobial Peptide Prodrugs[J]. Molecules, 2015, 20, 1210-1227.
[34]Ungaro F, d'Angelo I, Miro A, et al. Engineered PLGA nano-and micro-carriers for pulmonary delivery: challenges and promises[J]. J Pharm Pharmacol, 2012, 64, 1217-1235.
[35]Cho Y, Borgens R B. Polymer and nano-technology applications for repair and reconstruction of the central nervous system[J]. Exp Neurol, 2012, 233(1): 126-144.
[36]Carballo-Molina O A, Velasco I. Hydrogels as scaffolds and delivery systems to enhance axonal regeneration after injuries[J]. Front Cell Neurosci, 2015, 9: 1-12.
[37]Islam M A, Firdous J, Choi Y J, et al. Design and application of chitosan microspheres as oral and nasal vaccine carriers: an updated review[J]. Int J Nanomed, 2012, 7(7): 6077-6093.
[38]Yatuv R, Robinson M, Dayan-Tarshish I, et al. The use of PEGylated liposomes in the development of drug delivery applications for the treatment of hemophilia[J]. Int J Nanomed, 2010, 5(655): 581-591.
[39]Yoshitomi T, Nagasaki Y. Nitroxyl radical-containing nanoparticles for novel nanomedicine against oxidative stress injury[J]. Nanomedicine, 2011, 6(3): 509-518.
[40]Mei L, Jiang Y, Feng S S. Star-shaped block polymers as a molecular biomaterial for nanomedicine development[J]. Nanomedicine, 2014, 9(1): 9-12.
[41]Han S, Li M, Liu X, et al. Construction of amphiphilic copolymer nanoparticles based on gelatin as drug carriers for doxorubicin delivery[J]. Colloids Surf B Biointerfaces, 2013, 102(102c): 833-841.
[42] Tan G R, Feng S S, Leong D T. The reduction of anti-cancer drug antagonism by the spatial protection of drugs with PLAeTPGS nanoparticles[J]. Biomaterials, 2014, 35(9): 3044-3051.
[43]Alcala-Alcala S, Urban-Morlan Z, Aguilar-Rosas I, et al. A biodegradable polymeric system for peptide-protein delivery assembled with porous microspheres and nanoparticles, using an adsorption/infiltration process[J]. Int J Nanomed, 2013, 8(13): 2141-1251.
[44] Zhang X, Dong Y, Zeng X, et al. The effect of autophagy inhibitors on drug delivery using biodegradable polymer nanoparticles in cancer treatment[J]. Biomaterials, 2014, 35(6): 1932-1943.
[45] Yang J, Xie S X, Huang Y, et al. Prostate-targeted biodegradable nanoparticles loaded with androgen receptor silencing constructs eradicate xenograft tumors in mice[J]. Nanomedicine: Lond, 2012, 7(9): 1297-1309.
[46]Guo L, Zhang H, Wang F, et al. Targeted multidrug-resistance reversal in tumor based on PEG-PLL-PLGA polymer nano drug delivery system[J]. Int J Nanomed, 2015, 10: 4535-4547.
[47] Shieh M J, Hsu C Y, Huang L Y, et al. Reversal of doxorubicin-resistance by multifunctional nanoparticles in MCF-7/ADR cells[J]. J Control Release, 2011, 152(3): 418-425.
[48]Zhu H, Chen H, Zeng X, et al. Co-delivery of chemotherapeutic drugs with vitamin E TPGS by porous PLGA nanoparticles for enhanced chemotherapy against multi-drug resistance[J]. Biomaterials, 2014, 35(7): 2391-2400.
[49] Gui L, Zhao M, Wang Y, et al. Synthesis, nano-features, ex vivo anti-platelet aggregation and in vivo antithrombotic activities of poly-a,b-DL-aspartyl-L-arginine[J]. Med Chem Comm, 2012, 3(1): 102-108.
[50]Gui L, Zhao M, Wang Y, et al. Synthesis, nanofeatures, in vitro thrombus lysis activity and in vivo thrombolytic activity of poly-a,b-aspartyl-l-alanine[J]. Nanomedicine, 2010, 5(5): 703-714.
[51]Chen S, Wang Y, Li S, et al. Poly-a,b-aspartyl-Arg-Gly-Asp-Phe: a novel polymeric nanomedicine[J]. Med Chem Comm, 2015, 6(1): 182-186.
[52] Li L, Wu J, Zhao M, et al. Poly-α,β-DL-aspartyl-L-cysteine: A novel nanomaterial having a porous structure, special complexation capability for Pb(II), and selectivity of removing Pb(II)[J]. Chem Res Toxicol, 2012, 25(9): 1948-1954.
[53] Zhang H, Wang Y, Zhao M, et al. Synthesis and in vivo lead detoxification evaluation of Poly-α,β-DLaspartyl-L-methionine[J]. Chem Res Toxicol, 2012, 25(2): 471-477.
[54]Patil R, Portilla-Arias J, Ding H, et al. Cellular delivery of doxorubicin via pH-Controlled hydrazone linkage using multifunctional nano vehicle based on poly(β-L-Malic Acid)[J]. Int J Mol Sci, 2012, 13(9): 11681-11693.
[55]Chen G, Liu J, Yang Y, et al. Preparation of pH-sensitive nanoparticles of poly (methacrylic acid) (PMAA)/poly (vinyl pyrrolidone) (PVP) by ATRP-template miniemulsion polymerization in the aqueous solution[J]. Colloid Polym Sci, 2015, 293(7): 2035-2044.
[56]Poh S, Lin J B, Panitch A. Release of anti-inflammatory peptides from thermosensitive nanoparticles with degradable cross-links suppresses pro-inflammatory cytokine production[J]. Biomacromolecules, 2015, 16(4): 1191-1200.
[58]Malathi S, Nandhakumar P, Pandiyan V, et al. Novel PLGA-based nanoparticles for the oral delivery of insulin[J]. Int J Nanomed, 2015, 10: 2207-2218.
[59]Lewis D R, Kamisoglu K, York A W, et al. Polymer-Based Therapeutics: Nanoassemblies and Nanoparticles for management of atherosclerosis[J]. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2011, 3(4): 400-420.
[60]Scott D , Rohr J, Bae Y. Nanoparticulate formulations of mithramycin analogs for enhanced cytotoxicity[J]. Int J Nanomed, 2011, 6(6): 2757-2767.
[61] Cho H, Kwon G S. Polymeric micelles for neoadjuvant cancer therapy and tumor-primed optical Imaging[J]. ACS Nano, 2011, 5(11): 8721-8729.
[62]Gao X, Wang B, Wei X, et al. Preparation, characterization and application of star-shaped PCL/PEG micelles for the delivery of doxorubicin in the treatment of colon cancer[J]. Int J Nanomed, 2013, 8(1): 971.
[63]Kutty R V, Feng S S. Cetuximab conjugated vitamin E TPGS micelles for targeted delivery of docetaxel for treatment of triple negative breast cancers[J]. Biomaterials, 2013, 34(38): 10160.
[64]Wang B L, Gao X, Men K, et al. Treating acute cystitis with biodegradable micelle-encapsulated quercetin[J]. Int J Nanomed, 2012, 7(5): 2239.
[65] Kokuryo D, Nakashima S, Ozaki F, et al. Evaluation of thermo-triggered drug release in intramuscular-transplanted tumors using thermosensitive polymer-modified liposomes and MRI[J]. Nanomedicine, 2015, 11(1): 229.
[66]Wei X, Senanayake T H, Warren G, et al. Hyaluronic acid-based nanogel-drug conjugates with enhanced anticancer activity designed for targeting of CD44-positive and drug-resistant tumors[J]. Bioconjug Chem, 2013, 24(4): 658-668.
[67] Yallapu M M, Ebeling M C, Chauhan N, et al. Interaction of curcumin nanoformulations with human plasma proteins and erythrocytes[J]. Int J Nanomed, 2011, 6(1): 2779.
[68]Yan Y, Wang Y J, Wu J H, et al. Studies on anticancer activity of 17-AAG poly-butylcyanoacrylate nanoparticle[J].J Cap Med Univ, 2015, 36(2): 178-184.
[69]Shi F, Cui C Y, Wu J H, et al. Evaluation of anti-tumor activity of docetaxel loaded nanostructured lipid carriers with seal oil[J]. J Cap Med Univ, 2015, 36(2): 185-191.
[70]Xing L, Cui C Y, Wang Y J, et al. 6-Mercaptopurine/ verapamil-mesoporous silica and reversing multidrug resistance[J]. J Cap Med Univ, 2015, 36(2): 161-165.
编辑 慕 萌
Structure and feature of nano-medicines assembled by biodegradable polymers
Zhao Ming, Peng Shiqi*
(SchoolofPharmaceuticalSciences,CapitalMedicalUniversity,Beijing100069,China)
Major progress in the understanding of the onset and pathology of a series of diseases highlights the biodegradable polymers based nano-medicine having desirable pharmaceutical and pharmacological property, targeting disease site, crossing biological barriers, exhibiting multiple functions and monitoring real-time drug-distribution. These advantages of nano-medicines attract a great deal of research interests, and the polymeric biodegradable conjugates have been deeply emphasized by medicinal chemistry in particular. In this regard, the present review focuses on the assembling process and feature of biodegradable polymers based nano-medicines. To get insight into the assembling process of the biodegradable polymers and to visualize the feature of the formed nano-species we provided a number of figures.
biodegradable polymers; nano-medicine; nano-structure; assembly process; assembly mechanism
国家自然科学基金(81273379, 81373264,81673303),北京市教委重点项目(Z141100002114049,KZ201610025029),多肽及小分子北京市重点实验室(130011601603)。This study was supported by National Natural Science Foundation of China (81273379, 81373264,81673303), Key Project of Beijing Education Commission (Z141100002114049,KZ201610025029),Beijing Area Major Laboratory of Peptide And Small Molecular Drugs(130011601603).
时间:2017-01-17
10.3969/j.issn.1006-7795.2017.01.026]
R 94
2017-01-04)
*Corresponding author, E-mail:sqpeng@bjmu.edu.cn

