基于3,5-二((4′-羧基苄基)氧)苯甲酸和4′-(4-吡啶基)-2,2′∶6′,2″-三联吡啶为混合配体的两个配合物的水热合成与晶体结构
乔 宇 尉 兵 王璐瑶 李秀颖 车广波*, 刘春波 张兴晶
(1吉林师范大学化学学院,四平136000)
(2环境友好材料制备与应用教育部重点实验室,长春130103)
(3江苏大学绿色化学与化工技术研究院,镇江212013)
基于3,5-二((4′-羧基苄基)氧)苯甲酸和4′-(4-吡啶基)-2,2′∶6′,2″-三联吡啶为混合配体的两个配合物的水热合成与晶体结构
乔宇1,2尉兵1王璐瑶1李秀颖1,2车广波*,1,2刘春波*,3张兴晶1,2
(1吉林师范大学化学学院,四平136000)
(2环境友好材料制备与应用教育部重点实验室,长春130103)
(3江苏大学绿色化学与化工技术研究院,镇江212013)
在水热条件下,以3,5-二((4′-羧基苄基)氧)苯甲酸(H3bcb)和4′-(4-吡啶基)-2,2′∶6′,2″-三联吡啶(PYTPY)为混合配体构筑了2个过渡金属配合物[Co(H2bcb)2(PYTPY)]n(1)和[Mn(H2bcb)2(PYTPY)]n(2),利用元素分析、红外光谱以及单晶X射线衍射表征其结构。分析表明配合物1和2为一维链状结构。此外,2个配合物展示了优良的热稳定性。磁化率的测试结果表明,配合物1和2在2 K和8 K以下时展示了反铁磁相互作用。
过渡金属配合物;3,5-二((4′-羧基苄基)氧)苯甲酸;4′-(4-吡啶基)-2,2′∶6′,2″-三联吡啶;晶体结构;磁性
In recent years,rational design and synthesis of the complexes have been extensively studied not only for their fascinating architectures[1],but also for their potential applications in magnetism[2],catalysis[3], luminescence[4-5],adsorption[6]and separation[7].To the best of our knowledge,the construction of molecular architectures greatly depends on the coordination geometry of centralmetal and organic ligands.In this regard,the design or selection of suitable organic ligands is one of the keys for the construction of the complexes.Organic aromatic polycarboxylic acids and polypyridyl ligands have been extensively employed in the preparation of the complexes with novel structural motifs and interesting properties because of their versatile bridging or chelating coordination modes[8-10]. 3,5-bis((4′-carboxylbenzyl)oxy)benzoicacid(H3bcb)has both two twisty benzyloxy carboxylic acid moiety and a rigid benzoic acid moiety.The flexible moiety can freely twist around the C-C bond to meet the requirements of the coordination geometries,and the rigid moiety can help to construct and stabilize certain complexes architectures.Up to now,only a handful of complexes based on H3bcb ligand have been described[11-13].As an important polypyridyl ligand,4′-(4-pyridyl)-2,2′∶6′,2″-terpyridine(PYTPY)is excellent ligand to construct supramolecular architectures not only because it can coordinate to the metals easily, but also because the large conjugated aromatic rings system can easily formπ-πinteractions[14].Considering all of these above-mentioned,we consider that the simultaneous employment of H3bcb and PYTPY mixed ligands(Scheme 1)will contribute to the formation of various architectures and help researchers understand the process of self-assembly.Herein,we successfully apply this strategy and obtain two complexes, [Co(H2bcb)2(PYTPY)]n(1)and[Mn(H2bcb)2(PYTPY)]n(2).In this paper,we report the preparation,crystal structures,thermal stabilities and magnetic properties of complexes 1 and 2.

Scheme 1 Molecular structure of H3bcb and PYTPY ligands
1 Experimental
1.1M aterials and instruments
All reagents were used as purchased without further purification.Elemental analyses of carbon, hydrogen and nitrogen were performed on a Perkin-Elmer 240C elemental analyzer.IR spectra were obtained on a Perkin-Elmer 2400LSⅡspectrometer within the 4 000~400 cm-1region.Powder X-ray diffraction(PXRD)patterns of the samples were collected on a D/MAX-3C diffractometer with the Cu Kαradiation(λ=0.154 06 nm)at room temperature and 2θranging from 5°to 50°.Thermogravimetric analysis(TGA)was performed on a NETZSCH STA 449Canalyzer under air atmosphere.Magnetic analysis was performed on a SQUID MPMS3 analyzer under liquid helium refrigeration.
1.2Preparation of the complexes
1.2.1Synthesis of[Co(H2bcb)2(PYTPY)]n(1)
The complex 1 was prepared under hydrothermal condition.A mixture of Co(NO3)2·6H2O(0.029 1 g, 0.1 mmol),H3bcb(0.084 g,0.2 mmol),PYTPY(0.031 g,0.1mmol)and distilled water(15 mL)wasmixed in a 50 mL beaker.In the process of stirring,NaOH(0.1 mol·L-1)was dripped into the system until pH=4. After stirring for 0.5 h,themixture was sealed in a 25 mL Teflon-lined stainless steel autoclave and heated at 428 K for 72 h,then cooled at a rate of 5 K·h-1to room temperature.After filtration,the product was washed with distilled water and then dried at room temperature.Red block crystals were obtained with a yield of about 48%based on Co.Anal.Calcd.for C66H48N4O16Co(%):C,65.40;H,3.99;N,4.62.Found (%):C,65.35;H,4.06;N,4.60.IR spectrum(KBr, cm-1):3 431(m),1 709(s),1 682(s),1 577(m),1 541 (s),1 472(m),1 357(m),1 278(s),1 199(m),1 164 (s),1 041(m),909(m),821(m),786(s),751(w),707 (m),646(m),505(m).
1.2.2Synthesis of[Mn(H2bcb)2(PYTPY)]n(2)
An identical procedure with 1 was followed to prepare2 exceptCo(NO3)2·6H2Owas replaced byMnCl2·4H2O.Brown block crystals were obtained with a yield of about 53%based on Mn.Anal.Calcd.for C66H48N4O16Mn(%):C,65.62;H,4.00;N,4.64.Found (%):C,65.56;H,4.06;N,4.63.IR spectrum(KBr, cm-1):3 440(m),1 708(s),1 618(s),1 569(m),1 378 (m),1 297(s),1 158(s),1 041(s),903(m),827(m), 787(s),753(w),698(m),623(m),508(m).
1.3Single-crystal X-ray diffraction
Suitable single crystals of complexes 1 and 2 with dimensions of 0.331 mm×0.264 mm×0.202 mm and 0.295 mm×0.246 mm×0.197 mm were mounted on glass fibers for X-raymeasurement,respectively.Reflection data were collected at room temperature on a BrukerSMARTAPEXⅡCCD diffractometer,equipped with graphite-monochromatized Mo Kαradiation(λ= 0.071 073 nm)using theφ-ωscan technique.All absorption corrections were performed using the SADABS program[15].All the structureswere solved by direct methods with SHELXS-97 program[16]and refined by full-matrix least-squares techniques on F2with SHELXL 97[17].All of the non-hydrogen atoms were easily found from the different Fourier map and refined anisotropically,whereas the hydrogen atoms of the complexes were placed by geometrical considerations and were added to the structure factor calculation.Detailed crystallographic data and structure refinement parameters of complexes 1 and 2 are given in Table1,and the selected bond distances and bond angles are listed in Table2.
CCDC:1439250,1;1439249,2.

Table1 Crystal data and structure refinements for complexes 1 and 2
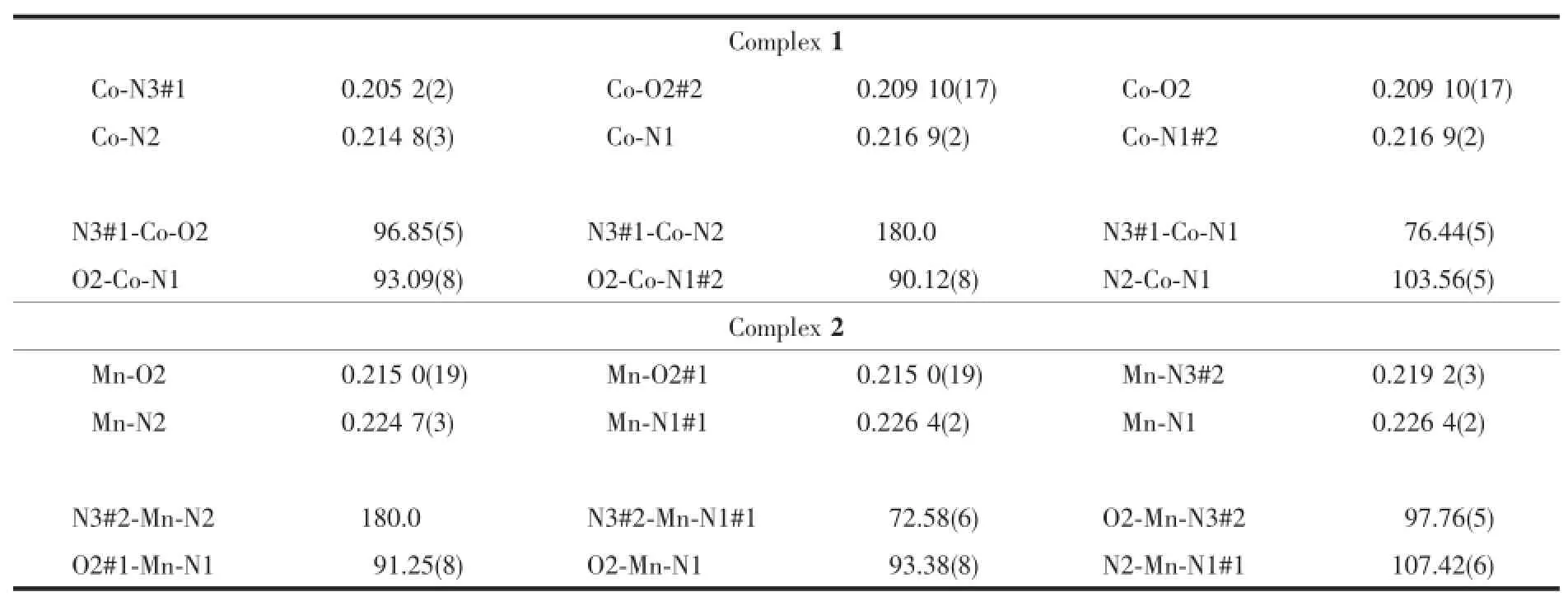
Table2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
2 Results and discussion
2.1Description of the crystal structure
Similar cell parameters with the same space group(Table1)and the results of crystallographic analysis confirm that 1 and 2 are isostructural.Thus, only the structure of 1 is described in detail as the typical example.
The single-crystal X-ray diffraction analysis reveals that complex 1 exhibits a one-dimensional chain structure,crystallizing in Orthorhombic space group Pccn.An asymmetric unit[Co(H2bcb)2(PYTPY)] contains one six-coordinated Co2+ion,two H2bcbligands and one PYTPY ligand.The coordination mode of Co2+is shown in Fig.1.The central Co2+is coordinated with two oxygen atoms(O2 and O2#1, symmetry codes:#1:-x+1/2,-y+1/2,z)from two monodentate bridging carboxyl group of two different H2bcb-ligands,four N atoms(N1,N1#1,N2,N3, symmetry codes:#1:-x+1/2,-y+1/2,z)from two different PYTPY ligands in a slightly distorted octahedral coordination.The average distances of Co-and Co-NPYTPYare 0.209 14(17)and 0.213 45(3) nm,respectively,all ofwhich are comparable to those reported forother Co2+complexes[18].The adjacentmetal center Co2+ions were bridged by PYTPY ligands, generating an infinite one dimensional(1D)fishbonelike chain along the c-axis(Fig.2),in which the PYTPY ligands and Co2+ions form themain skeleton, whereas H2bcb-ligands play the roles of the side bones.In complex 1,the larger conjugated system of PYTPY ligand plays a vital role in further extending and consolidating the molecular structure viaπ…π interactions.Theneighboring1D chainsinteract through π…πstacking between pyridine rings of PYTPY ligands to yield a two-dimensional(2D)layer structure. The centroid-to-centroid distance is 0.369 2 nm and dihedral angel is 3°between Cg(1)and Cg(1)#6 rings (Cg(1):N1→C30→C31→C32→C33→C34,symmetry codes:#6:-x+1/2,-y+3/2,z,Fig.3).It is worth mentioning that the intramolecular hydrogen bonds (O4-H4…O1#3 0.253 3 nm,163.65°,symmetry codes: #3:x,-y+1/2,z+1/2)and intermolecular hydrogen bonds(O6-H6A…O6#7 0.2641nm,161.03°,symmetry codes:#7:-x+3/2,-y+1/2,z)involving the carboxylic groups of H2bcb-ligands further consolidate 2D supramolecular structure of complex 1.

Fig.1 ORTEP drawing of complex 1 showing Co2+coordination environmentwith thermal ellipsoids at 30%probability

Fig.2 1D chain structure of complex 1 with hydrogen atoms omitted for clarity
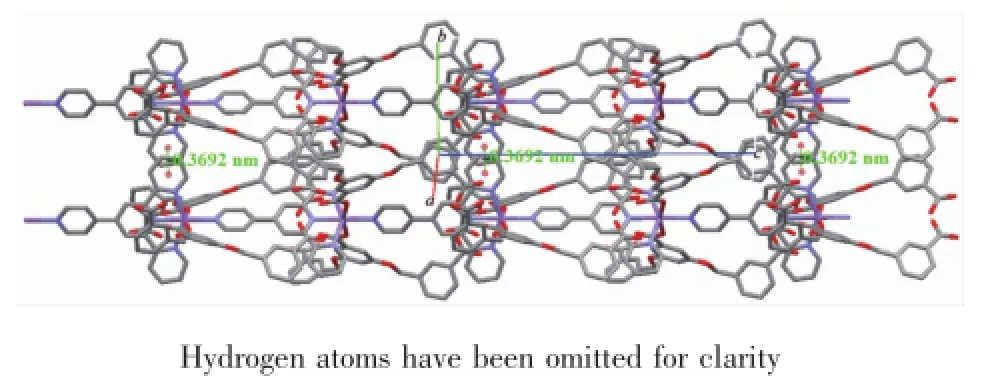
Fig.3 2D layer structure of complex 1 constructed by the π…πstacking interactions
2.2Powder XRD
The experimental PXRD patterns of complexes 1 and 2 match with their simulated ones,indicating the phase purity of the bulk materials.The variation in reflection intensities among the simulated and experimental patterns is due to the superiororientation of the powder sample during collection of the experimental PXRD data(Fig.4).

Fig.4 PXRD patterns of simulated and experimental of complexes 1 and 2
2.3TG analysis

Fig.5 TG curve of complex 1
The thermal stabilities of 1 and 2 are similar,so the TGA of 1 is described as the typical example.The thermal stability of complex 1 in air was examined by the TG techniques in the temperature range of 40~1 000℃.As shown in Fig.5,the complex 1 is thermally stable upon heating to 260℃.Then themain weight loss happened from 260 to 915℃corresponding to the release ofH2bcb-and PYTPY ligandswith aweight lossof94.90%(Calcd.95.14%:H2bcb-69.53%,PYTPY 25.61%).
2.4Magnetic properties
Temperature dependence of the molar magnetic susceptibilityχMof complexes 1 and 2 weremeasured in the temperature range of 2~300 K at 0.1 T and are shown asχM-1vs T andχMT vs T plots in Fig.6.

Fig.6 Plots ofχM-1vs T(○)andχMT vs T((Ⅱ))for 1 and 2
For complex 1,as the temperature decreases from room temperature,χMT is 0.34 cm3·mol-1·K,after which the value decreases until a value of 0.08 cm3· mol-1·K is reached at~2 K.The decline in theχMT value from 300 K is due to an antiferromagnetic(AFM)interaction between the neighboring Co2+centers[19]. The temperature dependence of the reciprocal susceptibility(χM-1)is fitted according to the Curie-Weiss law,χMT=C/(T-θ),affording a Curie constant C=0.578 7 cm3·mol-1·K and Weiss temperatureθ= -249.2 K.The negativeθvalue can be attributed to both AFM and exchange coupling.
For complex 2,theχMT product has values of 4.82 cm3·mol-1·K at 300 K,and undergoes a gradual decrease down to 3.8 cm3·mol-1·K at 8 K,due to the Boltzmann depopulation of the excited states and simultaneous population of the antiferromagnetically coupled ground state.Upon further cooling,χMT drops sharply,suggesting AFM interactions between the chains or the saturation effect[20].Themolar susceptibilities of Mn obey the Curie-Weiss law perfectly, with C=4.8 cm3·mol-1·K andθ=-12.97 K.Thenegative θvalue can be attributed to both AFM and exchange coupling.
3 Conclusions
In this work,two new complexes based on H3bcb and PYTPY ligandswere achieved under hydrothermal conditions.Complexes 1 and 2 possess 1D chain structures.It isworth noting that in this systemπ…π stacking has an important influence in linking the low-dimensional(1D)entities into a high dimensional (2D)structure.Moreover,the intramolecular and intermolecular hydrogen bonding interactions consolidated the 2D supramolecular network.In addition, magneticmeasurements on complexes 1 and 2 confirm a strong AFM behavior below 2 K and 8 K,respectively.In summary,our research demonstrates further that the ligands H3bcb and PYTPY could be a potential building block to construct novel coordination compounds with unusual architectures and interesting physical properties.
References:
[1]Tranchemontagne D J,O′Keeffe M,YaghiOM,et al.Chem. Soc.Rev.,2009,38:1257-1283
[2]Liu JW,Chen L F,CuiH,etal.Chem.Soc.Rev.,2014,43: 6011-6061
[3]Sun Q F,Iwasa J,Ogawa D,et al.Science,2010,328:1144-1147
[4]Desiraju GR.J.Am.Chem.Soc.,2013,135:9952-9967
[5]Zhang C L,Zhang M D,Qin L,et al.Cryst.Growth Des., 2014,14:491-499
[6]Qin L,Ju Z M,Wang Z J,et al.Cryst.Growth Des.,2014, 14:2742-2746
[7]Sun C Y,Wang X L,Qin C,et al.Chem.Eur.J.,2013,19: 3639-3643
[8]Pan Z R,Zheng H G,Wang TW,etal.Inorg.Chem.,2008, 47:9528-9536
[9]QIAO Yu(乔宇),MA Bo-Nan(马博男),LIXiu-Ying(李秀颖), et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31(6): 1245-1251
[10]Sun Y C,Wang Y F,Li Z,etal.J.Mol.Struct.,2014,1074: 416-421
[11]Liu J J,Lin Y J,Jin G X,et al.Organometallics,2014,33: 1283-1290
[12]Figgemeier E,Merz L,Hermann B A,et al.J.Phys.Chem. B,2003,107:1157-1162
[13]Leandro M O,Lourenco,Filipe A,et al.Acta Crystallogr. Sect.E,2015,71:330-335
[14]Hiroki K,ToshiN.Inorg.Chem.,2009,48:8593-8602
[15]SADABS,Bruker AXS Inc.,Madison,Wisconsin,USA, 1997.
[16]Sheldrick G M.SHELXS-97,Program for the Solution of Crystal Structure,University of Göttingen,Germany,1997.
[17]Sheldrick G M.SHELXS-97,Program for the Refinement of Crystal Structure,University of Göttingen,Germany,1997.
[18]LIXiu-Ying(李秀颖),HU Bo(胡波),CHE Guang-Bo(车广波),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30 (12):2818-2824
[19]Ganguly S,Mondal R.Cryst.Growth Des.,2015,15:2211-2222
[20]Zeng M H,Zhou Y L,Wu M C,et al.Inorg.Chem.,2010, 49:6436-6442
Hydrothermal Syntheses and Crystal Structures of Two Com p lexes Constructed from 3,5-Bis((4′-carboxylbenzyl)oxy)benzoic Acid and 4′-(4-Pyridyl)-2,2′∶6′,2″-terpyridine M ixed Ligands
QIAO Yu1,2WEIBing1WANG Lu-Yao1LIXiu-Ying1,2CHEGuang-Bo*,1,2LIU Chun-Bo*,3ZHANG Xing-Jing1,2
(1College of Chem istry,Jilin Normal University,Siping,Jilin 136000,China) (2Key Laboratory of Preparation and Applications of Environmental Friendly Materials (Jilin Normal University),Ministry of Education,Changchun 130103,China)
(3Institute of Green Chemistry&Chemical Technology,Jiangsu University,Zhenjiang,Jiangsu 212013,China)
Two transition metal complexes based on 3,5-bis((4′-carboxylbenzyl)oxy)benzoic acid(H3bcb)and 4′-(4-pyridyl)-2,2′∶6′,2″-terpyridine(PYTPY)mixed ligands,namely,[Co(H2bcb)2(PYTPY)]n(1)and[Mn(H2bcb)2(PYTPY)]n(2),have been synthesized under hydrothermal conditions and characterized by elemental analysis, infrared spectrum and single-crystal X-ray diffraction.Complexes 1 and 2 exhibit one-dimensional chain structures,respectively.Furthermore,these two complexes exhibit excellent thermal stabilities.They also display antiferromagnetic interaction below 2 K and 8 K confirmed throughmagnetic susceptibilitymeasurements.CCDC: 1439250,1;1439249,2.
transitionmetal complex;3,5-bis((4′-carboxylbenzyl)oxy)benzoic acid;4′-(4-pyridyl)-2,2′:6′,2″-terpyridine; crystal structure;magnetic property
O614.81+2;O614.7+11
A
1001-4861(2016)07-1261-06
10.11862/CJIC.2016.164
2016-01-20。收修改稿日期:2016-05-19。
国家自然科学基金(No.21576112)、吉林省科技厅(No.20130521019JH,201215219,20150623024TC-19)、吉林省教育厅(吉教科合字[2014]第152号)和四平市科技局(No.2015049)资助项目。
*通信联系人。E-mail:guangboche@jlnu.edu.cn,liuchunbo431@gmail.com

