饲粮中添加甜菜碱对热应激蛋鸡生产性能、蛋品质及血清生化指标的影响
郝生燕 刘陇生 王国栋 顾 娴 潘发明
(甘肃省农业科学院畜草与绿色农业研究所,兰州730070)
饲粮中添加甜菜碱对热应激蛋鸡生产性能、蛋品质及血清生化指标的影响
郝生燕 刘陇生 王国栋 顾 娴 潘发明
(甘肃省农业科学院畜草与绿色农业研究所,兰州730070)
本试验旨在研究饲粮中添加甜菜碱对热应激蛋鸡生产性能、蛋品质及血清生化指标的影响。选用健康的22周龄商品代罗曼褐蛋鸡600只,随机分成5组,每组8个重复,每个重复15只鸡。Ⅰ组为正对照组,饲喂基础饲粮,正常温热环境,温湿指数(THI)介于64.9~68.9;Ⅱ组为负对照组,饲喂基础饲粮,热应激环境,THI>72;Ⅲ~Ⅴ组分别在基础饲粮中添加200、400和600 mg/kg甜菜碱,均为热应激环境,THI>72。试验期为14周。结果表明,各组间平均日采食量、料蛋比和破蛋率差异不显著(P>0.05)。与Ⅰ组相比,Ⅱ组显著降低了入舍母鸡产蛋率、入舍母鸡产蛋重及血清总蛋白(TP)含量、碱性磷酸酶(AKP)活性(P<0.05),显著提高了血清中谷草转氨酶(GOT)、肌酸激酶(CK)和谷丙转氨酶(GPT)活性(P<0.05)。与Ⅱ组相比,Ⅳ组入舍母鸡产蛋率、入舍母鸡产蛋量和血清TP含量均显著提高(P<0.05),Ⅴ组入舍母鸡产蛋量及血清TP、白蛋白(ALB)含量也显著提高(P<0.05),而Ⅳ组和Ⅴ组的血清CK、GPT活性却显著降低(P<0.05),且Ⅴ组的血清甘油三酯(TG)含量也显著降低(P<0.05)。综上所述,热应激可使产蛋鸡的新陈代谢和生理机能发生变化,导致生产性能下降,而饲粮中添加甜菜碱可以提高入舍母鸡产蛋率和入舍母鸡产蛋重,并改善热应激对蛋鸡的损伤,饲粮中甜菜碱的适宜添加量为400 mg/kg。
甜菜碱;热应激;蛋鸡;生产性能;蛋品质;血清生化指标
环境温度和湿度是影响家禽生产性能的重要因素。温湿指数(temperature-humidity index,THI)在一定程度上反映了动物生产环境的舒适度,当温湿指数介于55~72时,动物所受的温湿度应激最小,表现为舒适;当温湿指数>72时,动物表现为热应激反应,温湿指数越大,热应激反应越大[1]。蛋鸡生产周期长,夏季高温易引起热应激,致使采食量、产蛋量、饲料转化率和孵化率等指标下降,氧化损伤加剧,并伴有肠道微生物区系失衡,严重时蛋鸡停产,给蛋鸡养殖造成重大经济损失[2-4]。此外,热应激可造成家禽小肠绒毛组织变性,肠细胞膜通透性增加,易感病原微生物,患病风险增加[5]。
甜菜碱即三甲基甘氨酸,进入机体可作为机体代谢过程中甲基的供体,而甲基是神经、免疫、肾脏和心血管系统必需的基团[6]。研究表明,甜菜碱参与体内蛋白质和脂肪的代谢,有改善饲粮适口性、降低机体脂肪蓄积、维持细胞渗透压以及调节细胞电解质平衡等生物学功能[7-9]。Klasing等[10]报道肉鸡感染球虫后,十二指肠肠绒毛高度、细胞渗透压、体增重均显著下降,而饲粮添加甜菜碱后上述症状均有所改善。类似的研究已在肉鸡上多有报道,研究发现,饲粮中添加甜菜碱有助于缓解肉鸡热应激损伤,降低热应激引起机体脱水产生的负面影响[11-12]。但目前关于甜菜碱对热应激蛋鸡生产性能及血清生化指标的影响鲜有报道。因此,本文通过研究甜菜碱对热应激蛋鸡生产性能、蛋品质及血清生化指标的影响,为甜菜碱在蛋鸡饲料中的应用提供参考数据。
1 材料与方法
1.1 试验动物及材料
选择体况良好、产蛋量相近的22周龄商品代罗曼褐蛋鸡600只,随机分成5个组,每组8个重复,每个重复15只鸡。Ⅰ组为正对照组,饲喂基础饲粮,正常温热环境,温湿指数介于64.9~68.9;Ⅱ组为负对照组,饲喂基础饲粮,热应激环境,温湿指数>72;试验组(Ⅲ~Ⅴ组)分别在基础饲粮中添加200、400和600 mg/kg甜菜碱,均为热应激环境,温湿指数>72。试验期为14周,其中预试期2周,正试期12周。
基础饲粮为玉米-大豆粕-杂粕型饲粮,参考中华人民共和国农业行业标准《鸡饲养标准》(NY/T 33—2004)中蛋鸡营养成分推荐值配制。计算配方时,饲料原料中干物质、粗蛋白质、钙和磷的含量使用实测值,其他指标参考《中国饲料成分及营养价值表》(第26版)。试验饲粮为粉料型,基础饲粮组成及营养水平见表1。试验用甜菜碱购于商业公司,纯度为98%。
1.2 饲养管理
试验地点为甘肃省兰州市榆中县宏艳养殖场。试验占用2个鸡舍,预试期,600只试验鸡饲养于舍1;正试期,负对照组和试验组480只鸡转入舍2饲养,2层笼养,每一层相连的5个小笼作为1个重复(15只鸡),各组安排时考虑位置效应。试验期间所有鸡只自由采食、自由饮水,自然光照加人工补光(16 h/d);人工喂料,每天3次(06:30、14:00和17:30);每天清粪1次,每周带鸡消毒1次,进行常规防疫和免疫。
试验时间为2015年6月2日到2015年9月7日,每天08:00、14:00、22:00采用干湿球温度计记录舍1、舍2的干球温度和湿球湿度,并按如下公式计算温湿指数,结果见表2。当温湿指数>72时,表明试验鸡处于慢性热应激状态。
温湿指数=0.72×(Td+Tw)+40.6。
式中:Td为干球温度;Tw为湿球湿度。

表1 基础饲粮组成及营养水平(风干基础)
预混料为每千克饲粮提供The premix provided the following per kg of the diet:VA 8 000 IU,VD31 600 IU,VE 5 IU,VK 0.5 IU,VB10.8 mg,VB22.5 mg,D-泛酸D-pantothenic acid 2.2 mg,烟酸 nicotinic acid 20 mg,VB63.0 mg,生物素 biotin 0.10 mg,叶酸 folic acid 0.25 mg,VB120.004 mg,胆碱 choline 500 mg,Mn (as manganese sulfate) 60 mg,I (as potassium iodide) 0.35 mg,Fe (as ferrous sulfate) 60 mg,Cu (as copper sulfate) 8 mg,Zn (as zinc sulfate) 80 mg,Se (as sodium selenite) 0.30 mg。
1.3 生产性能测定
试验期间以重复为单位每日记录采食量、产蛋数与产蛋量、废蛋个数(破、畸、碎、软、无壳)、淘汰与死亡鸡只数、死亡时间、体(尸)重,并计算期内入舍母鸡产蛋率、入舍母鸡产蛋量、平均日采食量、料蛋比和破蛋率。
1.4 蛋品质测定
分别于28、33周龄第7天,随机从各重复抽取6枚蛋进行蛋品质测定(24 h内测完)。采用蛋形指数测定仪(日本岛津)测量蛋的纵径、横径,并计算蛋形指数(纵径/横径);卵壳强度计(日本岛津)测定蛋壳强度;蛋壳厚度仪(日本岛津)测定蛋壳厚度;蛋白高度测定仪(日本岛津)测定蛋白高度,并按公式计算哈氏单位:
HU=100×log(H-1.7W0.37+7.57)。
式中:HU、H和W分别为哈氏单位、蛋白高度(mm)和蛋重(g)。
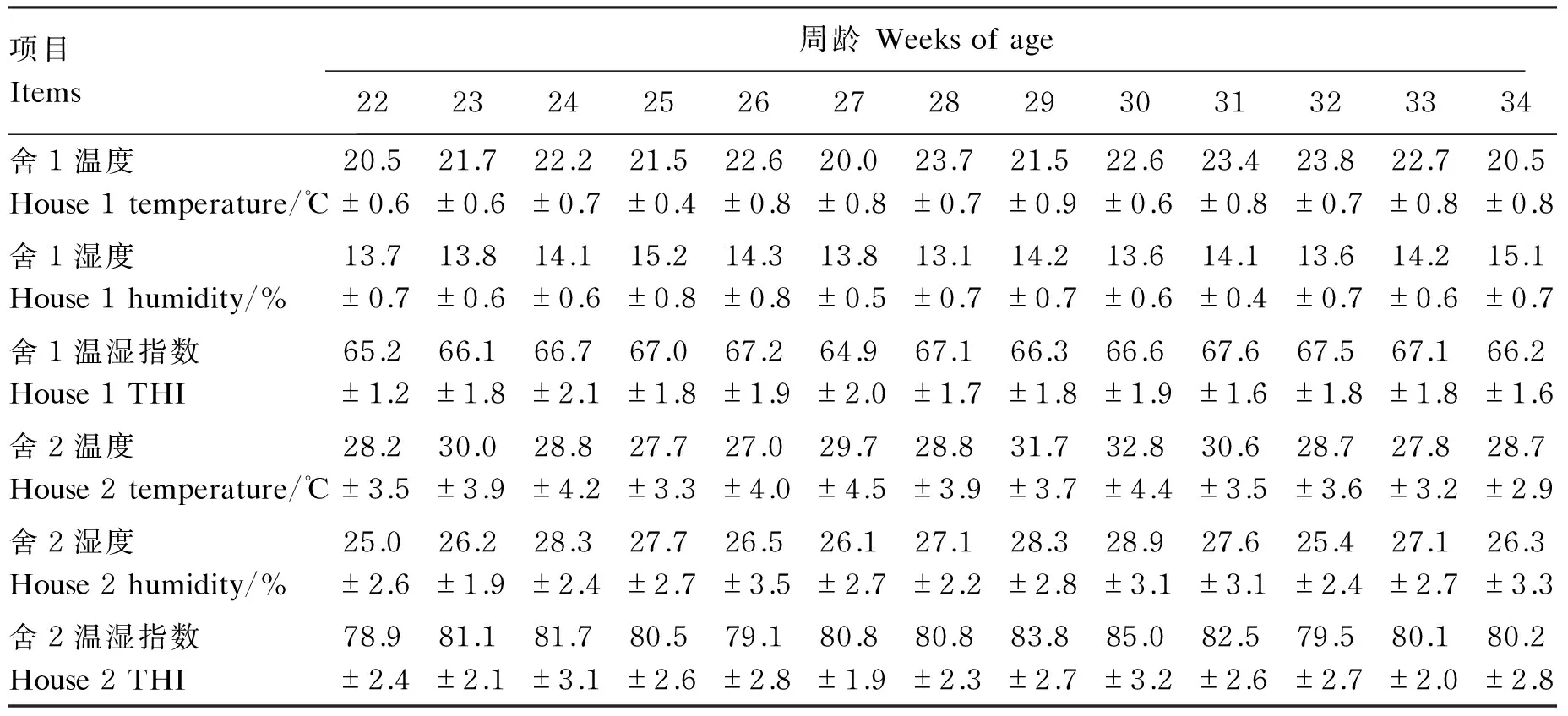
表2 试验期舍1、舍2的温度、湿度和温湿指数
1.5 血清生化指标测定
饲养试验结束时,从每个重复中抽取1只蛋鸡,空腹颈静脉采血,4 000/min离心15 min后分离血清,-20 ℃冰箱保存,用于检测血清常规生化指标,包括总蛋白(TP)、白蛋白(ALB)、球蛋白(GLO)、肌酸激酶(creatine kinase,CK)、碱性磷酸酶(alkaline phosphatase,AKP)、谷草转氨酶(oxaloacetic transaminase,GOT)、谷丙转氨酶(alanine aminotransferase,GPT)、甘油三酯(triglycerides,TG)、总胆固醇(total cholesterol,TC)、磷(phosphate,P)、钙(calcium,Ca)。检测用试剂盒购自南京建成生物工程研究所。
1.6 数据统计与分析
所有数据均经Excel 2013软件进行整理,再用SPSS 19.0的单因子方差分析(one-way ANOVA)过程进行分析,差异显著时用Tukey法作多重比较。结果表示为平均值±标准差,差异显著水平为P<0.05,0.05 2.1 饲粮中添加甜菜碱对热应激蛋鸡生产性能的影响 本试验中,仅有2只试验鸡死淘,且非试验处理效应所致,因此,表3中未列该数据。由表3可知,Ⅰ组入舍母鸡产蛋率和入舍母鸡产蛋量均显著高于Ⅱ组(P<0.05)。与Ⅱ组相比较,Ⅳ组入舍母鸡产蛋率和入舍母鸡产蛋量均显著提高(P<0.05),且Ⅴ组入舍母鸡产蛋量也显著提高(P<0.05)。各组间平均日采食量、料蛋比和破蛋率均差异不显著(P>0.05),但Ⅱ组料蛋比表现出提高的趋势(P=0.084)。 从图1可以看出,随着试验鸡周龄的增长,入舍母鸡产蛋率呈下降趋势,Ⅱ组下降趋势明显,且32~35周龄Ⅰ组和Ⅱ组间入舍母鸡产蛋率差异较大,而添加甜菜碱组(Ⅲ~Ⅴ组)下降趋势较缓,显示了甜菜碱对缓解试验鸡热应激的积极作用。从图2可以看出,试验期各组间试验鸡平均日采食量相当,Ⅱ组试验鸡平均日采食量也未受影响。从图3可以看出,随着试验鸡周龄的增长,料蛋比呈上升趋势,Ⅱ组上升趋势较其他组明显,同时添加甜菜碱组试验鸡料蛋比均有所改善,以Ⅴ组改善幅度最大。 表3 饲粮中添加甜菜碱对热应激蛋鸡生产性能的影响 同列数据肩标不同小写字母表示差异显著(P<0.05),相同或无字母表示差异不显著(P>0.05)。表4、表5同。 In the same column, values with different small letter superscripts mean significant difference (P<0.05), while with the same or no letter superscripts mean no significant difference (P>0.05). The same as Table 4 and Table 5. 图1 饲粮中添加甜菜碱对热应激蛋鸡入舍母鸡产蛋率的影响 图2 饲粮中添加甜菜碱对热应激蛋鸡平均日采食量的影响 2.2 饲粮中添加甜菜碱对热应激蛋鸡蛋品质的影响 饲粮中添加甜菜碱对热应激蛋鸡28、33周龄蛋品质的影响结果分别见表4、表5。由表4、表5可知,各组28和33周龄平均蛋重、蛋形指数、哈氏单位、蛋壳强度、蛋壳厚度、单位面积蛋壳重和蛋壳率均无显著差异(P>0.05),且各指标均在正常范围之内。 图3 饲粮中添加甜菜碱对热应激蛋鸡料蛋比的影响 组别Groups平均蛋重Averageeggweight/g蛋形指数Eggshapeindex哈氏单位Haughunits蛋壳强度Eggshellstrength/(kg/cm2)蛋壳厚度Eggshellthickness/mm单位面积蛋壳重Eggshellperunitareaweight/(g/cm2)蛋壳率Eggshellpercentage/%Ⅰ62.57±1.021.26±0.0198.11±2.844.12±0.180.39±0.010.078±0.0059.13±0.35Ⅱ62.09±1.151.24±0.0296.25±3.014.01±0.200.39±0.010.081±0.0079.18±0.27Ⅲ62.31±1.231.29±0.0198.03±3.094.07±0.320.39±0.020.080±0.0059.47±0.55Ⅳ61.85±1.051.26±0.0197.97±2.954.11±0.310.40±0.010.082±0.0069.27±0.36Ⅴ63.11±1.161.28±0.0198.28±3.174.09±0.170.39±0.010.080±0.0069.49±0.39P值P-value0.5730.8920.5540.7630.9210.7750.732 表5 饲粮中添加甜菜碱对热应激蛋鸡33周龄蛋品质的影响 2.3 饲粮中添加甜菜碱对热应激蛋鸡血清生化指标的影响 由表6可知,试验鸡受热应激影响后,Ⅱ组血清TP含量和AKP活性较Ⅰ组显著降低(P<0.05),而血清GOT、CK和GPT活性均显著升高(P<0.05)。与Ⅱ组相比,添加甜菜碱后,Ⅳ组、Ⅴ组血清TP含量和AKP活性(Ⅳ组除外)均显著提高(P<0.05),而血清CK、GPT活性均显著降低(P<0.05),且Ⅴ组血清ALB含量显著高于Ⅱ组(P<0.05),但Ⅴ组血清TG含量显著低于Ⅱ组(P<0.05)。 表6 饲粮中添加甜菜碱对热应激蛋鸡血清生化指标的影响 同行数据肩标不同小写字母表示差异显著(P<0.05),相同或无字母表示差异不显著(P>0.05)。 In the same row, values with different small letter superscripts mean significant difference (P<0.05), while with the same or no letter superscripts mean no significant difference (P>0.05). 3.1 饲粮中添加甜菜碱对热应激蛋鸡生产性能的影响 蛋鸡羽毛丰厚,皮肤无汗腺,新陈代谢旺盛,自身体温较高,依靠呼吸和蒸发散热,因此,高温是影响蛋鸡生产性能的主要环境因素[13]。早期关于热应激对家禽的不利影响主要以肉鸡为模式动物,在生产性能、生理指标和基因表达等方面均有文献报道[14-16]。Quinteiro-Filho等[17]报道当环境温度高于31 ℃(经测算温湿指数为89.3),试验鸡的采食量和体增重均显著下降。家禽遭受热应激后,行为上表现基本一致,如采食和行走时间缩短,饮水和休息时间延长,因而采食量和体增重也随之下降[18]。本试验中,热应激组试验鸡的平均日采食量较对照组未显著下降,该结果与Quinteiro-Filho等[17]、Mack等[18]报道结果不尽一致,其原因不明,可能与试验地昼夜环境温湿度差较大相关,高温湿指数时因热增耗试验鸡减少的采食量在低温湿指数时通过补偿采食进行了调节,有待于进一步证实。试验鸡产蛋率的下降主要由饲料转化率的下降所致。试验鸡遭受热应激后,机体抗氧化机能下降,产生的自由基导致器官和肠道绒毛损伤,进而影响了养分的消化利用[19-20]。 甜菜碱具有调节机体渗透压的作用[21]。当饲粮中添加甜菜碱后,甜菜碱被细胞吸收,防止水分的流失和盐类的进入,调节细胞渗透压和离子平衡,生产性能得到改善[22]。本试验中饲粮添加400 mg/kg甜菜碱的入舍母鸡产蛋量和入舍母鸡产蛋率均显著高于负对照组,证实了其抗应激作用。甜菜碱可以缓解或消除由高渗作用引起的DNA复制、蛋白质合成及细胞增殖速率下降,并且抑制高渗介质诱导的热休克蛋白(HSP)-70基因的表达[22]。另外,急性热应激条件下,添加甜菜碱还可提高机体对能量的利用效率[23]。由此可见,添加甜菜碱是一种缓解蛋鸡热应激的有效措施。 3.2 饲粮中添加甜菜碱对热应激蛋鸡蛋品质的影响 热应激状态下,蛋鸡对体液酸碱平衡调节的补偿机制共同作用于机体而达到稳定,尽管这些反应对其生存有效,但仍不能避免热应激对机体的影响,如更多血清流向外周组织,内部器官包括输卵管血流减少,导致蛋品质下降[24]。本试验中,试验鸡受热应激影响后,鸡蛋平均蛋重、蛋形指数、哈氏单位、蛋壳强度、蛋壳厚度、单位面积蛋壳重和蛋壳率等指标均有不同程度降低,但未达到显著水平,类似的结果早有报道[25]。添加甜菜碱后,上述指标均有所改善,且剂量越高,改善程度越好,主要是由于甜菜碱提供了活性甲基,而甲基是动物新陈代谢,尤其是蛋白质和脂肪代谢所必需的基团,机体的许多代谢反应,如肾上腺素、肉碱、肌酸的合成,以及DNA和RNA的甲基化均需要甲基,最终改善了细胞代谢能力[26]。 3.3 饲粮中添加甜菜碱对热应激蛋鸡血清生化指标的影响 在慢性热应激状态下,基础代谢降低,采食量减少,影响了体内正常物质的代谢过程,体内营养物质浓度减少,而细胞新陈代谢所需能量只有通过分解营养物质来提供,因而各种参与分解代谢的酶活性也显著增加[27]。血清酶绝大部分来自于动物的各种组织器官,其活性高低直接与相应组织器官的代谢水平和功能状态相关,机体的调节和适应能力在很大程度上取决于各组织器官的机能水平[28]。在热应激条件下,由于细胞膜的通透性升高导致细胞内酶释放入血的速度加快,而使血清GOT、CK和GPT的活性升高,促进糖代谢途径以产生大量能量的有氧氧化向无氧酵解方向进行,通过机体交感-肾上腺髓质系统和糖皮脂激素的调控,进而使血糖浓度升高[29]。同时,热应激降低鸡采食量及抑制甲状腺激素分泌,蛋白质合成会减少,从而降低血清中TP、ALB含量[28],这与本试验结果相一致。 本试验中,饲粮添加400和600 mg/kg甜菜碱后蛋鸡血清TP、ALB含量和AKP活性均较负对照组显著提高,血清CK、GPT活性和TG含量均显著降低,且与正对照组水平相当。血清TP和ALB含量的显著提高说明甜菜碱具有改善体液免疫和细胞免疫的双重作用,其增高的原因缘于甜菜碱甲基供体使得蛋白质代谢趋于正常[6,30]。添加甜菜碱后,热应激试验鸡血清AKP和CK活性恢复主要因甲状腺激素活性的提高和离子平衡稳定共同作用所致[30],而血清GPT活性和TG含量的降低证实了甜菜碱具有保护动物肝脏的作用,一方面甜菜碱降低了肝脏中脂肪生成酶的活性,促进了肝脏中载脂蛋白的合成和脂肪的迁移,进而降低了肝脏中TG的含量[31],另一方面甜菜碱可有效抑制肝脏炎症的发生[32]。 夏季高温使产蛋鸡的新陈代谢和生理机能发生变化,导致生产性能下降。饲粮中添加甜菜碱可以有效调节鸡体体液平衡,提高产蛋量和产蛋重,并改善了热应激对蛋鸡的损伤。综合来看,饲粮中甜菜碱的适宜添加量为400 mg/kg。 [1] 韩天龙,赵瑞霞,高翠英,等.养殖模式对冬季商品肉鸭舍温湿指数的影响[J].家畜生态学报,2015,36(2):80-83. [2] 李永洙,李进,张宁波,等.热应激环境下蛋鸡肠道微生物菌群多样性[J].生态学报,2015,35(5):1601-1609. [3] BURKHOIDER K M,THOMPSON K L,EINSTEIN M E,et al.Influence of stressors on normal intestinal microbiota,intestinal morphology,and susceptibility toSalmonellaenteritidis colonization in broilers[J].Poultry Science,2008,87(9):1734-1741. [4] LARA L J,ROSTAGNO M H.Impact of heat stress on poultry production[J].Animals,2013,3(2):356-369. [5] SONG J,XIAO K,KE Y L,et al.Effect of a probiotic mixture on intestinal microflora,morphology,and barrier integrity of broilers subjected to heat stress[J].Poultry Science,2014,93(3):581-588. [6] KIDD M T,FERKET P R,GARLICH J D.Nutritional and osmoregulatory functions of betaine[J].World’s Poultry Science Journal,1997,53(2):125-139. [7] PEKKINEN J,OLLI K,HUOTARI A,et al.Betaine supplementation causes increase in carnitine metabolites in the muscle and liver of mice fed a high-fat diet as studied by nontargeted LC-MS metabolomics approach[J].Molecular Nutrition & Food Research,2013,57(11):1959-1968. [8] 贺绍君,赵书景,李静,等.甜菜碱对热应激肉鸡生长性能、十二指肠消化酶活性及盲肠微生物区系的影响[J].动物营养学报,2014,26(12):3731-3739. [9] HUANG Y L,YANG J,XIAO F,et al.Effects of supplemental chromium source and concentration on growth performance,carcass traits,and meat quality of broilers under heat stress conditions[J].Biological Trace Element Research,2016,170(1):216-223. [10] KLASING K C,ADLER K L,REMUS J C,et al.Dietary betaine increases intraepithelial lymphocytes in the duodenum of coccidia-infected chicks and increases functional properties of phagocytes[J].The Journal of Nutrition,2002,132(8):2274-2282. [11] 鲍恩东,龚远英,HARTUNG J,等.肉鸡热应激病理损伤与应激蛋白(HSP70)相关性研究[J].中国农业科学,2004,37(2):301-305. [12] 于纪棉.持续热应激肉鸡组织中热休克蛋白表达规律与应激性损伤机理研究[D].博士学位论文.南京:南京农业大学,2009. [13] SHEN P N,LEI P K,LIU Y C,et al.Development of a temperature measurement system for a broiler flock with thermal imaging[J].Engineering in Agriculture,Environment and Food,2016,9(3):291-295. [14] WILLEMSEN H,SWENNEN Q,EVERAERT N,et al.Effects of dietary supplementation of methionine and its hydroxy analogDL-2-hydroxy-4-methylthiobutanoic acid on growth performance,plasma hormone levels,and the redox status of broiler chickens exposed to high temperatures[J].Poultry Science,2011,90(10):2311-2320. [15] ZHANG Z Y,JIA G Q,ZUO J J,et al.Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat[J].Poultry Science,2012,91(11):2931-2937. [16] SOHAIL M U,HUME M E,BYRD J A,et al.Effect of supplementation of prebiotic mannan-oligosaccharides and probiotic mixture on growth performance of broilers subjected to chronic heat stress[J].Poultry Science,2012,91(9):2235-2240. [17] QUINTEIRO-FILHO W M,RIBEIRO A,FERRAZ-DE-PAULA V,et al.Heat stress impairs performance parameters,induces intestinal injury,and decreases macrophage activity in broiler chickens[J].Poultry Science,2010,89(9):1905-1914. [18] MACK L A,FELVER-GANT J N,DENNIS R L,et al.Genetic variations alter production and behavioral responses following heat stress in 2 strains of laying hens[J].Poultry Science,2013,92(2):285-294. [19] LIN H,DE VOS D,DECUYPERE E,et al.Dynamic changes in parameters of redox balance after mild heat stress in aged laying hens (Gallusgallusdomesticus)[J].Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology,2008,147(1):30-35. [20] AZAD M A K,KIKUSATO M,MAEKAWA T,et al.Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress[J].Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2010,155(3):401-406. [21] EKLUND M,BAUER E,WAMATU J,et al.Potential nutritional and physiological functions of betaine in livestock[J].Nutrition Research Reviews,2005,18(1):31-48. [22] RIMOLDI S,LASAGNA E,SARTI F M,et al.Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions[J].Meta Gene,2015,6:17-25. [23] LIPINSKI K,SZRAMKO E,JEROCH H,et al.Effects of betaine on energy utilization in growing pigs—a review[J].Annals of Animal Science,2012,12(3):291-300. [24] EBEID T A,SUZUKI T,SUGIYAMA T.High ambient temperature influences eggshell quality and calbindin-D28k localization of eggshell gland and all intestinal segments of laying hens[J].Poultry Science,2012,91(9):2282-2287. [25] LIN H,MERTENS K,KEMPS B,et al.New approach of testing the effect of heat stress on eggshell quality:mechanical and material properties of eggshell and membrane[J].British Poultry Science,2004,45(4):476-482. [27] VINOTH A,THIRUNALASUNDARI T,THARIAN J A,et al.Effect of thermal manipulation during embryogenesis on liver heat shock protein expression in chronic heat stressed colored broiler chickens[J].Journal of Thermal Biology,2015,53:162-171. [28] 董淑丽,邓雯,雷雪芹,等.热应激对动物理化特性及生产性能的影响[J].河南科技大学学报:农学版,2003,23(1):59-62,66. [29] EGBUNIWE I C,AYO J O,KAWU M U,et al.Effects of betaine and ascorbic acid on tonic immobility,superoxide dismutase and glutathione peroxidase in broiler chickens during the hot-dry season[J].Journal of Veterinary Behavior,2016,12:60-65. [30] HASSAN R A,EBEUD T A,ABDEl-LATEIF A I,et al.Effect of dietary betaine supplementation on growth,carcass and immunity of New Zealand White rabbits under high ambient temperature[J].Livestock Science,2011,135(2/3):103-109 [31] 陈力,王丽君,谭耀宗,等.甜菜碱对脂肪变性HepG2细胞Hcy、SAM/SAH及脂代谢相关基因mRNA表达的影响[J].华南预防医学,2013,39(3):1-6. [32] SU S Y,DODSON M V,LI X B,et al.The effects of dietary betaine supplementation on fatty liver performance,serum parameters,histological changes,methylation status and the mRNA expression level of Spot14α in Landes goose fatty liver[J].Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology,2009,154(3):308-314. Author, HAO Shengyan, research assistant, E-mail: haoshengyan_happy@126.com (责任编辑 武海龙) Effects of Dietary Betaine on Performance, Egg Quality and Serum Biochemical Parameters of Laying Hens under Heat Stress Condition HAO Shengyan LIU Longsheng WANG Guodong GU Xian PAN Faming (InstituteofAnimalHusbandry,PastureandGreenAgriculture,GansuAcademyofAgriculturalScience,Lanzhou730070,China) This experiment was conducted to study the effects of dietary betaine on performance, egg quality and serum biochemical parameters of laying hens under heat stress condition. Six hundred 22-week-old commercial Roman laying hens were randomly divided into 5 groups with 8 replicates per group and 15 hens per replicate. The temperature humidity index (THI) of group Ⅰ (positive control group) was between 64.9 to 68.9, which was fed a basal diet in normal thermal environment; the THI of group Ⅱ (negative control group) was greater than 72, which was fed a basal diet under heat stress condition; while groups Ⅲ to Ⅴ were fed the basal diets supplemented with 200, 400 and 600 mg/kg betaine under heat stress condition, respectively, the THI of them were greater than 72 as well. The experiment lasted for 14 weeks. The results showed that there were no significant differences in average daily feed intake, feed/egg and broken egg rate among all groups (P>0.05). Compared with the group Ⅰ, the hen-housed laying rate, hen-housed egg yield, serum total protein (TP) content and serum alkaline phosphatase (AKP) activity of group Ⅱ were significantly decreased (P<0.05), but the activities of glutamic-oxalacetic transaminase (GOT), creatine kinase (CK) and glutamic-pyruvic transaminase (GPT) of group Ⅱ were significantly increased (P<0.05). Compared with the group Ⅱ, the hen-housed egg production, hen-housed egg yield and serum TP content of group Ⅳ were significantly increased (P<0.05), the hen-housed egg yield and the contents of TP and albumin (ALB) in serum of group Ⅴ were significantly increased (P<0.05), the activities of CK and GPT in serum of groups Ⅳ and Ⅴ were significantly decreased (P<0.05), the serum triglyceride (TG) content of group Ⅴ were significantly decreased (P<0.05). In conclusion, the metabolism and physiological function of layer are affected by heat stress, and which resulting in a decline in performance, but dietary betaine can improve the hen-housed egg production and hen-housed laying rate, and to improve the health of layer under heat stress. The dietary appropriate betaine level is 400 mg/kg.[ChineseJournalofAnimalNutrition, 2017, 29(1):184-192] betaine; heat stress; laying hens; performance; egg quality; serum biochemical parameters 10.3969/j.issn.1006-267x.2017.01.021 2016-07-12 甘肃省农业科学院中青年基金项目(2014GAAS33);甘肃省农业科学院农业科技创新专项(2013GAAS04) 郝生燕(1985—),女,山西大同人,研究实习员,硕士,从事动物营养与饲料科学研究。E-mail: haoshengyan_happy@126.com S831 A 1006-267X(2017)01-0184-092 结 果







3 讨 论
4 结 论

