Selenium supplementation within the periconception period: Influence on maternal liver and renal histoarchitecture
Mark Anthony C Mamon, Cheska May M Menodiado, Glenn L Siasu, Gliceria B. Ramos
1Biology Department, De La Salle University, Taft Avenue, Manila, Philippines, 1004
2Department of Biology, College of Arts and Sciences, University of the Philippines, Manila
Selenium supplementation within the periconception period: Influence on maternal liver and renal histoarchitecture
Mark Anthony C Mamon1, Cheska May M Menodiado1, Glenn L Siasu2, Gliceria B. Ramos1*
1Biology Department, De La Salle University, Taft Avenue, Manila, Philippines, 1004
2Department of Biology, College of Arts and Sciences, University of the Philippines, Manila
ARTICLE INFO
Article history:
Received 2016
Received in revised form 2016
Accepted 2016
Available online 2016
Maternal liver histoarchitecture Renal histoarchitecture Maternal selenium supplementation Periconception stages
Objective: To assess the histoarchitecture of the maternal liver and kidney as influenced by selenium supplementation within the periconception period and to determine which of the periconception stages of selenium intake is most favorable of the liver and kidney. Methods: Thirty-six 7-week-old female mice were divided into four groups where all groups were given 6.0 g of food pellets per day. Un-supplemented group (U) received no selenium in the food pellet, Pre-gestation supplemented group (P) was given 3.0 µg selenium/d for 21 d, Pre-gestation to Gestation supplemented group (PG) was given 3.0 µg selenium/ d for 37 d, and Gestation supplemented group (G) was given 3.0 µg selenium/d for 16 d only. Results: The occurrences of vacuolated hepatocytes and congested central veins in the liver were observed. The P and the G groups incurred the lowest and highest percent occurrence, respectively, of murine mothers that exhibited presence of vacuolated hepatocytes. The mean percent occurrence of congested central veins did not significantly vary among groups. The occurrences of shrunken and swollen glomeruli were observed in the kidney. The mean percent occurrence of shrunken glomeruli showed that P and G groups were significantly lower than those of U and PG groups. The mean percent occurrences of swollen glomeruli did not vary significantly among groups. Conclusion: The histological analyses of the liver and the kidney obtained from different stages of periconception indicate that selenium supplementation would be best during pregestation stage.
1. Introduction
Pregnancy causes physiological changes in the mother’s body to accommodate both maternal and fetal needs. Maternal metabolic processes and renal function change throughout pregnancy [1-3]. The high metabolic demand increases the production of reactive oxygen species [4]. Reactive oxygen species (ROS) are formed as a normal product of cellular metabolism like the oxidation of lipids that could cause damage and would lead to an increase in oxidative stress [5-7]. Oxidative stress during pregnancy may cause complications like pre-eclampsia, preterm labor and gestational diabetes [8]. Maternal micronutrient demand also increases. Its deficiency is associated with maternal complications like anemia, hypertension, and even death [9-10].
The change in the maternal mortality rate (MMR) of the Philippines is relatively low despite the observed decline from 1993 to 1998 until 2006 with 209, 172, and 162 deaths per 100 000 live births, respectively. As of 2015, one of the Millennium Development Goals is to achieve a maternal mortality rate of 52 deaths per 100 000 live births [11]. However, this seems to be unattainable because of the insufficient access of Filipino women to health care services. Thus, this study may augment means of improving maternal health via micronutrient supplementation.
Selenium, an essential micronutrient trace element, is involved in different biological processes like the regulation of thyroid hormone metabolism, enzymatic antioxidant defenses, and immune system functionality [12]. It is present in selenoproteins like glutathione peroxidase (GPx), thioredoxin reductase (TrxR), and iodothyronine deiodinase (DIO) that serve as enzymes [13]. Selenium is present
in both organic [selenocysteine (SeCys) and selenomethionine (SeMet)] and inorganic forms [selenium element (Se), selenite (SeO(OH)2) and selenate (SeO2(OH)2)] [14]. The organic forms, especially SeMet which is present in selenium-yeast, were found to be more effective in antioxidant function, absorption, and retention in tissues than the inorganic forms. SeMet has other advantages over the other forms wherein it can be incorporated into proteins and it can be easily excreted from the body. Thus, SeMet is a good form of supplementation [15-17].
Selenium deficiency is correlated with some diseases and disorders like Keshan disease, Kashin-Beck disease, cancer, cardiovascular, immune disorders, and infertility [15]. On the other hand, oversupplementation, accidental ingestion of high doses, or high levels in food can cause selenium toxicity [18]. Several studies reported that selenium is important for normal reproductive function and for the prevention of compromised pregnancies as it reduces the risk of miscarriage, preeclampsia, gestational diabetes, pregnancy-induced hypertension, and premature rupture of membranes (PROM) [19-20]. Amidst these reported beneficial effects of selenium on the female reproductive health, there is a recent recommendation [21] to determine the best timing of selenium supplementation that is best supportive of embryonic development and implantation. Hence, this study was designed to investigate a possible influence of selenium on maternal health when administered within periconception period. The results of this study may provide evidence on the best possible stage of periconception period for selenium supplementation that would not compromise the maternal hepato-renal histoarchitecture and function.
2. Materials and methods
2.1. Test animals
Thirty-six ICR female mice, 8-week of age and eighteen ICR male mice, 15-week of age were obtained from the Marine Science Institute–Natural Products Laboratory, University of the Philippines (Diliman). All mice were maintained and kept inside the Animal House of De La Salle University (Manila) at room temperature, in a 12-h light-dark cycle. Each mouse in the un-supplemented group (U) and the three supplemented groups which are the Pregestation supplementation only (P), Pregestation to Gestation supplementation (PG), and Gestation supplementation only (G) were given with the basal diet of 6 g of food pellets per day and filtered drinking water ad libitum. A basal diet of 6.0 g per day for a mouse weighing 10–25 g was based on previous study [22].
The proper handling of the test animals was based on the Rules and Regulations on the Conduct of Scientific Procedures Using Animals by the Department of Agriculture, Philippines.
2.2. Selenium micronutrient
The selenium tablets were purchased from General Nutrition Center (GNC Philippines). A tablet contains 200 µg selenium yeast and 35 mg calcium. The other components include cellulose and dicalcium phosphate. The tablet does not have preservatives, sodium, wheat, gluten, soy, sugar, nor artificial flavors.
2.3. Supplementation regimen
The selenium supplement was prepared by dissolving a 200 µg tablet in 10.0 mL filtered water. A 0.15 mL of the solution would contain 3.0 µg of selenium. The 3.0 µg is the dosage that was proven to provide protection against oxidative stress and damage in rat [23]. The 0.15 mL solution was coated in the 2.0 g of food pellets for initial consumption. The remaining 4.0 g of the daily basal food requirement were given after complete consumption of the 2.0 g initial food supply. The U group was given with 6.0 g of food pellets without selenium supplement. Each mouse in the supplemented groups was given with 6.0 g of food pellets with selenium supplement of 3.0 µg selenium/d for 21 d (P group); for 37 d (PG group); for 16 d (G group).
2.4. Mating
On the 22nd day of the experimental period, one male mouse and two female mice were joined together in a cage at 1 800 h. The presence of vaginal copulatory plugs, which indicate a successful mating, was checked the following day at 0700-0800H. The plugpositive females were considered to be pregnant with embryos aged at 0.5 d post coitus (dpc).
2.5. Isolation of maternal liver and kidney and histological slide preparation
All female mice were sacrificed via cervical dislocation at ED16 (embryonic day 16). The liver and kidney were isolated and fixed in 10% buffered formalin. The fixed tissues were processed by the Histopathology Laboratory of Philippine Kidney Dialysis Foundation in Quezon City, Philippines using the standard histological procedures of Hematoxylin and Eosin staining.
2.6. Histological analyses of maternal liver and kidney
The tissue samples were examined and documented at 400x using an Olympus light microscope. The % occurrence of murine mothers that exhibited vacuolated hepatocytes, and the mean % occurrence of congested central veins were determined. The mean % occurrences of swollen and shrunken glomeruli were determined. For both the maternal liver and kidney, tissue samples from each murine mother were assessed. % occurrence of murine mothers with vacuolated hepatocytes = Mothers with vacuolated hepatocytes / Total number of murine mothers x 100%.
Mean % occurrence of congested central veins = Mean number of congested central veins / Total number of central veins x 100%.
Mean % occurrence of shrunken glomeruli = Mean number of shrunken glomeruli / Total number of glomeruli x 100%. Mean % occurrence of swollen glomeruli = Mean number of swollen glomeruli / Total number of glomeruli x 100%.
2.7. Statistical analysis
The data on mean % occurrence of congested central veins and shrunken and swollen glomeruli were subjected to Kruskal-Wallis test and Mann-Whitney test using the Statistical Package for the Social Sciences (SPSS) Version 22 to determine the significant differences among all groups. The level of significance in all cases was P < 0.05.
3. Results
3.1. Histological analysis of maternal liver
The liver tissue samples of the murine mothers of all groups exhibited both normal and abnormal histology. A normal histoarchitecture is characterized by the parenchematous arrangement of hepatocytes forming hepatic plates that are radiating from the central vein. The hepatocytes appear intact with their distinct polygonal shape, centrally located nuclei and granular cytoplasm (Figure 1). The abnormal histological features that were observed were the occurrences of vacuolated hepatocytes and dilated sinusoids. It was observed that most of the nuclei were moved to its periphery. Occurrences of congested central veins were also noticed (Figure 2). The mean percent occurrence of congested hepatic central veins of the U group, P group, PG group, and G group was 6%, 4%, 7%, and 9%, respectively, and no significant difference was found between them. In terms of % occurrence of murine mothers exhibiting vacuolated hepatocytes, the P group had the lowest among the supplemented groups (55%). The G group had the highest % occurrence (88%) while the PG group had a % occurrence similar with that of U group (both 77%). In terms of mean % occurrence of congested central veins, all groups were not significantly different from each other.
3.2. Histological analysis of maternal kidney
The kidney tissue samples of the murine mothers from all groups exhibited both normal and abnormal histological characteristics. Normal histoarchitecture is characterized by the presence of intact glomeruli and unobliterated Bowman’s space (Figure 3). The observed abnormal histological characteristics were the occurrences of shrunken and swollen glomeruli (Figures 4 & 5). In terms of mean percent occurrence of shrunken glomeruli, groups P and G were significantly lower than U group (both 1% vs 12%, P < 0.05). Among the three groups with supplementation, groups P and G were significantly lower than PG group (both 1% vs 9%, P < 0.05). In terms of mean percent occurrence of swollen glomeruli, all groups were not significantly different from each other. The mean percent occurrence of swollen glomeruli of the U, P, PG, and G groups was 21%, 16%, 25%, and 31%, respectively.

Figure 1. Histology of the liver of all groups: the un-supplemented group (U), pre-gestation group (P), pre-gestation to gestation group (PG), and gestation group (G) exhibiting normal histoarchitecture without vacuolations and intact array of hepatic plates (400x). CV (Central vein); HP (Hepatic plates).
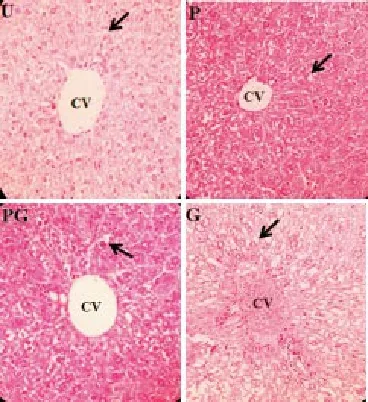
Figure 2. Histology of the liver of all groups: the un-supplemented group (U), pre-gestation group (P), pre-gestation to gestation group (PG), and gestation group (G) showing hepatic vacuolations and dilated sinusoids (400x). CV (Central vein); Arrows (Hepatic vacuolations).

Figure 3. Histology of the kidney of all groups: the un-supplemented group (U), pre-gestation group (P), pre-gestation to gestation group (PG), and gestation group (G) exhibiting intact glomeruli and unobliterated Bowman’s space (400x).G (Glomerulus); * (Bowman’s space).
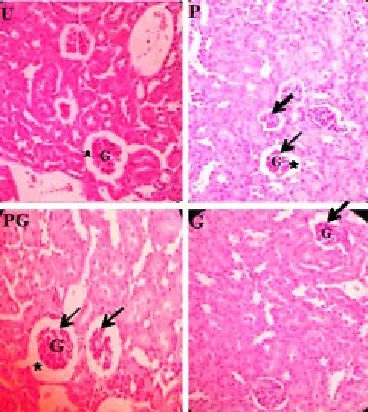
Figure 4. Histology of the kidney of all groups: the un-supplemented group (U), pre-gestation group (P), pre-gestation to gestation group (PG), and gestation group (G) exhibiting shrunken glomeruli as made evident by enlarged Bowman’s space relative to the intact glomeruli (400x).G (Glomerulus); * (Bowman’s space); Arrows (Shrunken glomeruli).
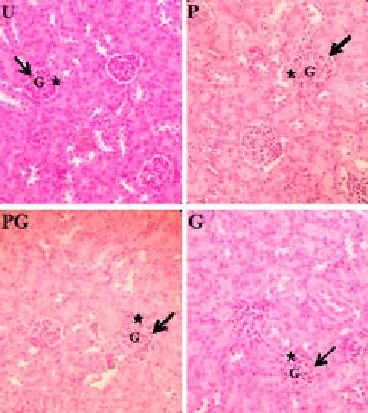
Figure 5. Histology of the kidney of all groups: the un-supplemented group (U), pre-gestation group (P), pre-gestation to gestation group (PG), and gestation group (G) exhibits swollen glomeruli as made evident by obliterated Bowman’s space relative to the intact glomeruli (400x).G (Glomerulus); * (Bowman’s space); Arrows (Swollen glomeruli).
4. Discussion
The histological response of P supplemented group may indicate the antioxidant protection of SeMet to the murine mothers. SeMet is converted to selenoproteins in which glutathione peroxidases (GPx) is one of its families [13,17]. Glutathione peroxidases are enzymes involved in cellular antioxidant activities by detoxifying and decomposing reactive oxygen species (ROS) [6,24,25]. Lipid hydroxyperoxides, a type of reactive oxygen species (ROS), are products of lipid oxidation which is an uncontrolled process in the body wherein oxygen radicals targets unsaturated lipids like triglycerides and fatty acids [6,26,27]. The liver normally increases its lipid production during pregnancy [28-30]. This would then cause a high lipid accumulation in the hepatocytes which is a characteristic of hepatic vacuolations. Hepatic vacuolation is an indication of impairment in triglyceride secretion which causes blockage and an indication of decrease in carrier lipoprotein synthesis [31,32]. Very low density lipoprotein (VLDL) serves as the carrier lipoprotein of triglyceride in the liver for its secretion and is synthesized by the assembly of triglycerides, microsomal triglyceride transfer protein (MTP) and apolipoprotein B (apo B) [33]. These VLDL components may be damaged by lipid hydroperoxides as it interacts with lipids and proteins. Glutathione peroxidases are considered as the major
biological defense against lipid hydroperoxides [27]. This mechanism may not only protect the hepatocytes from oxidative stress but also it may improve triglyceride secretion by the liver thus decreasing the occurrence of hepatic vacuolations.
The highest percent occurrence of hepatic vacuolations in G group may be due to a shorter duration of selenium supplementation which lasted for only 16 d (gestation period) as compared with that of the other treatment groups: P group lasted for 21 d (pre-gestation period) while PG group lasted for 37 d (pre-gestation to gestation period). Thus, the supplementation during the gestation period only may not be sufficient for the antioxidant action of the SeMet to function optimally considering that during the gestation stage, there is a high lipid production. Due to the high lipid production, SeMet becomes insufficient to balance the excessive level of reactive oxygen species in the form of lipid hydroperoxides.
The percent occurrence incurred in PG group which is similar with that of U group may indicate that long term selenium supplementation may have preparatory protection for the high lipid production during the gestation period. Therefore, among the treatment groups, PG group had an intermediate value of % occurrence of murine mothers that exhibited vacuolated hepatocytes which was higher than P group but lower than G group. Thus, this % occurrence is comparable with that of U group which can be interpreted as the possible percentage of murine pregnant mothers that exhibit hepatic vacuolations in the absence of selenium supplementation and/or other micronutrient supplement. Congested central veins are associated with a change in the presence or amount of fatty acids [34]. As previously stated, SeMet, as an antioxidant, may have improved triglyceride secretion by allowing normal assembly of very low density lipoproteins (VLDL) which serve as the carrier lipoprotein of triglyceride in the liver [33]. Thus, an efficient triglyceride secretion may have decreased the occurrence of congested central veins. The observed occurrence of congested central veins amongst all the treated groups that were not significantly different from the un-supplemented group may further support this assumption.
The significant difference of the mean percent occurrence of shrunken glomeruli in P and G groups as compared with that of U group may indicate an efficient excretion of trimethylselenonium ion (TMSe), the urinary metabolite of SeMet. Trimethylselenonium ion (TMSe) may cause toxicity but it is considered less toxic than the other forms of metabolites [35]. PG group, which exhibited the highest mean percent occurrence of shrunken glomeruli, had the longest period of selenium supplementation among all the three supplemented groups. Hence, the period of supplementation appears to have influenced the significant differences in the occurrence of shrunken glomeruli among the three treatment groups. Shrunken glomerulus is caused by a broken attachment of the capsular basement membrane to the tubular basement membrane which is considered as “atubular glomeruli” and non-functional [36,37]. Atubular glomeruli are associated with toxic nephropathy which suggests that the glomerulotubular junction becomes susceptible to toxic injury [38]. During pregnancy, the renal function is altered wherein the effective renal plasma flow (ERPF) is decreased, especially during the late stage [2]. The effective renal plasma flow is measured by the clearance of a substance which is defined as the volume of plasma from which that substance is completely removed by the kidney per unit time [39]. In this study, the supplementation during gestation, which was up to the 16th day that was considered as the late stage of pregnancy, could then decreases the excretion of trimethylselenonium ion (TMSe). However, the short duration of supplementation (16 d) may have caused a less toxic effect that led to lower occurrence of shrunken glomeruli as compared with pregestation-to-gestation (37 d) that incurred significantly higher occurrence of damaged glomeruli. Over-supplementation may cause selenium toxicity [18].
Swollen glomeruli are caused by colloid accumulation which leads to glomerular damage or injury [40]. A colloid is a substance with a high molecular weight that remains intravascularly [41]. Organic molecules forms colloids caused by their large size [42]. Trimethylselenonium ion, the urinary metabolite of SeMet, is considered an organic selenium compound hence the supplementation may have caused colloidal accumulation in the glomeruli [43,44]. The observed occurrences of swollen glomeruli amongst all the treated groups which were not significantly different from that of the un-supplemented group may indicate that this alteration maybe attributed to some factors other than selenium supplement. It could also be interpreted as a normal histological occurrence for homeostasis.
Based on the histological analyses of the maternal liver and the kidney, it appears that selenium supplementation was most favorable when given during the pregestation stage.
Conflict of interest statement
We declare that we have no conflict of interest.
[1] Felig P, Kim Y, Lynch V, Hendler R. Amino acid metabolism during starvation in human pregnancy. J Clin Invest 1972; 51: 1195-1202.
[2] Dunlop W. Renal physiology in pregnancy. Postgrad Med J 1979; 55: 329-332.
[3] Davidson J. The kidney in pregnancy: A review. J Royal Soc Med 1983; 76: 485-501.
[4] Saikumar P, Jaya B, Renuka MR. Oxidative stress in pregnancy. IOSRJDMS 2013; 3(6): 12-13.
[5] Sies H. Oxidative stress: Oxidant and antioxidants. Experimt Physiol 1998; 82: 291-295.
[6] Battin E, Brumaghim J. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem Biophys 2009; 55(1): 1-23.
[7] Thannickal V, Fanburg B. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000; 279(6): 1005-1028.
[8] Agarwal A, Prabakaran SA. Oxidative stress and antioxidants in male infertility: a difficult balance. Iranian J Reprod Med 2005; 3(1): 1-8.
[9] Black R. Micronutrients in pregnancy. Br J Nutr 2001; 85(Suppl 2): S193-197.
[10] Barker DJ. Maternal nutrition, fetal nutrition and disease in later life. Nutrition 1997; 13(9): 807-813.
[11] United Nations Development Programme. Improve Maternal Health. 2013. [Online]. Available from http://www.ph.undp.org/content/ philippines/en/home/mdgoverview/overview/mdg5.html. [Accessed on September 28, 2015].
[12] Rayman M. The importance of selenium to human health. Lancet 2000; 356(9225): 233-241.
[13] Lu J, Holmgren A. The thioredoxin antioxidant system. Free Rad Biol Med 2014; 66: 75-87.
[14] Suzuki K. Metabolomics of selenium: Se metabolites based on speciation studies. J Health Sci 2005; 51(2): 107-114.
[15] Mehdi Y, Hornick J, Istasse L, Dufrasne I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013; 18: 3292-3311.
[16] Turanov A, Xu X, Carlson B, Yoo M, Gladyshev V, Hatfield D. Biosynthesis of selenocysteine, the 21st amino acid in the genetic code, and a novel pathway for cysteine biosynthesis. Adv Nutr 2011; 2(2): 122-128.
[17] Schrauzer G. Selenomethionine: a review of its nutritional significance, metabolism and toxicity. J Nutr 2000; 130(7): 1653-1656.
[18] Fairweather-Tait S, Bao Y, Broadley M, Collings R, Ford D, Hesketh J, et al. Selenium in human health and disease. Antioxid Redox Signal 2011; 14(7): 1337-1383.
[19] Barrington J, Lindsay P, James D, Smith S, Roberts A. Selenium deficiency and miscarriage: A possible link? BJOG 2014; 103(2): 130-132.
[20] Palmieri C, Szarek J. Effect of maternal selenium supplementation on pregnancy in humans and livestock. J Element 2011; 16: 143-156.
[21] Mistry H, Pipkin F, Redman C, Poston L. Selenium in reproductive health. Am J Obstet Gynecol 2012; 206(1): 21-30.
[22] Sahni S, Marg B. Guidelines for care and use of animals in scientific research. New Delhi: Indian Nat Sci Acad; 2000.
[23] Soudani N, Amara IB, Sefi M, Boudawara T, Zeghal N. Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp Toxicol Pathol 2011; 63(6): 541-548.
[24] Baker D. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition 1997; 13(9): 807-813.
[25] Mustacich D, Powis G. Thioredoxin reductase. Biochem J 2000; 346(1): 1-8.
[26] Frankel E. Lipid oxidation: mechanisms, products and biological significance. J Am Oil Chem’ Soc 1984; 61(12): 1908-1917.
[27] Fischer C. Lipid Hydroperoxide (LOOH) of the Fatty Acid (FA) Nature. The University of Iowa, Iowa; 2003.
[28] Herrera E. Metabolic adaptations in pregnancy and their implications for the availability of substrates to the fetus. Eur J Clin Nutr 2000; 54: 47-51. [29] Girard J, Cuendet E, Marliss B, Kervran A, Rieutort M, Assan R. The J Clin Invest 1973; 52: 3190-3200.
[30] Bell A. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J Anim Sci 1995; 73: 2804-2819.
[31] Molina E, Balander R, Fitzgerald S, Giesy J, Kannan K, Mitchell R, et al. Effects of air cell injection of perfluorooctane sulfonate before incubation on development of the white leghorn chicken (Gallus domesticus) embryo. Environ Toxicol and Chem 2005; 25(1): 227-232.
[32] Osweiler GD. Toxicology. Philadelphia: Lippinkott Williams Wilkins; 1996.
[33] Swift L. Role of Liver in Triglyceride Homeostasis. [Online]. Available from: http://www.mc.vanderbilt.edu/diabetes/msshortcourse/ presentations/7232012_Swift.pdf. [Accessed on July 16-26, 2012].
[34] Shenoy K, Somayaji S, Bairy K. Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharm 2001; 33: 260-266.
[35] Nakamuro K, Okuno T, Hasegawa T. Metabolism of selenoamino acids and contribution to selenium methylation to their toxicity. J Health Sci 2000; 46(6): 418-421.
[36] Sutherland Mr, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RSC, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol 2011; 22(7): 1365-1374.
[37] Meyer TW. Tubular injury in glomerular disease. Kidney Int 2003; 63: 774-787.
[38] Chevalier RL, Forbes MS. Generation and evolution of atubular glomeruli in the progression of renal disorders. J Am Soc Nephrol 2009; 19(2): 197-126.
[39] Widmaier E, Raff H, Strang K. Vander’s human physiology: The mechanisms of body function. 13th ed. New York: The McGraw-Hill Companies Inc.; 2014.
[40] Vassalli P, Morris RH, Mccluskey RT. The pathogenic role of fibrin deposition in the glomerular lesions of toxemia of pregnancy. J Exp Med 1963; 118: 467-478.
[41] Mitra S, Khandelwal P. Are all colloids same? How to select the right colloid? Indian J Anaesth 2009; 53(5): 592.
[42] Lieser K. Nuclear and radiochemistry: Fundamentals and applications. 2nd ed. New York: John Wiley & Sons Ltd.; 2008.
[43] Cornelis R, Caruso J, Crews H, Heumann K. Handbook of elemental speciation II: Species in the environment, food, medicine, and occupational health. West Sussex: John Wiley & Sons Ltd.; 2005.
[44] National Research Council Subcommittee on Selenium. Selenium in nutrition. Revised Ed. Washington (DC): National Academies Press (US); 1983.
ment heading
10.1016/j.apjr.2016.06.014
*Corresponding author: Gliceria B. Ramos, Biology Department, College of Science, De La Salle University, Taft Avenue, Manila, Philippines, 1004.
Tel: 63 2 5360228
Fax: 63 2 5360228
E-mail: gliceria.ramos@dlsu.edu.ph
 Asian Pacific Journal of Reproduction2016年4期
Asian Pacific Journal of Reproduction2016年4期
- Asian Pacific Journal of Reproduction的其它文章
- Spontaneous pregnancy after vaginoplasty in a patient presenting a congenital vaginal aplasia
- Evaluation of polymorphonuclear (PMN) cells in cervical sample as a diagnostic technique for detection of subclinical endometritis in dairy cattle
- In vitro polyembryony induction on a critically endangered fern, Pteris tripartita Sw.
- Natural honey as a cryoprotectant to improve Arab stallion post-thawing sperm parameters
- Effects of pomegranate juice in Tris-based extender on cattle semen quality after chilling and cryopreservation
- The relationship between trace mineral concentrations of amniotic fluid with placenta traits in the pregnancy toxemia Ghezel ewes
