靶向MAPK信号通路调控脂肪细胞分化的microRNAs
张秀秀,郭云涛,黄万龙,李 嫒,苗向阳
(中国农业科学院北京畜牧兽医研究所,北京 100193)
靶向MAPK信号通路调控脂肪细胞分化的microRNAs
张秀秀,郭云涛,黄万龙,李 嫒,苗向阳*
(中国农业科学院北京畜牧兽医研究所,北京 100193)
脂肪细胞分化是一个多能间充质干细胞(MSCs)逐渐向成熟脂肪细胞分化的复杂过程,该过程受很多转录因子、激素以及信号通路相关分子的严格调控。体内外的试验表明,microRNAs(miRNAs)也参与了脂肪细胞分化的调节,且可以靶向转录因子和信号通路中的关键分子发挥作用。丝裂原活化蛋白激酶(MAPK)信号通路是真核细胞将胞外信号转导至胞内引起细胞反应的一类重要信号系统,研究证明,miRNAs可以靶向MAPK信号通路中的某些基因,影响该通路的信号转导,参与脂肪细胞分化的调控。因此本文总结了近几年有关miRNA改变MAPK信号转导,实现调控脂肪细胞分化功能的研究,以期为深入了解脂肪细胞分化的机制,为治疗脂肪型疾病提供新的思路。
microRNA; MAPK信号通路; 靶基因; 脂肪细胞分化
脂肪组织不仅是一个储存能量的组织,还是一个内分泌组织,调控着体内的代谢平衡,在肥胖的状态下,脂肪组织会增生,表现为脂肪细胞数目的增多和体积增大,这会导致葡萄糖和脂肪代谢的失调,最终可能导致机体能量代谢失调,增加胰岛素拮抗,高血压以及血脂异常的风险[1-2]。因此对脂肪细胞分化机制的研究有助于治疗肥胖疾病。
脂肪细胞分化分为很多个阶段,第一步,MSCs被诱导分化为前体脂肪细胞,MSCs是一种多能干细胞,一旦被决定分化为脂肪细胞,就失去了分化为其他细胞类型的能力;其次前体脂肪细胞进行有丝分裂进入克隆增殖期;最后进入生长停滞期或终末分化期,分化为成熟的脂肪细胞。这个过程受一系列转录因子如CCAAT增强子结合蛋白(C/EBPs)、过氧化物酶体增殖剂激活受体γ(PPARγ)、信号通路及miRNA的严格调控(图1),其中重要的信号通路有MAPK信号通路、Wnt信号通路、Insulin信号通路等。近年来研究发现MAPK信号通路在脂肪细胞分化中也发挥重要作用,成为研究脂肪细胞分化调控机制的热点,且有研究证明miRNA调控脂肪细胞分化的很多靶基因都富集于MAPK通路中,所以本文着重探讨靶向MAPK调控脂肪细胞分化的miRNAs,以期为今后的试验作指导,为脂肪相关疾病的预防和治疗以及动物体脂肪沉积的调控提供新的思路。
1 MAPK信号转导通路概述
丝裂原活化蛋白激酶(Mitogen-activated protein kinases,MAPKs)是细胞内重要的信号通路之一,是哺乳动物体内广泛存在的一类丝/苏氨酸(Ser/Thr)蛋白激酶,可使多种核转录因子和蛋白激酶磷酸化,且能被一系列的细胞外信号或刺激所激活,如物理应激,炎性细胞因子,生长因子,细菌复合物等。目前在哺乳动物中已鉴定了4条MAPK信号转导通路,即ERK1/2信号通路、JNK通路、p38 MAPK通路和ERK5/BMK1通路,它们由不同的刺激因素激活,形成不同的转导途径,激活各不相同的转录因子,介导不同的生物学效应,参与细胞的增殖、分化、凋亡及细胞间的功能同步等一系列生理过程,在动物的生长发育、炎性反应等多种生命活动中发挥重要作用[3-6]。MAPK信号转导是以三级激酶级联的方式进行的,首先MAPKKK受有丝分裂原刺激磷酸化而激活,在此基础上MAPKKK磷酸化激活 MAPKK,最后由MAPKK磷酸化 MAPK,然后激活的MAPK作用于相应的转录因子,调控特定的基因表达。研究发现MAPK信号通路在脂肪细胞分化的各个阶段均有参与,且发挥了重要作用。
2 MAPK信号通路对脂肪细胞分化的调控
四条MAPK信号通路都与脂肪细胞分化或脂肪代谢相关,ERK1/2是最早发现的MAPK家族成员,其对细胞成脂分化的调控比较复杂,很多研究都得到了完全相反的结果,E. Turpin 与V. A. Constant等[7-8]发现ERK1/2可能抑制了脂肪细胞分化,而也有研究证明ERK1/2在脂肪细胞分化早期发挥积极的促进作用[9-12],具体调控机制可能是脂肪细胞分化早期激活的ERK1/2促进了C/EBPα和PPARγ的表达[13],从而促进脂肪细胞分化,而在后期激活的ERK1/2会磷酸化PPARγ使其失活[14-15],导致了脂肪细胞分化的抑制,所以ERK1/2对脂肪细胞分化的调控作用可能取决于该通路被激活的时间。p38MAPK是1993年J. L.Brewster等[16]发现的相对分子质量为38 ku的酪氨酸磷酸化的蛋白激酶,包括 p38α、p38β、p38γ、p38δ 4种亚型。M. Aouadi和J.Ji等[17-18]发现p38MAPK可以促进人类前体脂肪细胞和3T3-L1前体脂肪细胞的成脂分化,而M. Aouadi等[19]也发现p38MAPK抑制了小鼠胚胎成纤维细胞及成年鼠脂肪前体细胞成脂分化,可见p38MAPK通路对脂肪细胞分化的调控与物种和细胞类型有关。JNK(c-Jun氨基末端激酶)是相对分子质量54 ku的丝/苏氨酸蛋白激酶,S. Tominaga等[20]证实JNK抑制剂SP600125促进了人类间充质干细胞(hMSCs)成脂分化,M. Feng等[21]发现微管亲和调节蛋白激酶4(Mark4)可以通过激活JNK1信号通路来促进脂肪细胞分化,说明JNK信号通路在调节脂肪细胞分化方面发挥了重要作用。此外,ERK5/BMK1通路,一类非典型的MAPK通路,也参与了脂肪细胞分化及脂肪代谢的调控[22-23]。综上可得,这些MAPK信号通路通过不同途径,不同分子的参与,调控了脂肪细胞分化,为预防和治疗肥胖相关疾病提供了重要的靶点。
3 miRNAs的特点及调控机制
miRNA 是一种长为18~22 nt的内源性非编码RNA,它可通过与靶基因mRNA的3′非翻译区(3′UTR)互补配对,抑制靶基因的翻译,对基因表达进行转录后调控[24-26],在动物细胞的增殖、分化、凋亡和代谢等许多生物学过程中发挥重要作用[27-29]。大量研究显示miRNA 也参与调控动物脂肪细胞的分化[30],这些miRNAs在脂肪细胞分化的早期或者后期通过靶向作用信号通路中的分子或转录因子发挥调节功能。Y. F. Tang等[31]发现在脂肪干细胞(ADSCs)成脂分化期间miR-31和miR-326分别通过靶向转录因子C/EBPα和信号分子RASSF1来调节ADSCs分化,S. Y. Kim与E. K. Lee等[32-33]证明miR-27a和miR-130a也可以靶向转录因子PPARγ抑制脂肪细胞分化。此外有研究表明,部分miRNAs可以靶向MAPK信号通路中的信号分子ERK5、ERK1/2等在脂肪细胞分化中发挥重要调控作用。因此本文着重阐述了靶向MAPK调控脂肪细胞分化的miRNAs,有助于我们对脂肪细胞分化机制的进一步探索和研究。
4 靶向MAPK信号通路调节脂肪细胞分化的miRNAs
4.1 miRNA-143靶向MAP2K2-ERK5
C. Esau等[34]利用反义RNA寡核苷酸(ASOs)转染技术以及芯片技术分析了参与人类脂肪细胞分化的miRNAs。结果显示miR-143在人成熟脂肪细胞和前体脂肪细胞中存在显著差异表达,且在成熟脂肪细胞中上调,说明miR-143可能促进脂肪细胞分化。此外Esau等还发现ERK5的蛋白表达水平在转染有miR-143 ASO的细胞中明显高于对照组,推测ERK5可能是miR-143的一个作用靶基因。在Esau研究的基础上,L. Chen等[35]首次证明了miR-143对脂肪干细胞(ADSC)成脂分化的作用不是持续不变的,而是取决于发挥作用的阶段,即miR-143在ADSC分化的克隆增殖期产生抑制作用,在生长停滞期或终末分化期发挥促进作用。为了探索其机制,L. Chen等利用生物信息学方法和试验方法证实MAP2K2是miR-143调节脂肪细胞分化的一个直接靶基因,而其下游的ERK5是miR-143的间接靶基因[35],所以推测在ADSC克隆增殖期miR-143抑制了MAP2K2的表达,导致其下游ERK5活性降低,脂肪细胞分化受到抑制,而在终末分化期,miR-143抑制了MAP2K2-ERK5的活性后,ERK5介导的PPARγ磷酸化减少,从而促进了脂肪细胞分化。综上可得miR-143通过调节MAP2K2-ERK5通路在脂肪细胞分化过程中扮演重要角色。
4.2 miRNA-21靶向SPRY2-ERK-MAPK
miR-21是脂肪细胞分化的正调控因子[36],其调控机制不断地被探索,近年来也有研究证明miR-21靶向TGF-β信号通路中的TGFRB2来调节脂肪细胞分化[37]。在此基础上Y. Mei等[38]发现ERK-MAPK信号通路也是miR-21的靶标,在MSC成脂分化的早期过表达miR-21,发现脂肪细胞分化标志基因PPARγ和ap2的表达水平显著增加,且ERK-MAPK信号通路的活性显著增强[38],由于ERK-MAPK通路在脂肪细胞分化早期发挥促进作用[13],故miR-21可能与ERK-MAPK共同参与促进了MSC成脂分化。生物信息学方法预测可知miR-21的靶基因大多与ERK-MAPK通路有关,利用转染技术和荧光素酶报告分析法证明SPRY2是miR-21的直接靶基因[38],SPRY2蛋白是SPRY家族的成员,是ERK-MAPK通路的负调控者[39],故miR-21是通过直接抑制SPRY2活性来调节和维持ERK-MAPK通路活性,且三者构成一个反馈回路网络,共同促进脂肪细胞分化。
4.3 miR-375靶向ERK1/2
研究表明,miR-375参与了胰岛素分泌的调节[40],且对于维持内环境稳态以及抑制神经突细胞分化有着非常重要的作用[41-43]。近来H. Y. Ling等[44]探索了miR-375在脂肪细胞分化中的作用,芯片技术检测结果显示miR-375在3T3-L1成熟脂肪细胞中上调,且在3T3-L1前体脂肪细胞中过表达miR-375后发现脂肪细胞分化标志基因PPARγ、C/EBPα、aP2表达量显著增加,说明miR-375可以促进3T3-L1脂肪细胞的分化。此外H. Y. Ling等[44]还发现miR-375的过表达抑制ERK1/2的磷酸化,然而敲除 miR-375可以显著促进ERK1/2磷酸化,且降低了PPARγ、C/EBPα、aP2的表达量,故推测ERK1/2介导了miR-375对脂肪细胞分化的调控。然而由于ERK1/2的表达量不受miR-375影响,故miR-375调控脂肪细胞分化是直接靶向ERK1/2还是其他未知的靶基因仍有待探索。
4.4 其他通过MAPK调控脂肪细胞分化的miRNAs
miR-14可以调控胰岛细胞的内分泌,调节果蝇体内的脂肪代谢及能量代谢[45],P. Xu等[46]发现在果蝇中抑制miR-14的表达会导致脂肪细胞中脂滴数和甘油三酯积累的增加,即miR-14对脂肪代谢有抑制作用,且是通过靶基因p38和MAPK实现的[46]。此外, miR-27a和miR-27b也可以通过间接影响MAPK信号通路的信号转导参与调控脂肪细胞的分化. MiR-27a/b是脂肪细胞分化的负调控因子[47], T. Kang等[48]利用生物信息学方法和荧光素酶检测试验证明抑制素(PHB)是miR-27a和miR-27b的靶基因,PHB在脂肪细胞中高度表达,与脂肪细胞分化有密切的关系[49]。在3T3-L1细胞中过表达PHB抑制了胰岛素诱导的成脂分化,但是在胰岛素缺乏的情况下,PHB会通过上调MAPK/ERK信号通路促进脂肪细胞分化。在脂肪来源干细胞(ASC)中转染miR-27使得PHB沉默,脂肪细胞的分化就会被抑制[50],推测在胰岛素缺乏的情况下,PHB可以作用于下游的MAPK信号通路来促进脂肪的分化,而miR-27a和miR-27b通过抑制PHB间接靶向MAPK通路抑制脂肪细胞分化。此外,miR-448[51]和miR-378[52]也可以通过直接或间接调节MAPK信号分子来调控脂肪细胞分化。综上所述,miR-143、miR-21、miR-375、miR-27a/b等miRNAs通过直接或间接靶向MAPK信号通路中的相关分子,参与了脂肪细胞分化的调控(表1)。
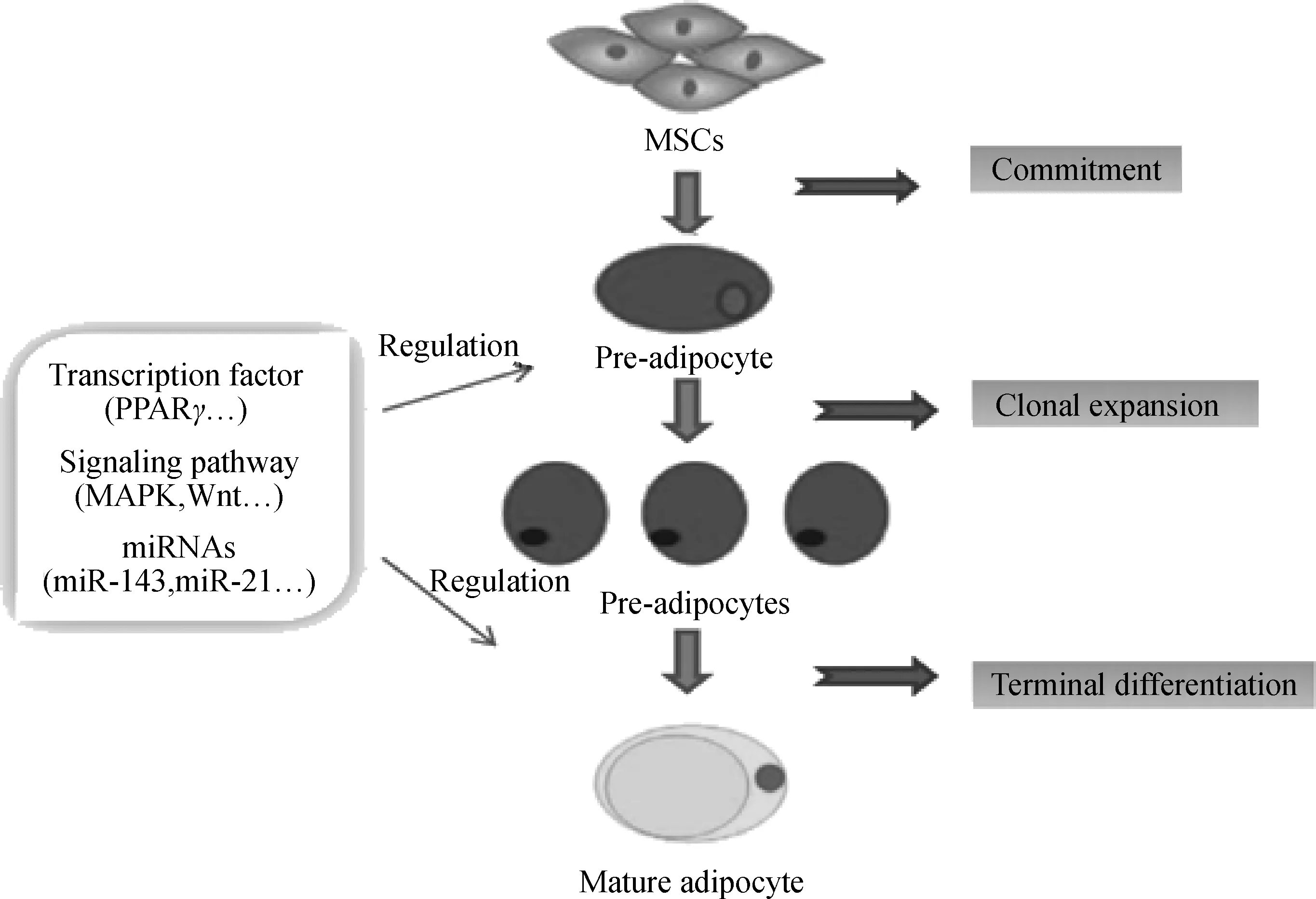
图1 MSCs成脂分化过程中的调控因素Fig.1 The regulatory factors during adipogenic differentiation of MSCs
表1 通过靶向MAPK信号通路中的靶基因调节脂肪细胞分化的miRNAs
Table 1 miRNAs in the regulation of adipocyte differentiation via targeting genes in MAPK signaling pathway
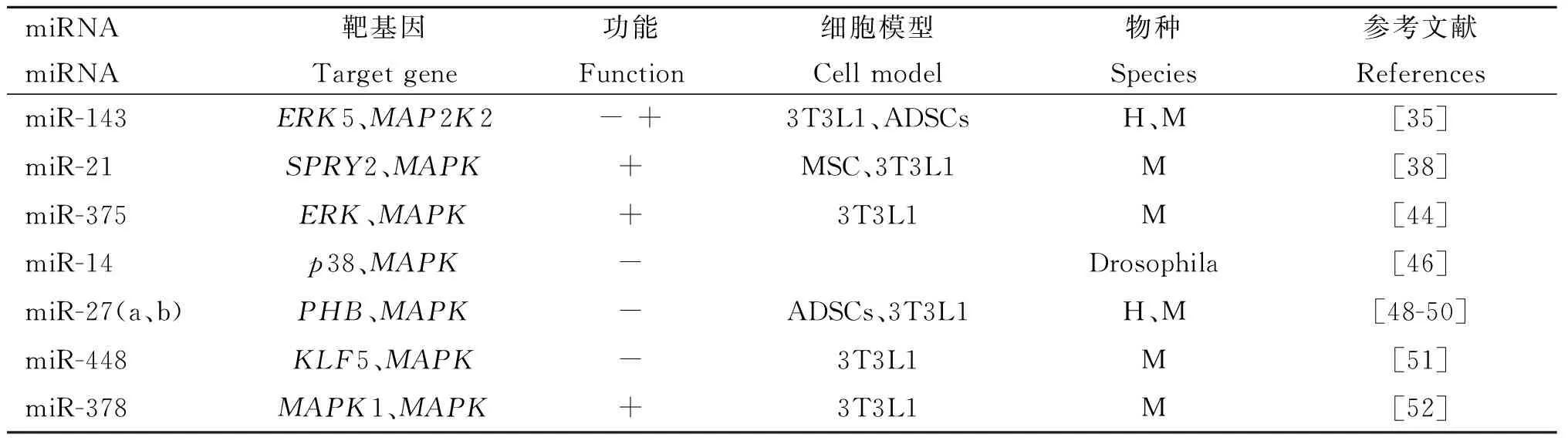
miRNAmiRNA靶基因Targetgene功能Function细胞模型Cellmodel物种Species参考文献ReferencesmiR-143ERK5、MAP2K2-+3T3L1、ADSCsH、M[35]miR-21SPRY2、MAPK+MSC、3T3L1M[38]miR-375ERK、MAPK+3T3L1M[44]miR-14p38、MAPK-Drosophila[46]miR-27(a、b)PHB、MAPK-ADSCs、3T3L1H、M[48-50]miR-448KLF5、MAPK-3T3L1M[51]miR-378MAPK1、MAPK+3T3L1M[52]
+.miRNA促进脂肪细胞分化; -.miRNA抑制脂肪细胞分化;- +.miRNA在脂肪细胞分化的早期发挥抑制作用,而在分化后期发挥促进作用。H.人;M.小鼠
+. Promoting adipocyte differentiation by miRNA; -. Inhibiting adipocyte differentiation by miRNA ; - +. Inhibiting effect and promoting effect, respectively during the early stage and late stage of adipocyte differentiation by miRNA. H. Human; M. Mouse
5 展望
肥胖、Ⅱ型糖尿病等代谢性疾病已经成为危害人类健康的杀手,其病理过程与脂肪细胞分化失调及脂代谢紊乱密切相关,目前脂肪细胞分化的机制已经成为了研究的热点。而miRNA对脂肪细胞分化的调控机制更是吸引了越来越多的科研工作者。试验证明miRNA可以通过靶向转录因子和信号通路中的关键分子影响脂肪细胞分化,故针对特定的miRNA寻找下游靶基因,研究其如何调控脂肪细胞分化成为了研究重点。随着分子生物学领域的不断发展,利用新一代测序技术研究miRNA已经越来越受欢迎,该技术快速、准确,在发现新的 miRNA 方面具有突出优势,且测序成本逐年降低。本实验室前期已经完成了日本黑毛和牛与荷斯坦牛皮下脂肪组织miRNA的Illumina测序,日本黑毛和牛与荷斯坦牛同为世界知名牛种,但在脂肪沉积方面二者却形成了显著差异,为研究脂肪细胞分化机制提供良好的素材。测序结果共鉴定出17个差异表达的已知miRNAs,以及15个新的miRNAs,对差异表达miRNAs的靶基因进行KEGG Pathway富集分析,发现某些差异表达miRNAs可能通过靶向甘油磷脂代谢,脂肪酸代谢及PPAR[53]等脂肪细胞分化相关信号通路中的重要基因调节了牛脂肪细胞分化或脂肪代谢,影响了牛的脂肪沉积,为研究脂肪代谢疾病提供有用的信息。
影响脂肪细胞分化的信号通路非常多,MAPK是其中较关键的一个通路,此外pRB-E2F[54]、Wnt[55]以及本试验中鉴定出来的PPAR信号通路也在脂肪细胞分化中发挥了重要的作用,这些通路之间存在广泛的“cross talk”,且与miRNA形成一个庞大的网络共同调控脂肪细胞分化,本文着重阐述了靶向MAPK信号通路调节脂肪细胞分化的miRNAs,希望有助于调控网络的研究,有助于miRNA调控脂肪细胞分化机制的阐明,为防治人类肥胖及其相关疾病提供新的靶点。目前,miRNA已经被用于研发新一代治疗疾病的药物。Santaris医药公司进行了靶向miRNA药物的首次人类临床试验,他们将SPC3649,一种miR-122的反义锁核苷酸(locked nucleic acid,LNA)用于丙型肝炎的治疗,反义锁核苷酸能够沉默相关的miRNAs[56],miR-122可以影响丙型肝炎病毒的复制,也可以调控胆固醇的合成[57],基于这些特点miR-122已经成为了第一代基于miRNA治疗代谢性疾病的发展对象,尽管安全、有效。但是靶向miRNA的药物治疗仍然处于研究的初期,每次药物试验非常昂贵且失败风险很大,故将靶向miRNA药物应用于临床仍需很大的努力。
未来miRNA调控脂肪细胞分化机制的研究已经不仅仅局限于对下游靶基因的寻找和验证,其上游的转录因子、细胞因子以及环境因素等也逐渐受到学者们的关注,miRNA与其下游的靶基因、上游的转录因子和细胞因子等构成了复杂的网络共同发挥调控作用。随着研究的深入,miRNA调控脂肪细胞分化的机制会越来越清楚,再加上靶向miRNA药物的治疗技术日趋成熟,相信不久以后利用miRNA抵御和治疗肥胖将成为现实。
[1] GUILHERME A, VIRBASIUS J V, PURI V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes[J].NatRevMolCellBiol, 2008, 9(5): 367-377.
[2] ABOOUF M A, HAMDY N M, AMIN A I, et al. Genotype screening of APLN rs3115757 variant in Egyptian women population reveals an association with obesity and insulin resistance[J].DiabetesResClinPract, 2015, 109(1): 40-47.
[3] CHEN H, XU X, TENG J, et al. CXCR4 inhibitor attenuates allergen-induced lung inflammation by down-regulating MMP-9 and ERK1/2[J].IntJClinExpPathol, 2015, 8(6): 6700-6707.
[4] BAO M H, ZHANG Y W, ZHOU H H. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway[J].JEthnopharmacol, 2013, 146(2): 543-551.
[5] LIU J, CHANG F, LI F, et al. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK[J].BiochemBiophysResCommun, 2015, 463(3): 262-267.
[6] HU K H, LI W X, SUN M Y, et al. Cadmium induced apoptosis in MG63 cells by increasing ROS, activation of p38 MAPK and inhibition of ERK 1/2 pathways[J].CellPhysiolBiochem, 2015, 36(2): 642-654.
[7] TURPIN E, MUSCAT A, VATIER C, et al. Carbamazepine directly inhibits adipocyte differentiation through activation of the ERK 1/2 pathway[J].BrJPharmacol, 2013, 168(1): 139-150.
[8] CONSTANT V A, GAGNON A, YARMO M, et al. The antiadipogenic effect of macrophage-conditioned medium depends on ERK1/2 activation[J].Metabolism, 2008, 57(4): 465-472.
[9] GWON S Y, AHN J Y, JUNG C H, et al. Shikonin suppresses ERK 1/2 phosphorylation during the early stages of adipocyte differentiation in 3T3-L1 cells[J].BMCComplementAlternMed, 2013, 13:207.
[10] KWAK D H, LEE J H, KIM D G, et al. Inhibitory effects of hwangryunhaedok-tang in 3T3-L1 adipogenesis by regulation of Raf/MEK1/ERK1/2 pathway and PDK1/Akt phosphorylation[J].EvidBasedComplementAlternatMed, 2013, 2013: 413906.
[11] LIAO Q C, LI Y L, QIN Y F, et al. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal- regulated kinases 1/2 activity[J].JCellBiochem, 2008, 104(5): 1853-1864.
[12] CHOI J S, KIM J H, ALI M Y, et al. Anti-adipogenic effect of epiberberine is mediated by regulation of the Raf/MEK1/2/ERK1/2 and AMPKalpha/Akt pathways[J].ArchPharmRes, 2015, 38(12):2153-2162.
[13] PRUSTY D, PARK B H, DAVIS K E, et al. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma(PPAR gamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes[J].JBiolChem, 2002, 277(48): 46226-46232.
[14] TANABE Y, KOGA M, SAITO M,et al. Inhibition of adipocyte differentiation by mechanical stretching through ERK-mediated downregulation of PPARγ2[J].JCellSci, 2004, 117(Pt 16): 3605-3614.
[15] REGINATO M J, KRAKOW S L, BAILEY S T, et al. Prostaglandins promote and block adipogenesis through opposing effects on peroxisome proliferator-activated receptor gamma[J].JBiolChem, 1998, 273(4): 1855-1858.
[16] BREWSTER J L, DE VALOIR T, DWYER N D, et al. An osmosensing signal transduction pathway in yeast[J].Science, 1993, 259(5102): 1760-1763.
[17] AOUADI M, JAGER J, LAURENT K, et al. p38MAP Kinase activity is required for human primary adipocyte differentiation[J].FEBSLett, 2007, 581(29): 5591-5596.
[18] JI J, ZHU J, HU X, et al. (2S)-7,4'-dihydroxy-8-prenylflavan stimulates adipogenesis and glucose uptake through p38MAPK pathway in 3T3-L1 cells[J].BiochemBiophysResCommun, 2015, 460(3): 578-582.
[19] AOUADI M, LAURENT K, PROT M, et al. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages[J].Diabetes, 2006, 55(2): 281-289.
[20] TOMINAGA S, YAMAGUCHI T, TAKAHASHI S, et al. Negative regulation of adipogenesis from human mesenchymal stem cells by Jun N-terminal kinase[J].BiochemBiophysResCommun, 2005, 326(2): 499-504.
[21] FENG M, TIAN L, GAN L, et al. Mark4 promotes adipogenesis and triggers apoptosis in 3T3-L1 adipocytes by activating JNK1 and inhibiting p38MAPK pathways[J].BiolCell, 2014, 106(9): 294-307.
[22] ZHU H, GUARIGLIA S, LI W, et al. Role of extracellular signal-regulated kinase 5 in adipocyte signaling[J].JBiolChem, 2014, 289(9): 6311-6322.
[23] SHARMA G, GOALSTONE M L. Dominant negative FTase (DNFTalpha) inhibits ERK5, MEF2C and CREB activation in adipogenesis[J].MolCellEndocrinol, 2005, 245(1-2): 93-104.
[24] VALENCIA-SANCHEZ M A, LIU J, HANNON G J, et al. Control of translation and mRNA degradation by miRNAs and siRNAs[J].GenesDev, 2006, 20(5): 515-524.
[25] BARCKMANN B, SIMONELIG M. Control of maternal mRNA stability in germ cells and early embryos[J].BiochimBiophysActa, 2013, 1829(6-7): 714-724.
[26] LOH B, JONAS S, IZAURRALDE E. The SMG5-SMG7 heterodimer directly recruits the CCR4-NOT deadenylase complex to mRNAs containing nonsense codons via interaction with POP2[J].GenesDev, 2013, 27(19): 2125-2138.
[27] XUE Z, ZHAO J, NIU L, et al. Up-regulation of miR-300 promotes proliferation and invasion of osteosarcoma by targeting BRD7[J].PLoSOne, 2015, 10(5): e0127682.
[28] VIMALRAJ S, SELVAMURUGAN N. Regulation of proliferation and apoptosis in human osteoblastic cells by microRNA-15b[J].IntJBiolMacromol, 2015, 79:490-497.
[29] ZHAO M, SUN L, CHEN S, et al. Borna disease virus infection impacts microRNAs associated with nervous system development, cell differentiation, proliferation and apoptosis in the hippocampi of neonatal rats[J].MolMedRep, 2015, 12(3):3697-3703.
[30] 贾夏丽,潘洋洋,乔利英, 等. 脂肪分化相关信号通路及microRNA调节研究进展[J]. 畜牧兽医学报, 2015,46(4): 518-525.
JIA X L, PAN Y Y, QIAO L Y, et al. Research progress in signaling pathways and microRNA regulation of adipocyte differentiation[J].ActaVeterinariaetZootechnicaSinica, 2015, 46(4): 518-525.(in Chinese)
[31] TANG Y F, ZHANG Y, LI X Y, et al. Expression of miR-31, miR-125b-5p, and miR-326 in the adipogenic differentiation process of adipose-derived stem cells[J].OMICS, 2009, 13(4): 331-336.
[32] KIM S Y, KIM A Y, LEE H W, et al. miR-27a is a negative regulator of adipocyte differentiation via suppressing PPARgamma expression[J].BiochemBiophysResCommun, 2010, 392(3): 323-328.
[33] LEE E K, LEE M J, ABDELMOHSEN K, et al. miR-130 suppresses adipogenesis by inhibiting peroxisome proliferator-activated receptor gamma expression[J].MolCellBiol, 2011, 31(4): 626-638.
[34] ESAU C, KANG X, PERALTA E, et al. MicroRNA-143 regulates adipocyte differentiation[J].JBiolChem, 2004, 279(50): 52361-52365.
[35] CHEN L, HOU J, YE L, et al. MicroRNA-143 regulates adipogenesis by modulating the MAP2K5-ERK5 signaling[J].SciRep, 2014, 4:3819.
[36] SEEGER T, FISCHER A, MUHLY- REINHOLZ M, et al. Long-term inhibition of miR-21 leads to reduction of obesity in db/db mice[J].Obesity(SilverSpring), 2014, 22(11): 2352-2360.
[37] KIM Y J, HWANG S J, BAE Y C, et al. MiR-21 regulates adipogenic differentiation through the modulation of TGF-beta signaling in mesenchymal stem cells derived from human adipose tissue[J].StemCells, 2009, 27(12): 3093-3102.
[38] MEI Y, BIAN C, LI J, et al. miR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation[J].JCellBiochem, 2013, 114(6): 1374-1384.
[39] CASCI T, VINS J, FREEMAN M. Sprouty, an intracellular inhibitor of Ras signaling[J].Cell, 1999, 96(5): 655-665.
[40] POY M N, ELIASSON L, KRUTZFELDT J, et al. A pancreatic islet-specific microRNA regulates insulin secretion[J].Nature, 2004, 432(7014): 226-230.
[41] LYNN F C. Meta-regulation: microRNA regulation of glucose and lipid metabolism[J].TrendsEndocrinolMetab, 2009, 20(9): 452-459.
[42] EL OUAAMARI A, BAROUKH N, MARTENS G A, et al. miR-375 targets 3′-phosphoinositide-dependent protein kinase-1 and regulates glucose-induced biological responses in pancreatic beta-cells[J].Diabetes, 2008, 57(10): 2708-2717.
[43] ABDELMOHSEN K, HUTCHISON E R, LEE E K, et al. miR-375 inhibits differentiation of neurites by lowering HuD levels[J].MolCellBiol, 2010, 30(17): 4197-4210.
[44] LING H Y, WEN G B, FENG S D, et al. MicroRNA-375 promotes 3T3-L1 adipocyte differentiation through modulation of extracellular signal-regulated kinase signalling[J].ClinExpPharmacolPhysiol, 2011, 38(4): 239-246.
[45] VARGHESE J, LIM S F, COHEN S M. Drosophila miR-14 regulates insulin production and metabolism through its target, sugarbabe[J].GenesDev, 2010, 24(24): 2748-2753.
[46] XU P, VERNOOY S Y, GUO M, et al. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism[J].CurrBiol, 2003, 13(9): 790-795.
[47] 陈 晨,胡雄贵,朱 吉, 等. 猪脂肪发育相关miRNAs的功能研究进展[J]. 畜牧兽医学报, 2015,46(12): 2117-2126.
CHEN C, HU X G, ZHU J, et al. Progress on the research of miRNAs associated with fat development in pigs[J].ActaVeterinariaetZootechnicaSinica, 2015,46(12): 2117-2126.(in Chinese)
[48] KANG T, LU W, XU W, et al. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells[J].JBiolChem, 2013, 288(48): 34394-34402.
[49] ANDE S R, XU Z, GU Y, et al. Prohibitin has an important role in adipocyte differentiation[J].IntJObes(Lond), 2012, 36(9): 1236-1244.
[50] LIN Q, GAO Z, ALARCON R M, et al. A role of miR-27 in the regulation of adipogenesis[J].FEBSJ, 2009, 276(8): 2348-2358.
[51] KINOSHITA M, ONO K, HORIE T, et al. Regulation of adipocyte differentiation by activation of serotonin (5-HT) receptors 5-HT2AR and 5-HT2CR and involvement of microRNA-448 mediated repression of KLF5[J].MolEndocrinol, 2010, 24(10): 1978-1987.
[52] HUANG N, WANG J, XIE W, et al. MiR-378a-3p enhances adipogenesis by targeting mitogen-activated protein kinase 1[J].BiochemBiophysResCommun, 2015, 457(1): 37-42.
[53] PARK H J, YUN J, JANG S H, et al. Coprinus comatus cap inhibits adipocyte differentiation via regulation of PPARγ and Akt signaling pathway[J].PLoSOne, 2014, 9(9): e105809.
[54] WANG Q, LI Y C, WANG J, et al. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130[J].ProcNatlAcadSciUSA, 2008, 105(8): 2889-2894.
[55] CHEN C, PENG Y, PENG Y, et al. miR-135a-5p inhibits 3T3-L1 adipogenesis through activation of canonical Wnt/beta-catenin signaling[J].JMolEndocrinol, 2014, 52(3): 311-320.
[57] WAHID F, SHEHZAD A, KHAN T, et al. MicroRNAs: synthesis, mechanism, function, and recent clinical trials[J].BiochimBiophysActa, 2010, 1803(11): 1231-1243.
(编辑 郭云雁)
Regulation of Adipocyte Differentiation via microRNAs Targeting MAPK Signaling Pathway
ZHANG Xiu-xiu, GUO Yun-tao, HUANG Wan-long, LI Yuan, MIAO Xiang-yang*
(InstituteofAnimalScience,ChineseAcademyofAgriculturalSciences,Beijing100193,China)
Adipocyte differentiation is a complicated process in which pluripotent mesenchymal stem cells (MSCs) differentiate into mature adipocytes. The process of adipocyte differentiation is strictly regulated by a number of transcription factors, hormones and signaling pathway molecules.Invivoandinvitroresearch has revealed that microRNAs (miRNAs) are also involved in adipocyte differentiation and play a role by targeting transcription factors and key signaling molecules. MAPK signaling pathway is one of important signaling systems which transduce the extracellular signal to intracellular space and cause cell response. The studies showed that, miRNAs can target certain genes in MAPK and affect its signal transduction, thus regulating adipocyte differentiation. Therefore, a summary of researches how miRNAs change the signal transduction of MAPK pathway and regulate adipocyte differentiation was performed in order to further understand the adipocyte differentiation mechanism and offer new ideas for curing the fat-associated diseases.
microRNA; MAPK signaling pathway; target gene; adipocyte differentiation
10.11843/j.issn.0366-6964.2016.11.002
2016-03-01
转基因生物新品种培育科技重大专项(2009ZX08008-004B;2008ZX08008-003);国家“863”计划项目(2008AA10Z140);国家自然科学基金项目(30571339);中国农业科学院农业科技创新项目(ASTIP-IAS05);国家重点基础研究发展计划(“973”计划)(2015CB943100);中国农业科学院创新基金项目(2004-院-1);中央级公益性科研院所基本科研业务费专项资金项目(2013ywf-yb-5;2013ywf-zd-2)
张秀秀(1989-),女,山西大同人,硕士,主要从事转基因与细胞工程研究,E-mail:13261953358@163.com
*通信作者:苗向阳,研究员,博士,博士生导师,主要从事基因工程与功能基因组学及转基因动物研究,E-mail:mxy32@sohu.com
S813.2
A
0366-6964(2016)11-2159-08

