改性凹凸棒土/纳米铁复合材料去除地下水的研究
董 磊,龚 磊,林 莉,冯 雪,李青云
(1.长江水利委员会长江科学院流域水环境研究所,湖北 武汉 430010; 2.长江水利委员会长江科学院流域水资源与生态环境科学湖北省重点实验室,湖北 武汉 430010;3.长江水利委员会长江科学院党群办公室,湖北 武汉 430010)
董 磊1,2,龚 磊3,林 莉1,2,冯 雪1,2,李青云1,2
(1.长江水利委员会长江科学院流域水环境研究所,湖北 武汉 430010; 2.长江水利委员会长江科学院流域水资源与生态环境科学湖北省重点实验室,湖北 武汉 430010;3.长江水利委员会长江科学院党群办公室,湖北 武汉 430010)
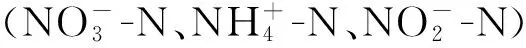

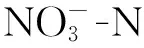
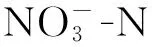
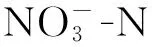
凹凸棒土理想结构式是[Mg5][Si8O20](OH)2(OH2)4·4H2O,属于含水富镁铝硅酸盐纤维状黏土矿物[13],直径一般为20~50 nm,长几百纳米至几微米,是天然的纳米材料。凹凸棒土内部具有发育的微孔孔道,其成分、形态、结构、物理化学性质都比较特殊。凹凸棒土的吸附性与比表面积成正相关,将其进行酸改性或热活化均可增大比表面积。酸改性可使凹凸棒土的八面体层不断溶解,进而提高矿物的微孔隙及微孔比表面积,增强凹凸棒土的吸附性能[14]。热改性可使凹凸棒土内部的有机质及其部分矿物质分解,孔道变得疏松,孔隙容积和比表面积增加,其吸附性随之增强[15]。
基于对凹凸棒土的独特结构和吸附性能的认识,许多学者将凹凸棒土作为吸附剂进行了深入的研究[16-19]。王金明等[16-17]分别用酸、微波加热等方法对凹凸棒土进行改性,用于吸附核素Sr2+、苯酚。Sanchez等[18-19]对凹凸棒土进行改性,并对改性后的吸附性能进行研究。总体而言,凹凸棒土具有结构独特和吸附性能良好的优点,已经取得的成果表明凹凸棒土是一种优良的水处理吸附剂,特别是经过改性后的凹凸棒土吸附性能更佳。
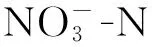
1 试验材料与仪器
1.1 试验材料
实验所用凹凸棒土由江苏玖川纳米材料科技发展有限公司提供。硝酸钾、亚硝酸钠、氢氧化钠、硫酸锌、碘化钾、碘化汞、酒石酸钾钠、硼氢化钠、无水乙醇、氨基磺酸、4-氨基苯磺酸胺、N-(1-萘基)-乙二胺二盐酸盐、六水合氯化铁、六偏磷酸钠等均为分析纯,实验用水为脱氧去离子水。
1.2 试验仪器
万分之一电子天平(美国DENVER,TP-21);低速台式离心机(上海安亭科学,TDL-40B);紫外可见分光光度计(日本岛津,UV2600);电热鼓风干燥箱(上海博迅实业,BGZ-240);多参数分析仪(上海雷磁,DZB-718);水浴恒温振荡器(江苏金坛普天,SHY-2A);冷冻干燥机(美国 GOLD SIM,FD5-3B)。
1.3 改性凹凸棒土、纳米铁和复合材料的制备方法
1.3.1 凹凸棒土的提纯
凹凸棒土研磨过200目筛后,将凹凸棒土配成悬浮液,在悬浮液中加入六偏磷酸钠作为分散剂[20-21],磁力搅拌,50℃下超声波处理后静置,取上层乳白色悬浮液离心处理,将离心后的沉淀物在105℃条件下干燥6 h后研磨过200目筛,得到提纯后凹凸棒土。
1.3.2 凹凸棒土的酸改性
相关研究采用不同浓度的盐酸对凹凸棒土进行酸处理[22-23]。本试验优化后采用浓度为1 mol/L的盐酸对提纯后凹凸棒土酸改性,凹凸棒土与盐酸的固液比为1∶10。磁力搅拌,50℃超声波处理,离心过滤,离心后的沉淀物采用去离子水洗涤,洗涤至中性,然后将所得沉淀物在105℃条件下干燥并过200目筛,得到粒径不大于200目的酸改性凹凸棒土。
1.3.3 凹凸棒土的热改性
相关研究表明对凹凸棒土进行热活化改性时不宜超过500℃,且活化时间3 h较为合适[24-25]。因此,本试验对提纯后的凹凸棒土进行热改性时,焙烧温度设置为100~500℃,焙烧时间3 h,冷却后,过200目筛,得到粒径不大于200目的热改性凹凸棒土。
1.3.4 复合材料和纳米铁的制备
复合材料和纳米铁均采用液相还原法制备,参考相关文献[26-27],称取2 g纯化改性凹凸棒土,用50 mL的去离子水和乙醇混合溶液(体积比4∶1)溶解,按质量比为1∶10、1∶5、1∶3、1∶2、1∶1加入六水合氯化铁,搅拌2 h;并向其中滴加100 mL一定浓度的KBH4溶液,滴加速度控制在0.2 mL/s左右,反应完毕后继续搅拌1 h,保证KBH4与Fe3+充分反应,反应见式(1),整个试验过程均在氩气保护下进行。
12H++6H2
(1)
反应结束后,离心过滤,所得沉淀物用乙醇洗涤3~4次,再真空过滤干燥。纳米铁在相同条件下制备,但不加纯化改性的凹凸棒土。复合材料和纳米铁均储存于密封棕色玻璃瓶(通氩气除氧)。
1.4 试验方法
试验中用锡纸包覆反应离心管,避免光照,以模拟地下水的黑暗环境;向反应溶液中通氩气,去除反应溶液中的DO以模拟地下水DO环境。
1.4.1 改性凹凸棒土选择试验
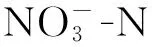
1.4.2 复合材料制备试验
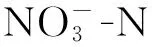
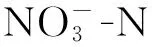
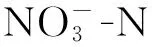
2 结果与讨论
2.1 复合材料的制备及性能
2.1.1 凹凸棒土改性条件的确定
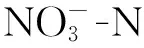

图1 3种改性条件下凹凸棒土对去除率

图2 不同焙烧温度下凹凸棒土对的吸附性能
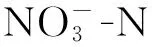

图3 4种改性条件下凹凸棒土对去除率
综上,本研究选用的凹凸棒土采用先提纯,后热活化的方式进行改性,热活化条件:焙烧温度270℃,焙烧时间3 h。
2.1.2 复合材料制备方案的确定
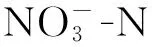
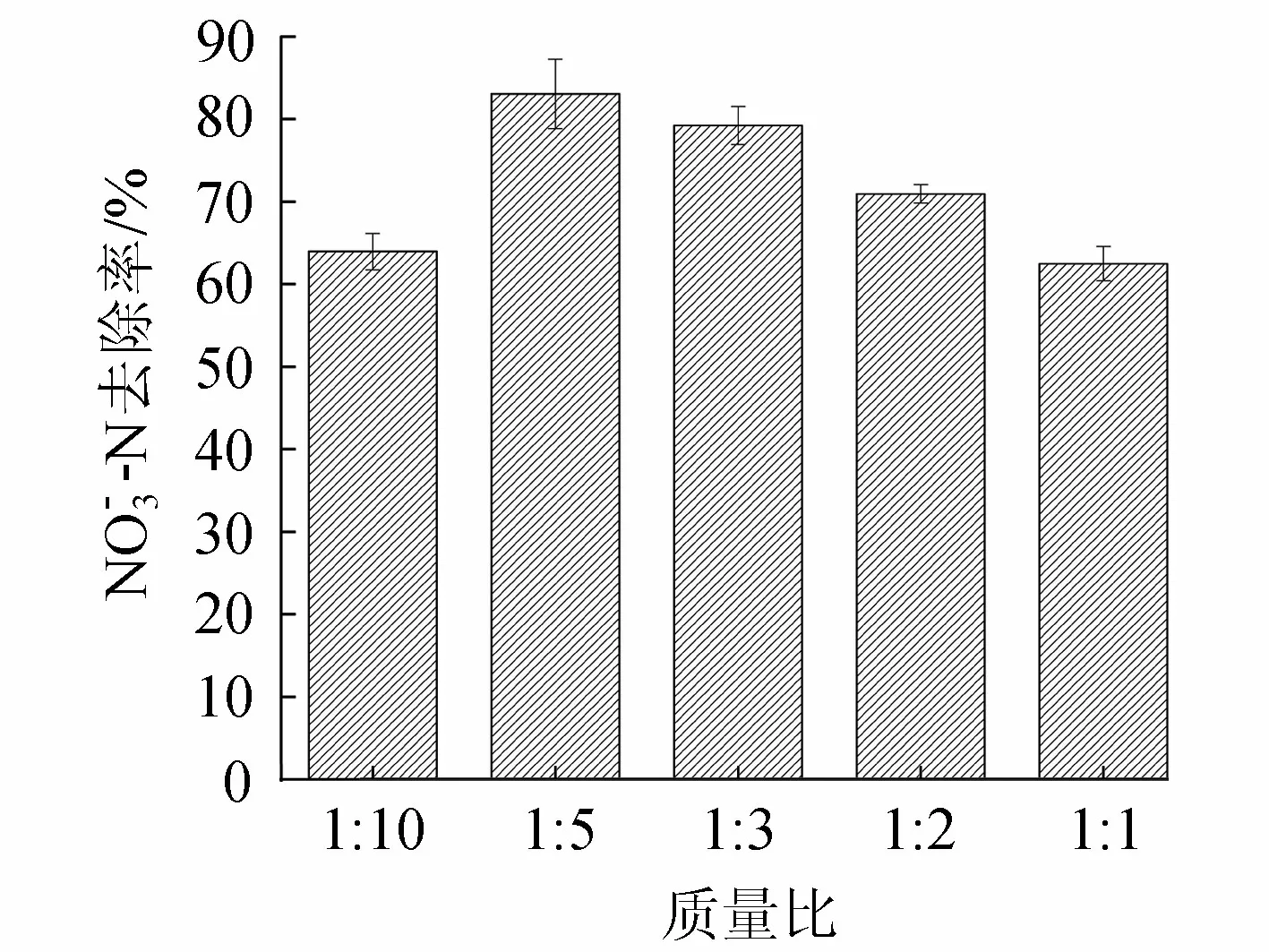
图4 不同质量比下复合材料对去除率
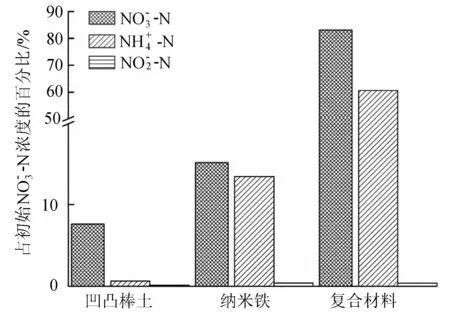
图5 不同材料下“三氮”变化情况
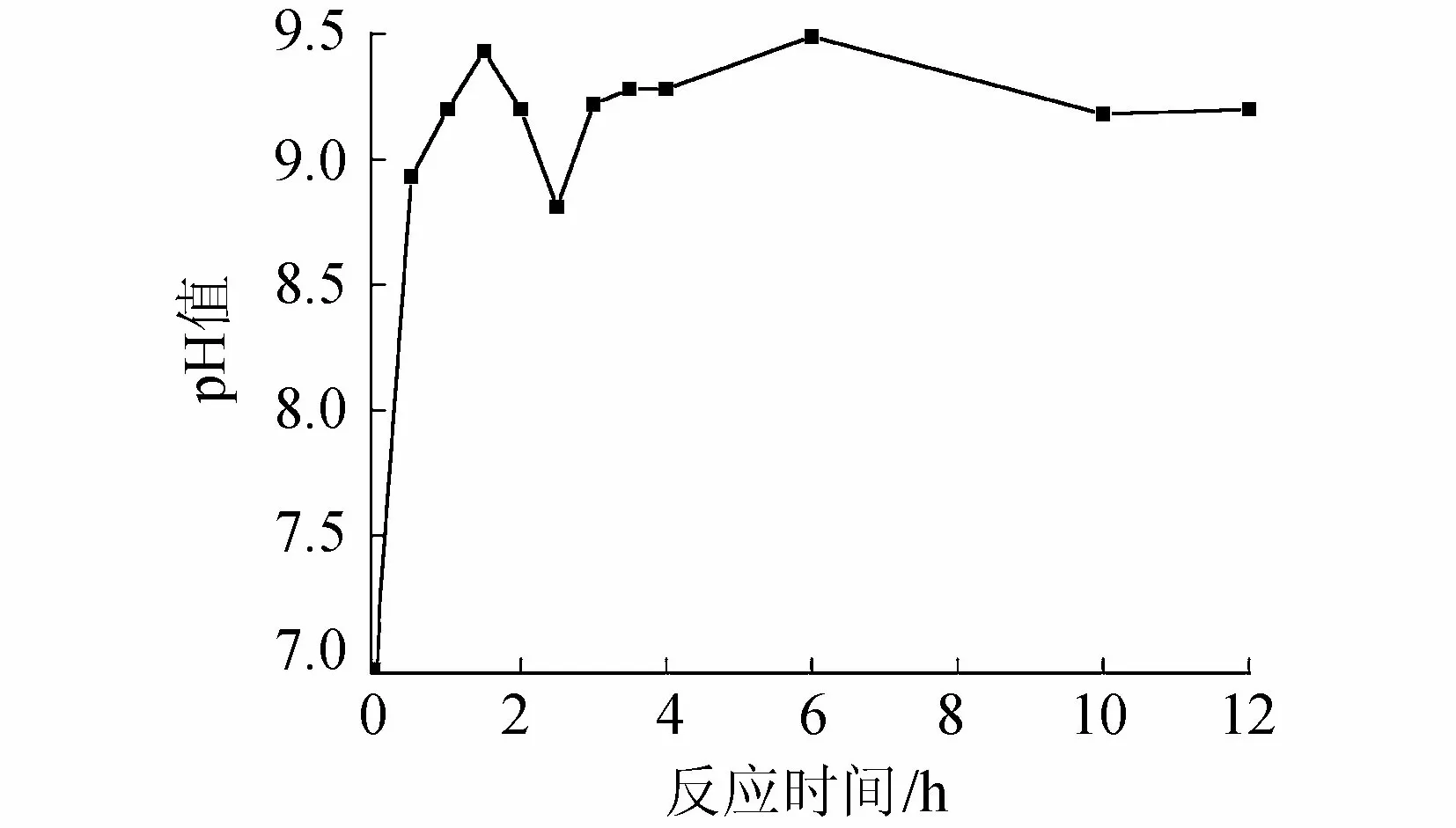
图6 反应过程中溶液pH值变化
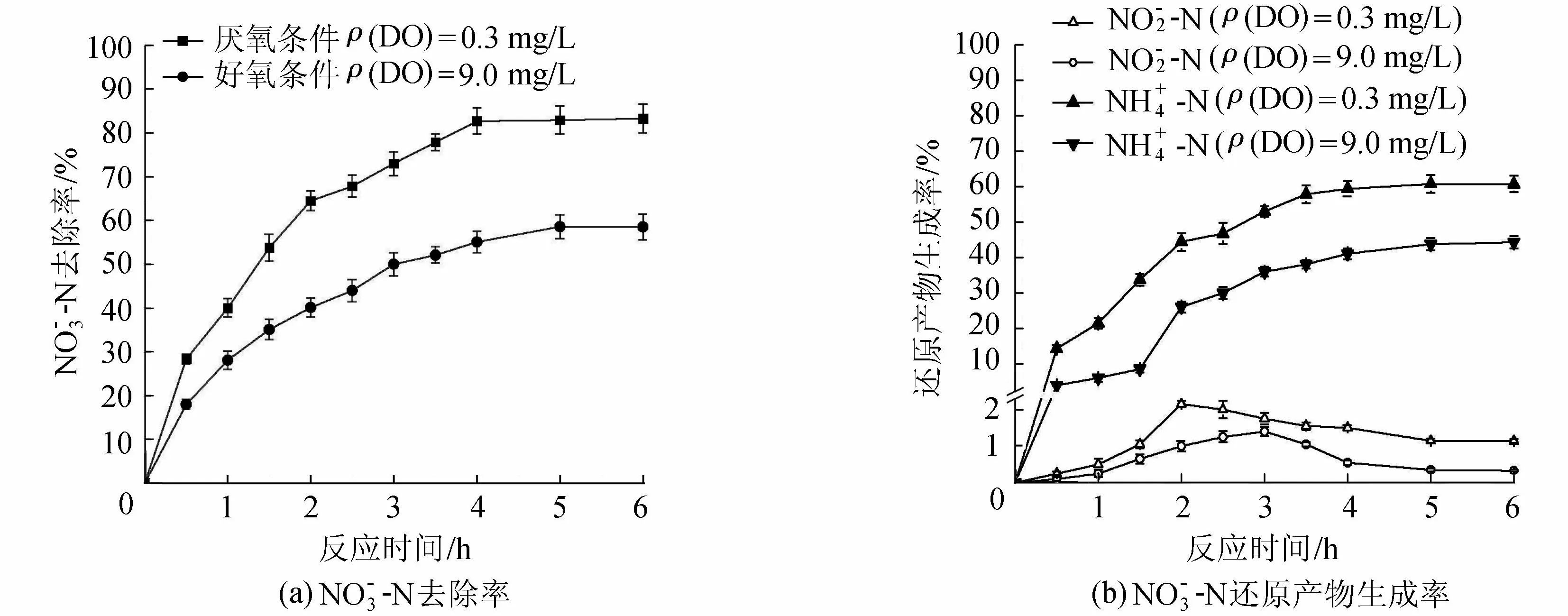
图7 DO对去除率及其还原产物生成率的影响
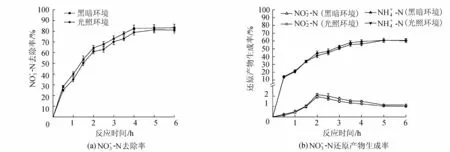
图8 光照对去除率及其还原产物生成率的影响
(2)
(3)
(4)
2 Fe0+2H2O+O2→2Fe(OH)2
(5)
4Fe(OH)2+O2+2H2O→4Fe(OH)3
(6)

图9 温度对去除率及其还原产物生成率的影响
Fe0+2H2O→Fe2++H2+2OH-
(7)
(8)
(9)
3 结 论
a. 确立凹凸棒土改性条件、凹凸棒土与六水合氯化铁最佳质量比等关键试验参数,优化了复合材料制备方案;复合材料较单独纳米铁稳定性好,不易凝聚成团,不容易被氧化。
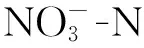
[ 1 ] RIVETT M O, BUSS S R, MORGAN P, et al. Nitrate attenuation in groundwater: a review of biogeochemical controlling processes [J]. Water Research, 2008, 42 (16): 4215-4232.
[ 2 ] KAPOOR A, VIRARAGHAVAN T. Nitrate removal from drinking water-review [J]. Journal of Environmental Engineering, 1997, 123(4): 371-380.
[ 3 ] CHO D W, SONG H, SCHWARTZ F W, et al. The role of magnetite nanoparticles in the reduction of nitrate in groundwater by zero-valent iron [J]. Chemosphere, 2015, 125(5): 41-49.
[ 4 ] LIN Xiurong, CAO Lixiang, XIONG Jian, et al. Interactions of denitrifying bacteria, actinomycetes, and fungi on nitrate removal in mix-culturing systems [J]. Water Air and Soil Pollution, 2012, 223(6): 2995-3007.
[ 5 ] SCHMIDT C A, CLARK M W. Efficacy of a denitrification wall to treat continuously high nitrate loads [J]. Ecological Engineering, 2012, 42(3): 203-211.
[ 6 ] WESTERHOFF P, JAMES J. Nitrate removal in zero-valent iron packed columns [J]. Water Research, 2003, 37(8): 1818-1830.
[ 7 ] BUROW K R, NOLAN B T, RUPERT M G, et al. Nitrate in groundwater of the United States, 1991-2003 [J]. Environmental Science & Technology, 2010, 44(13): 4988-4997.
[ 8 ] ZHANG Huan, JIN Zhaohui, HAN Lu, et al. Synthesis of nanoscale zero valent iron supported on exfoliated graphite for removal of nitrate [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(s1): 345-349.
[ 9 ] 李铁龙, 孙丽莉, 金朝晖, 等. 纳米铁系双金属复合材料还原水中硝酸盐氮[J]. 吉林大学学报(工学版), 2009, 39(2): 362-367.(LI Tielong, SUN Lili, JIN Zhaohui, et al. Nitrate reduction in water by iron-system bimetallic nanoparticles [J]. Journal of Jilin University (Engineering and Technology Edition), 2009, 39(2): 362-367.(in Chinese))
[10] GMEZA M, HONTORIA E, GONZALEZ-LOPEZ J. Effect of dissolved oxygen concentration on nitrate removal from groundwater using a denitrifying submerged filter [J]. Journal of Hazardous Materials, 2002, 90(3): 267-278.
[11] 王玉焕, 廉新颖, 李秀金, 等. 地下水环境因素对包覆型纳米铁降解NO-3-N的影响[J]. 环境科学研究, 2014, 27(11): 1367-1372.(WANG Yuhuan, LIAN Xinying, LI Xiujin, et al. Effects of groundwater environmental factors on NO-3-N reduction using coated nano iron [J]. Research of Environmental Sciences, 2014, 27(11): 1367-1372.(in Chinese))
[12] FATEMINIA F S, FALAMAKI C. Zero valent nano-sized iron clinoptilolite modified with zero valent copper for reductive nitrate removal[J]. Process Safety and Environmental Protection, 2013, 91(4): 304-310.
[13] 仰榴青. 国产凹凸棒土的研究[J]. 江苏理工大学学报, 1995, 16(1): 55-60.(YANG Liuqing. Study on chinese attapulgites [J]. Journal of Jiangsu University of Science and Technology, 1995, 16(1): 55-60.(in Chinese))
[14] 张秀丽, 王明珊, 廖立兵. 凹凸棒石吸附地下水中氨氮的实验研究[J].非金属矿, 2010, 33(6): 64-67. (ZHANG Xiuli, WANG Mingshan, LIAO Libing. Experimental research on adsorption of ammonium nitrogen from groundwater by using attapulgite [J]. Non-Metallic Mines, 2010, 33(6): 64-67.(in Chinese))
[15] 陈天虎, 王健, 庆承松, 等. 热处理对凹凸棒石结构、形貌和表面性质的影响[J]. 硅酸盐学报, 2006, 34(11): 1406-1410. (CHEN Tianhu, WANG Jian, QING Chengsong, et al. Effect of heat treatment on structure, morphology and surface properties of palygorskite [J]. Journal of the Chinese Ceramic Society, 2006, 34(11): 1406-1410.(in Chinese))
[16] 王金明, 易发成. 改性凹凸棒石对模拟核素Sr2+的吸附性能的研究[J]. 水处理技术, 2006, 32(10): 207-214.(WANG Jinming, YI Facheng. Studies on adsorption capacity of modified attapulgite to simulated nuclide strontium [J]. Technology of Water Treatment, 2006, 32(10): 207-214.(in Chinese))
[17] 齐治国, 史高峰, 白利民. 微波改性凹凸棒石黏土对废水中苯酚的吸附研究[J]. 非金属矿, 2007, 30(4): 56-59. (QI Zhiguo, SHI Gaofeng, BAI Limin. Study on absorption of phenol in wastewater by microwave modified attapulgite clay [J]. Non-Metallic Mines, 2007, 30(4): 56-59.(in Chinese))
[18] SANCHEZ M J, RODRIGUEZ-CRUZ M S, ANDRADES M S, et al. Efficiency of different clay minerals modified with a cationic surfactant in the adsorption of pesticides: influence of clay type and pesticide hydrophobicity [J]. Applied Clay Science, 2006, 31(3-4): 216-228.
[19] VICO L I, ACEBAL S G. Some aspects about the adsorption of quinoline on fibrous silicates and patagonian saponite [J]. Applied Clay Science, 2006, 33(2): 142-148.
[20] 金叶玲, 陈静, 钱运华, 等. 超声水热法制备高纯超细凹凸棒石黏土[J]. 非金属矿, 2005, 28(3): 42-44. (JIN Yeling, CHEN Jing, QIAN Yunhua, et al. Preparing ultrafine & pure attapulgite clay by ultrasonic water-heat method[J]. Non-Metallic Mines, 2005, 28(3): 42-44.(in Chinese))
[21] 刘 蓉, 胡 盛, 杨 眉. 六偏磷酸钠提纯凹凸棒石的试验研究[J]. 非金属矿, 2010, 33 (3): 39-41. (LIU Rong, HU Sheng, YANG Mei. Study on purification of attapulgite by sodium hexametahposphate [J]. Non-Metallic Mines, 2010, 33 (3): 39-41.(in Chinese))
[22] BARRIOS M S, GONZALEZ L V, RODRIGUEZ M A, et al. Acid activation of a palygorskite with HCl: development of physico-chemical, textural and surface properties [J]. Applied Clay Science, 1995, 10(3): 247-258.
[23] FRINI-SRASRA N, SRASRA E. Acid treatment of south Tunisian palygorskite: removal of Cd(II) from aqueous and phosphoric acid solutions [J]. Desalination, 2010, 250(1): 26-34.
[24] WANG Wenji, CHEN Hao, WANG Aiqin. Adsorption charaeteristic of Cd(II) from aqueous solution onto activated palygorskite [J]. Separation and Purification Technology, 2007, 55(2): 157-164.
[25] 郑建东, 常彬彬, 吴霖生. 高温改性凹凸棒石对Cr(VI)的吸附研究[J]. 化工新型材料, 2012, 40(12): 76-78.(ZHENG Jiandong, CHANG Binbin, WU Linsheng. Study on adsorption of Cr6+in solution with attapulgite clay modified at high temperature [J]. New Chemical Materials, 2012, 40(12): 76-78.(in Chinese))
[26] SHI Lina, ZHANG Xin, CHEN Zuliang. Removal of chromium (VI) from wastewater using bentonite-supported nanoscale zero-valent iron [J]. Water Research, 2011, 45(2): 886-892.
[27] FU Rongbing, YANG Yingpin, XU Zhen, et al. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI) [J]. Chemosphere, 2015, 138: 726-734.
[28] HUANG C P, WANG H W, CHIU P C. Nitrate reduction by metallic iron [J]. Water Research, 1998, 32(8): 2257-2264.
[29] YANG G C, LEE H L. Chemical reduction of nitrate by nanosized iron: kinetics and pathways [J]. Water Research, 2005, 39(5): 884-894.
[30] RYU A, JEONG S W, JANG A, et al. Reduction of highly concentrated nitrate using nanoscale zero-valent iron: effects of aggregation and catalyst on reactivity [J]. Applied Catalysis B: Environmental, 2011, 105(1-2):128-135.
[31] 李培峰, 李文帅, 刘春颖, 等. 水体中亚硝酸盐的光降解[J]. 环境化学, 2011, 30(11): 1883-1888.(LI Peifeng, LI Wenshuai, LIU Chunying, et al. The photodecomposition of nitrite in water [J]. Environmental Chemistry, 2011, 30(11): 1883-1888.(in Chinese)
[32] 李铁龙, 刘海水, 金朝晖, 等. 纳米铁去除水中硝酸盐氮的批实验[J]. 吉林大学学报(工学版), 2006, 36(2):264-268. (LI Tielong, LIU Haishui, JIN Zhaohui, et al. Batch experiment on reduction of nitrate in water by nanoscale zero valent iron particles [J]. Journal of Jilin University (Engineering and Technology Edition), 2006, 36(2):264-268.(in Chinese)
[33] 廖娣劼, 杨琦, 尚海涛. 纳米铁去除水中硝酸盐的动力学研究[J]. 环境工程学报, 2009, 3(6): 985-989.(LIAO Dijie, YANG Qi, SHANG Haitao, et al. Kinetics of removing nitrate by nanoscale zero-valent iron [J]. Chinese Journal of Environmental Engineering, 2009, 3(6): 985-989.(in Chinese)
[34] JIANG Zhenmao, LV Lu, ZHANG Weiming, et al. Nitrate reduction using nanosized zero-valent iron supported by polystyrene resins: role of surface functional groups [J]. Water Research, 2011, 45(6): 2191-2198.
[35] LIU H B, CHEN T H, CHANG D Y, et al. Nitrate reduction over nanoscale zero-valent iron prepared by hydrogen reduction of goethite [J]. Materials Chemistry and Physics, 2012, 133(1): 205-211.
Study of removal of nitrate nitrogen from groundwater using modified attapulgite-Fe nano composite material
DONG Lei1,2, GONG Lei3, LIN Li1,2, FENG Xue1,2, LI Qingyun1,2
(1.BasinWaterEnvironmentalResearchDepartment,ChangjiangRiverScientificResearchInstitute,ChangjiangWaterResourcesCommission,Wuhan430010,China; 2.KeyLabofBasinWaterResourceandEco-EnvironmentalScienceinHubeiProvince,ChangjiangRiverScientificResearchInstitute,ChangjiangWaterResourcesCommission,Wuhan430010,China; 3.PartyandMassLineOffice,ChangjiangRiverScientificResearchInstitute,ChangjiangWaterResourcesCommission,Wuhan430010,China)
In this study, we synthesized modified attapulgite-Fe nano composite material (A-NZVI) using the liquid-phase reduction method, and investigated the stability of A-NZVI and changes of three kinds of nitrogen (NO3--N, NH4+-N, and NO2--N) in the reaction process. We also analyzed the effects of groundwater environmental factors (dissolved oxygen, temperature, and light) on the removal of NO3--N with A-NZVI. In the simulation of groundwater environment, the three kinds of materials were ranked in the following descending order according to their reactivity in removing NO3--N: A-NZVI, nanoscale zero-valent iron, and modified attapulgite. The conversion rate of NH4+-N was low, with nearly no NO2--N generated, when using A-NZVI. Dissolved oxygen and temperature had significant influence on the removal of NO3--N from groundwater with A-NZVI. When in the light and dark environment, the removal rate of NO3--N and the amounts of generated NH4+-N and NO2--N had no significant difference. The findings of the study provided a theoretical basis and technical support for restoration projects for groundwater with NO3--N pollution.
groundwater; nitrate nitrogen; modified attapulgite-Fe nano composite material; environmental factor
10.3880/j.issn.1004-6933.2017.01.014
国家自然科学基金(41302204);中央级公益性科研院所基本科研业务费项目(CKSF2014029/SH)
董磊(1987—),男,工程师,硕士,主要从事水环境治理。E-mail: dongleigushi@163.com
X523
A
1004-6933(2017)01-0067-08
2016-04-23 编辑:王 芳)

