糖尿病肾病治疗的研究进展*
李翔 杨沅君 刘杰夫 董茜
糖尿病肾病治疗的研究进展*
李翔①杨沅君②刘杰夫③董茜①
糖尿病肾病是糖尿病最常见的并发症,也是导致慢性肾衰竭的重要原因,但临床的治疗方法效果局限。目前的研究从抑制钠-葡萄糖协同转运蛋白2、蛋白激酶C、多元醇通路以及晚期糖化终末产物等方面,试图干扰发病过程中的炎性反应、氧化应激、免疫应答和组织纤维化来特异性治疗。干细胞移植方法也为进一步治疗糖尿病肾病提供了新希望。
糖尿病肾病; 治疗; 发病机制; 干细胞
First-author’s address:Department of Clinical Medicine,Xuzhou Medical University,Xuzhou 221000,China
随着经济的发展、人们生活习惯的改变和生活节奏加快,糖尿病(diabetes mellitus,DM)的发病率日益上升。糖尿病肾病(diabetic nephropathy,DN)是DM最常见的微血管并发症,也是导致终末期肾病(end-stage renal disease,ESRD)的最常见原因,严重威胁人类的健康[1]。然而,目前临床上的治疗方法有很大的局限性,不能有效地减缓病情的发展[2]。因此,有越来越多的学者致力于探索治疗DN的新思路(见图1)。现就近年来治疗DN的研究进展予以综述。
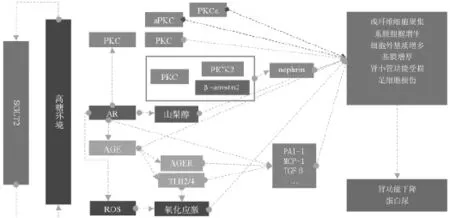
图1 针对病因的可用治疗靶点
1 针对病因的治疗
1.1钠-葡萄糖协同转运蛋白2 在高糖或高胰岛素环境下,肾近端小管表面表达的钠-葡萄糖协同转运蛋白2(sodium-glucose transporter 2,SGLT2)升高,导致其介导的对Na+和葡萄糖的重吸收程度升高[3],这提示使用SGLT2抑制剂,能够阻断钠和糖的共转运,改善患者的高血糖和高血压水平。临床试验联合SGLT2抑制剂和RAAS阻断表现出了极佳的心血管和肾脏保护效果[4-5],具体的作用机制不清,可能与血管紧张素转换酶Ⅱ、血管紧张素1-7/1-9的升高[6]、以及球管反馈有关,但文献[7-8]研究表明其具有增加生殖系统感染和骨折的风险。
1.2蛋白激酶C 蛋白激酶C(protein kinase C,PKC)作为一细胞质酶,广泛存在于机体组织器官及细胞中。其活性包括调节血管内皮细胞通透性和收缩性、ECM的合成、细胞增殖和调亡、血管新生、白细胞黏附、激活和抑制细胞因子和细胞生长等。PKC有多种亚型,其中PKCα、β被认为与DN的发病密切相关。PKCα与PICK2、β-arrestin2结合,使得nephrin蛋白的内吞作用增强,从而导致蛋白尿[9]。Meier等[10]发现:PKCβ基因敲除的DN大鼠的蛋白尿并没有明显改善,但肾小球增生和细胞外基质的产生减少。Menne等[11]综合两者的结果,同时抑制PKCα和PKCβ,较好的阻止了DN的发展。另外,近年来还发现PKCε、aPKC在肾脏保护中具有重要作用[12-13]。
1.3多元醇通路 多元醇通路由醛糖还原酶(aldose reductase,AR)及其受体NADPH和山梨糖醇脱氢酶及其受体NAD组成。肾小球细胞内持续的高糖环境会导致AR活化。作为多元醇通路的限速酶,AR活化使葡萄糖大量转化为山梨醇,引起细胞渗透性损伤[14]。另外,AR在调节多元醇通路的同时也在糖代谢、细胞外基质病理变化中起重要作用。大量AR的激活会引起AGE、ROS以及TGF-β的增多[15],导致氧化应激和组织纤维化。还有研究表明,AR可促进PKC的激活,通过上述途径加速肾小球的损伤。因此多元醇通路将是较好的治疗DN的靶点。
1.4晚期糖化终末产物 高血糖和氧化应激会促进内源性晚期糖基化终产物(advanced glycation end products,AGE)的产生[16]。AGE在组织中的积聚,会造成肾小球基底膜结构改变、电荷屏障减弱、足细胞受损、细胞外基质增生,并最终导致肾小球硬化和蛋白尿。而AGE除了自身的积聚会导致组织损伤外,与其受体AGER或Toll样受体2和4结合后,还会激活一系列的胞内信号转导通路[17],释放炎性因子并导致氧化应激。体外实验将AGE与肾小管上皮细胞共同培养,可见TGF-β、PAI-1、MCP-1的高表达[18]。在文献[19-20]DN的动物实验中,抑制AGE的形成或破坏其诱导的交联反应都具有肾脏保护作用。目前的研究致力于抑制内源性AGE的生成,以及寻找新的AGER拮抗剂,用于治疗糖尿病引起的微血管并发症。新的高亲和力DNA适体用于阻断AGE生成的方法也展现出了良好的应用前景[21]。
2 干细胞治疗
尽管不断地研究新的药物作用靶点给治疗DN提供了很好的思路,但到目前为止疗效还是很有限[22],因此急需开辟新的途径以求进一步的治疗。干细胞移植方法能够促进受损的肾脏组织、细胞的再生或直接分化为相应细胞来修复肾脏,给进一步治疗DN提供了希望,因而受到了人们的关注。
胚胎干细胞(embryonic stem cell,ESCs)是从早期胚胎(原肠胚期之前)或原始性腺中分离出来的一类细胞,它具有体外培养无限增殖、自我更新和多向分化的潜能。ESCs除了能分化为胰岛素分泌细胞外[23],在特定的生长因子或诱导剂的作用下还能够向肾脏细胞谱系分化,如近端小管样细胞[24],但由其带来的伦理问题一直限制着它的发展。
间充质干细胞(mesenchymal stem cells,MSCs)既具有多向分化潜能、免疫抑制和异体移植的安全性[25-27],又避免了ESCs的伦理问题,近年来被广泛用于多种疾病治疗的研究。MSCs能够分化为胰岛素分泌细胞、肾小管上皮细胞、上皮细胞和足细胞[28-30]。文献[31]研究报告指出MSCs的移植能够改善蛋白尿、血清肌酐/尿素氮比值、肾小球高压、系膜增生和纤维化。然而MSCs移植疗法仍有许多缺点:来自不同供体的MSCs的异质性很大,而且在慢性心血管系统疾病的患者和慢性肾病模型的大鼠中,MSCs的增殖和分化能力会降低[32],这些都会影响其治疗效果,需要继续研究来解决这些问题。由尿液中分离出来的干细胞—尿源性干细胞(urinederived stem cells,USCs)是近来发现的新的干细胞亚型[33]。从尿中提取干细胞更加地安全和经济。USCs具有较长的端粒酶长度,自我更新能力和增生能力都很强,但是关于其应用于DN的治疗暂无报道。
3 小结
DN的发生发展涉及多种发病机制的参与,不断深入研究发病机制,将为DN的治疗提供更多治疗靶点以及早期诊断的标志。近来有研究报道自噬参与了糖尿病肾病的发病过程,其作用将是未来研究热点。随着DN研究不断深入,干细胞、基因疗法不断发展,相信在不久的将来,糖尿病肾病的患者一定会得到更好的治疗。
[1] Duran-Salgado M B,Rubio-Guerra A F.Diabetic nephropathy and inflammation[J].World Journal of Diabetes,2014,5(3):393-398.
[2] Molitch M E,Defronzo R A,Franz M J,et al.Nephropathy in diabetes[J].Diabetes Care,2004,27(Suppl 1):S79-S83.
[3] Brown J.Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes[J].Diabetes,2006,54(12):3427-3434.
[4] Rosenstein R,Hough A.Empagliflozin,cardiovascular outcomes,and mortality in type 2 diabetes[J].New England Journal of Medicine,2015,373(22):2117-2128.
[5] Wanner C,Inzucchi S E,Lachin J M,et al.Empagliflozin and progression of kidney disease in type 2 diabetes[J].New England Journal of Medicine,2016,375(4):323-334.
[6] Tikellis C,Bialkowski K,Pete J,et al.ACE2 deficiency modifies renoprotection afforded by ACE inhibition in experimental diabetes[J].Diabetes,2008,57(4):1018-1025.
[7] Kohan D E,Fioretto P,Tang W,et al.Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control[J].Kidney International,2014,85(4):962-971.
[8] Watts N B,Bilezikian J P,Usiskin K,et al.Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus[J].The Journal of Clinical Endocrinology & Metabolism,2015,101(1):157-166.
[9] Quack I,Woznowski M,Potthoff S A,et al.PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia[J]. Journal of Biological Chemistry,2011,286(15):12 959-12 970.
[10] Meier M,Park J K,Overheu D,et al.Deletion of protein kinase C-beta isoform in vivo reduces renal hypertrophy but not albuminuria in the streptozotocin-induced diabetic mouse model[J].Diabetes,2007,56(2):346-354.
[11] Menne J,Shushakova N, Bartels J, et al. Dual inhibition of classical protein kinase C-α and protein kinase C-β isoforms protects against experimental murine diabetic nephropathy[J]. Diabetes,2013,62(4):1167-1174.
[12] Meier M,Menne J,Park J K,et al.Deletion of protein kinase C-ε signaling pathway induces glomerulo-sclerosis and tubulointerstitial fibrosis in vivo[J].Journal of the American Society of Nephrology Jasn,2007,18(4):1190-1198.
[13] Doublier S,Salvidio G,Lupia E,et al.Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II[J].Diabetes,2003,52(4):1023-1030.
[14] De-Min Y U,Liu D M,Liu X H,et al.Effect of aldose reductase inhibitor on rat kidney aldose reductase gene expression[J].Journal of Tianjin Medical University,2005,11(4):4.
[15] Xie P,Sun L Oates P J,et al.Pathobiology of renal-specific oxidoreductase/myo-inositol oxygenase in diabetic nephropathy:its implications in tubulointerstitial fibrosis[J].Ajp Renal Physiology,2010,298(6):F1393-F1404.
[16] Chilelli N C,Burlina S,Lapolla A.AGEs,rather than hyperglycemia, are responsible formicrovascular complications in diabetes: A“glycoxidation-centric”point of view[J].Nutrition Metabolism & Cardiovascular Diseases Nmcd,2013,23(10):913-919.
[17] Miyata T,Ueda Y,Asahi K,et al.Mechanism of the inhibitory effect of OPB-9195 on advanced glycation end product and advanced lipoxidation end product formation[J].Journal of the American Society of Nephrology,2000,11(9):1719-1725.
[18] Sasai Y,Iwakawa K,Yanagida K,et al.Advanced glycation endproducts stimulate renal epithelial cells to release chemokines that recruit macrophages, leading to renal fibrosis[J].Bioscience Biotechnology and Biochemistry,2012,76(9):1741-1745.
[19] Stracke H,Hammes H P,Werkmann D,et al.Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats[J]. Experimental & Clinical Endocrinology & Diabetes,2001,109(6):330-336.
[20] Kihm L P,Mü llerkrebs S,Klein J,et al.Benfotiamine protects against peritoneal and kidney damage in peritoneal dialysis[J]. Journal of the American Society of Nephrology,2011,22(5):914-926.
[21] Taguchi K,Fukami K,Yamagishi S,et al.DNA Aptamer Raised Against AGEs Blocks the Progression of Experimental Diabetic Nephropathy[J].Diabetes,2013,62(9):3241-3250.
[22] Liu Y,Tang S C W.Recent Progress in Stem Cell Therapy for Diabetic Nephropathy[J].Kidney Diseases,2015,2(1):20-27.
[23] Jiang W,Yan S,Zhao D X,et al.In vitro derivation of functional insulin-producing cells from human embryonic stem cells[J].Cell Research,2007,17(4):333-344.
[24] Narayanan K,Schumacher K M,Tasnim F,et al.Human embryonic stem cells differentiate into functional renal proximal tubular-like cells[J].Kidney International,2013,83(4):593-603.
[25] Aziz M T A,Wassef M A A,Ahmed H H,et al.The role of bone marrow derived-mesenchymal stem cells in attenuation of kidney function in rats with diabetic nephropathy[J].Diabetology& Metabolic Syndrome,2014,6(1):1-10.
[26] Fang Y,Tian X,Bai S,et al.Autologous transplantation of adipose-derived mesenchymal stem cells,ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting,oxidative stress,pro-inflammatory cytokines and the p38 MAPK signaling pathway[J].International Journal of Molecular Medicine,2012,30(1):85-92.
[27] Tögel F E,Westenfelder C.Kidney protection and regeneration following acute injury:progress through stem cell therapy[J]. American Journal of Kidney Diseases,2012,60(6):1012-1022.
[28] Karnieli O,Izhar-Prato Y,Bulvik S,et al.Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation[J].Stem Cells,2007,25(11):2837-2844.
[29] Poulsom R,Forbes S J,Hodivala-Dilke K,et al.Bone marrow contributes to renal parenchymal turnover and regeneration[J]. Journal of Pathology,2001,195(195):229-235.
[30] Prockop D J.Repair of tissues by adult stem/progenitor cells(MSCs):controversies, myths, and changing paradigms[J]. Molecular Therapy,2009,17(17):939-946.
[31] Lv S,Cheng J,Sun A,et al.Mesenchymal stem cells transplantation ameliorates glomerular injury in streptozotocininduced diabetic nephropathy in rats via inhibiting oxidative stress[J].International Immuno- pharmacology,2014,104(1):275-282.
[32] Yamada A,Yokoo T,Yokote S,et al.Comparison of multipotency and molecular profile of MSCs between CKD and healthy rats[J].Human Cell,2014,27(2):59-67.
[33] Bharadwaj S,Liu G,Shi Y,et al.Multipotential differentiation of human urine-derived stem cells:Potential for therapeutic applications in urology[J].Stem Cells,2013,31(9):1840-1856.
Research Progress in the Treatment of Diabetic Nephropathy/
LI Xiang,YANG Yuan-jun,LIU Jiefu,et al.//
Medical Innovation of China,2016,13(30):142-145
Diabetic nephropathy is the most common complication of diabetes,and it is also an important cause of chronic renal failure,but the clinical treatment effect is limited.The current study from the inhibition of sodium glucose cotransporter 2,protein kinase C,polyol pathway and advanced glycation end products and so on,to inflammatory reaction and interference in the pathogenesis of oxidative stress,immune response and tissue fibrosis to specific treatment.Stem cell transplantation provides a new hope for the treatment of diabetic nephropathy.
Diabetic nephropathy; Therapeutics; Pathogeneses; Stem cell
10.3969/j.issn.1674-4985.2016.30.040
江苏高校品牌专业建设工程一期资助项目(PPZY2015B161)
①徐州医科大学临床医学系 江苏 徐州 221000
②徐州医科大学麻醉学院
③徐州医科大学影像学院
董茜
(2016-06-24) (本文编辑:郎威)

