掺杂和取代对红色荧光材料SrA l12O19∶M n4+发光性能的影响
辛小东 魏恒伟 赵文慧 刘中仕 李文先*, 焦 桓*, 荆西平*,
(1内蒙古大学化学与化工学院,呼和浩特010021)
(2陕西省大分子科学重点实验室,陕西师范大学化学与化工学院,西安710062)
(3稀土材料化学与应用国家重点实验室,北京大学化学与分子工程学院,北京100871)
(4欧司朗(中国)有限公司,上海200082)
掺杂和取代对红色荧光材料SrA l12O19∶M n4+发光性能的影响
辛小东1魏恒伟2赵文慧3刘中仕4李文先*,1焦桓*,2荆西平*,3
(1内蒙古大学化学与化工学院,呼和浩特010021)
(2陕西省大分子科学重点实验室,陕西师范大学化学与化工学院,西安710062)
(3稀土材料化学与应用国家重点实验室,北京大学化学与分子工程学院,北京100871)
(4欧司朗(中国)有限公司,上海200082)
SrAl12O19∶Mn4+是一种用于高显色性白光发光二极管的候选红色荧光材料。本论文研究了Mg2+、Zn2+和Ge4+离子的掺杂效应以及Ga3+、Ca2+和Ba2+离子的取代效应对SrAl12O19∶Mn4+荧光材料性能的影响。样品通过高温固相反应制备,焙烧温度在1 250~1 500℃之间。利用X射线衍射技术表征了材料的相纯度,用荧光激发光谱和发射光谱表征了材料的荧光性能。研究结果指出,与未进行Mg2+或Zn2+掺杂的样品相比,Mg2+或Zn2+离子对A l3+格位的掺杂可以使材料的发光强度提高~60%,其原因被认为是掺杂促进了激活剂Mn4+离子进入晶格,其过程可以表示为:MO+MnO2⇌MAl′+MnAl·+3OO×(M=Mg,Zn),电子顺磁共振谱支持这一结果。Ge4+离子的掺杂使材料的发光性能明显下降。Ga3+离子可以取代Al3+离子形成全范围的固溶体,其中少量Ga3+离子的掺杂可以使材料的荧光发射强度提高~13%,而掺杂量进一步提高使材料的荧光性能下降。Ca2+和Ba2+对Sr2+的取代仅形成有限范围的固溶体。Ca2+的取代使材料的发光性能提高;而Ba2+的取代使材料的发光强度下降。
红色荧光粉;白光发光二极管;SrAl12O19;Mn4+掺杂
0 Introduction
White light-emitting diodes(W-LEDs)are considered as the next generation solid-state lightings, because they havemany advantages over conventional lighting devices,such as high brightness,high power efficiency,low applied voltage,and long lasting life[1-2]. In 1996,Nakamura and Fasol in Nichia combined a blue LED chip with a yellow phosphor Y3Al5O12∶Ce3+(YAG∶Ce)produced a white lighting[3].After then,the researches on the W-LED phosphors are blooming like mushrooms after rain[4-9].However,the color rendering index(Ra)of theW-LEDs assembled by the blue LED chips with YAG∶Ce yellow phosphor is not high(Ra≤85)for the warm white LED applications because their emission spectra are lack of red components[10]. Therefore,for improving solid state lighting technology,it is imperative to explore novel red phosphors suitable for the LED excitations.
Many researches on the red phosphors for theWLED applications have been reported[11-14].Eu2+doped sulfide red phosphors(such as CaS∶Eu2+)were firstly considered[15-16].This kind of phosphors show emission maximum at~620 nm under the blue light excitation, which are suitable for the use in W-LEDs. However,due to their unstability,their applications in W-LEDs were limited.Eu3+doped molybdate red phosphors[11-12,17-18](such as CaMoO4∶Eu3+)also attracted a lot of interests.The excitation efficiency of these phosphors was not very high due to the f-f transitions of Eu3+in near UV and blue range.Recently,Mn4+doped fluoride phosphors(such as K2TiF6∶Mn4+[19]) attracted scientists′interests.These phosphors were synthesized in aqueous solutions,thus their thermal stability needed to be improved.Actually,Eu2+doped nitride and oxynitride red phosphors(such as Sr2Si5N8∶Eu2+[20],SrSi2N2O2∶Eu2+[21]and Sr3Si2O4N2∶Eu2+[22])played the important role for improving Ra index ofW-LEDs. These phosphors are normally synthesized at high pressure with O2and H2O free conditions,whichmake the phosphors very expensive.
It is a strong aspiration to find low cost red phosphors for the W-LED applications.Nowadays, Mn4+doped oxide phosphors come into researchers′sight.The red phosphors 3.5MgO·0.5MgF2·GeO2∶Mn4+[23]and 6MgO·As2O5∶Mn4+[24]were early reported in 1950′s~60′s,which could be excited by the blue light and gave emission maximum at 655 nm.Since Ge is an expensive element and As oxides are toxic,these two phosphors are not satisfied for the W-LED applications.Recently-reported red phosphors of alkaline earth aluminates(such as CaAl12O19∶Mn4+[25-26], SrAl12O19∶Mn4+[27],and Sr4Al14O25∶Mn4+[28])maybeeffective alternatives.Actually,CaAl12O19∶Mn4+and SrAl12O19∶Mn4+were early reported in 1970′s[29].Among these aluminate phosphors,SrAl12O19∶Mn4+has been reported on the use in the W-LED fabrication by coating this phosphor on a commercial blue LED chip+YAG∶Ce yellow phosphor[27],which suggested that SrAl12O19∶Mn4+is a promising red phosphor candidate for fabricating W-LEDs with high Ra index.Actually,this phosphor has better excitation efficiency around 330 nm,thus it ismore suitable to be used as a red component in the tri-colorW-LEDs assembled by UV-LED chips.Itwas reported that the emissions of the UV-LEDs could cover from 410 nm down to 320 nm[30-31].However,it is imperative to further improve the luminescence properties of this phosphor.
In this study,the doped samples on Al site (SrAl11.99-xMxO19∶0.01Mn4+)and replaced sampleson both Al site[SrAl11.91(1-y)M11.91yO19∶0.01Mn4+,0.08Mg2+]and Sr site(Sr1-zMzAl11.91O19∶0.01Mn4+,0.08Mg2+)were synthesized by conventional solid state reactions,where M represents variousmetal ions.For the doping systems, x varied less than 0.12,whereas for the replacing systems,y and z varied in the whole ranges from 0.00 to 1.00.In the replacing systems,small amount of Mg2+was added for charge compensation.The phase relations of the samples were analyzed by X-ray diffraction(XRD)and the photoluminescence(PL) properties were investigated by PL excitation and emission spectra.
1 Experimental
1.1Samp le preparation
SrCO3,Al2O3and MnCO3were used as starting materials for preparing SrAl11O19∶Mn4+phosphors, meanwhile H3BO3was selected as flux.Mg(OH)2· 4MgCO3·6H2O,ZnO,GeO2,Ga2O3,CaCO3and BaCO3were chosen as the doping or replacing reagents. Among the above chemicals,Mg(OH)2·4MgCO3·6H2O, ZnO,MnCO3and H3BO3are in A.R.(analytical reagent)grade;GeO2and Ga2O3are in 4N(99.99%) grade;all others are in L.P.(luminescent pure)grade. All the chemicals were weighed in the stoichiometric ratio with 4%(w/w)H3BO3as flux and ground in an agate mortar with a pestle.After fully ground,the samples were put into corundum crucibles and then heated in an muffle furnace.For most samples,the heating process was conducted at 1 500℃for 5 h, while for the samples in the Ga replacing system SrAl11.99(1-y)Ga11.99yO19∶0.01Mn4+,0.08Mg2+,when y varied in the range less than 0.60,the heating process was conducted at1 500℃for 5 h,whereas when y varied in the ranges from 0.60 to 1.00,the heating temperature was reduced to 1 250℃(3 h)in order to avoid sample melting.During the heating processes, Mn2+in MnCO3was oxidized to Mn4+.
1.2Sam p le characterization
The phase purity of the samples was characterized using an X-ray diffractometer(XRD, Rigaku D/Max2400,Japan)with Cu Kαradiation(λ= 0.154 18 nm,40 kV and 100 mA).The data were collected with the scanning rate 2°·min-1.PL excitation and emission spectra of the phosphor samples were measured on a Hitachi F7000 fluorescence spectrophotometer(Japan)equipped with a 150W Xe lamp as an excitation source and the PL intensitieswere calculated by integrating all emissions in the spectra.Mn2+ions in the samples were checked by an electron paramagnetic resonance spectroscope (EPR,Bruker EMX,Germany)with frequency of 9.442 076 GHz.
2 Results and discussion
2.1PL properties of the Al-site doped system s
Initially,the optimized activator content of Mn4+was investigated.The experimental data indicated that in the SrAl12-wO19∶w Mn4+system,the PL intensity approached maximum at w=0.01(the data are not shown).Fig.1(a)represents a typical XRD pattern in the system(for the sample SrAl11.99O19∶0.01Mn4+).The pattern matches perfectly with that of the related JCPDS data 26-976,indicating that the doped sample prepared in this work is the pure phase of SrAl12O19. The hostmaterial SrAl12O19has the magnetoplumbitetype structure with the hexagonal unit cell(space group P63/mmc):a=0.556 66 nm,c=2.200 18 nm(Fig. 2)[32].This structure is constructed by four closepacked O layers and one close-packed SrO3layer with the sequence(chhhc)2,in which c refers the cubic close packing layer and h refers the hexagonal close packing layer.Most of the Al atoms take octahedroninterstitial positions and tetrahedron-interstitial positions,forming AlO6octahedrons and AlO4tetrahedrons,respectively.Whereas in the SrO3layer, the Al atoms take the trigon-interstitial positions constructed by three O atoms,forming AlO5trigonal bipyramids combined with two O atoms in the two adjacent O layers.The number ratio nAlO6∶nAlO4∶nAlO5=9∶2∶1.In the SrAl12O19host,there are two cations:Sr2+has the radius of 0.113 nm(CN=6)and Al3+has the radius of 0.053 5 nm(CN=6)[33].The radius of the activator Mn4+is 0.053 nm(CN=6)[33],similar to that ofAl3+,thus we believe that in the SrAl11.99O19∶0.01Mn4+phosphors,Mn4+is doped into the Al3+site.Obviously the valences of Mn4+and Al3+are different,thus doping Mn4+into Al3+may cause defects VAl‴or Oi″for balancing the charge difference:3MnO2⇌3MnAl·+ VAl‴+6OO×or 2MnO2⇌2MnAl·+Oi″+3OO×.But at this stage,we could not give details about the defect formations.

Fig.1 XRD patterns of selected samples in the SrAl12-wO19:w Mn4+system(a),the SrAl11.99-xMxO19∶0.01Mn4+(M=Mg,Zn,Ge) systems(b~d)and related JCPDS data(e)

Fig.2 Projection of the crystal structure of SrAl12O19
The PL excitation and emission spectra of SrAl11.99O19∶0.01Mn4+are represented in Fig.3.In SrAl12O19,most of the Al3+ions take the octahedral sites and it is believed that the Mn4+ions are likely located at Al3+sites.As we know that Mn4+has d3configuration,approximately the Tanabe-Sugano diagram of the d3configuration in the octahedral crystal field[34-35]can be consulted for the Mn4+spectrum assignment.Refer to the Tanabe-Sugano diagram as well as Brik′s work for CaAl12O19∶Mn4+[36]and Y2Sn2O7∶Mn4+[37],as well as Wang′s work for SrAl12O19∶Mn4+[27],the assignments for the excitation and emission spectra are given in Fig.3.The strongest excitation band at 330 nm is assigned to the4A2→4T1transition overlapped by the charge transfer transition Mn4+←O2-;the excitation bands at 395 and 465 nm are assigned to the4A2→2T2transition and the4A2→4T2transition,respectively.These three excitation bands wellmatch the emission bands of themost used LEDs, such as GaN(λEM=465 nm),InGaN(λEM=400 nm),and AlGaN/AlInGaN(λEM=330 nm),respectively[27].In the emission spectrum,two strong line emissions appear at 659 nm and 643 nm with some shouldered emissions, which are assigned to the2T1,2E→4A2transitions with the phonon sideband transition.

Fig.3 Excitation(EX)and emission(EM)spectra of SrAl11.99O19∶0.01Mn4+

Fig.4 Variations of the PL intensity with the M contents x in the SrAl11.99-xMxO19∶0.01Mn4+(M=Mg,Zn,Ge) systems
Doping effects of Mg2+,Zn2+and Ge4+on the PL properties of the SrAl11.99-xMxO19∶0.01Mn4+(M=Mg,Zn, Ge;x=0.0~0.12)systems were investigated.XRD data indicated that all the samples in the studied region were phase pure and XRD patterns for some selected samples are shown in Fig.1.The excitation and emission spectra of all the samples are identical to those shown in Fig.3and the variations of the PL intensity with the M contents are represented in Fig.4. For the Mg2+and Zn2+doped systems,the doping increases the PL intensities.The optimized doping contents are x=0.08 for the Mg2+doped system and x= 0.06 for the Zn2+doped system,respectively,and the PL intensities are enhanced about 60%,compared with the sample without the Mg2+or Zn2+doping.The Ge4+doping reduces the PL intensity.For the sample with x=0.05,the PL intensity is reduced about 30%. The Mg2+,Zn2+and Ge4+have radii of 0.072,0.088 and 0.067 nm(CN=6)[33]and they are all slightly larger than that of Al3+,butmuch smaller than that of Sr2+,thus it is deduced that these three cations prefer to take the Al3+positions.It is mentioned above that the Mn4+doping causes charge imbalance in the lattice.Doping Mg2+or Zn2+with Mn4+may play the role of charge compensation:MO+MnO2⇌MAl′+MnAl·+ 3OO×(M=Mg,Zn).Due to this charge composition effect,Mg2+and Zn2+may help Mn2+change its valence to Mn4+and also help Mn4+enter the lattice,so that the PL intensity increases.EPRmeasurements support the above discussion.Fig.5shows the EPR spectra of SrAl12O19∶Mn4+with and without Mg2+/Zn2+doping.For the Mg2+/Zn2+undoped sample,the typical sestet EPR signal of Mn2+(g=2.241)is clearly observed,whereas for the Mg2+or Zn2+doped sample,such sestet signal disappeared.This discussion for the Mg2+doping were also given by Pan[25]and Murata[38]for CaAl12O19∶Mn4+and Wang[27]for SrAl12O19∶Mn4+,respectively.The Ge4+co-dopingmay cause charge imbalance even more,so that the PL intensity decreases.
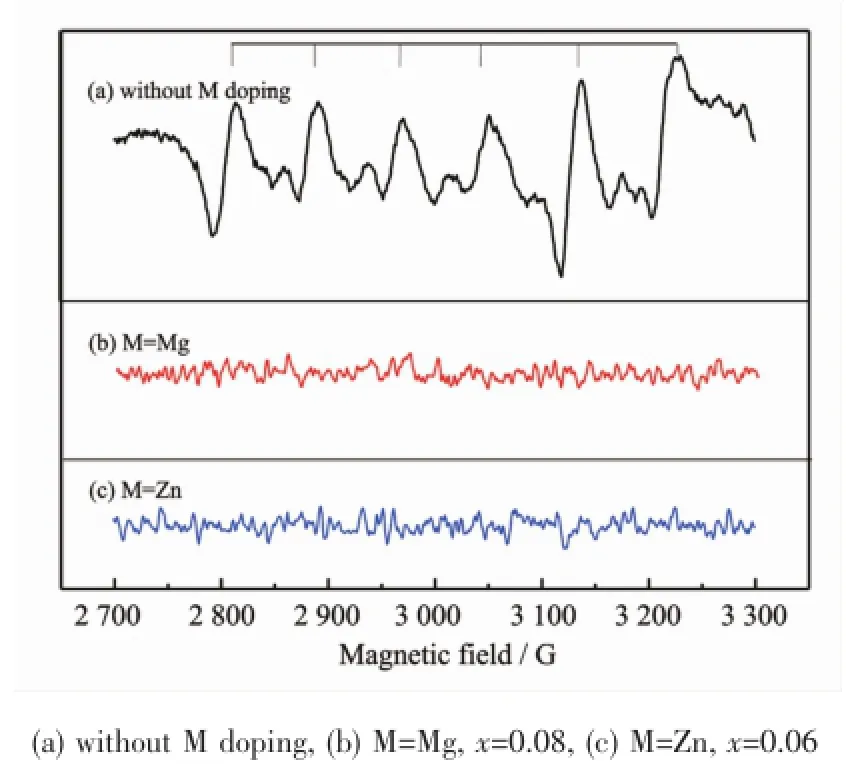
Fig.5 EPR spectra of SrAl11.99-xMxO19∶0.01Mn4+
2.2PL properties of the Al site replaced system
PL properties of the Al site replaced system SrAl11.91(1-y)Ga11.91yO19∶0.01Mn4+,0.08Mg2+were explored. SrGa12O19also has hexagonal unit cell with isostructure to SrAl12O19.It has space group P63/mmc with the cell parameters a=0.579 6 nm,c=2.284 nm (JCPDS 26-983).The XRD patterns of all the samples in the system are in accordance with JCPDS 26-976 for SrAl12O19and JCPDS 26-983 for SrGa12O19(Fig.6) and with an increase of the Ga3+content y from 0.00 to 1.00,the XRD peaks shift to lower 2θvalues gradually.Based on these patterns,the unit cell volumes were calculated and the variation of the cell volume with the Ga3+content y is represented in Fig.7. The cell volume increases continuously with an increase of the Ga3+content,since the radius of Ga3+(0.062 nm,CN=6)[33]is larger than that of Al3+,which indicates that the whole range solid solutions are formed in this system.Apparently Ga3+and Al3+have the same valence,thus the replacement of Ga3+for Al3+neither causes new charge imbalance,nor compensates the charge imbalance induced by the Mn4+doping.The PL intensity varies with the Ga3+content y,as shown in Fig.8.The inset of Fig.8shows when small amount of Ga3+is doped(Ga3+content y varies from 0.000 4 to 0.004 0),the PL intensity may increase,e.g.at y=0.000 8,the PL intensity increases about 13%.Whereas when the y value further increases,the PL intensity dramatically decreases.For the Mn4+doped SrGa12O19,no luminescence was observed.

Fig.6 XRD patterns of selected samp les in the SrA l11.91(1-y)Ga11.91yO19∶0.01Mn4+,0.08Mg2+system and related JCPDS data
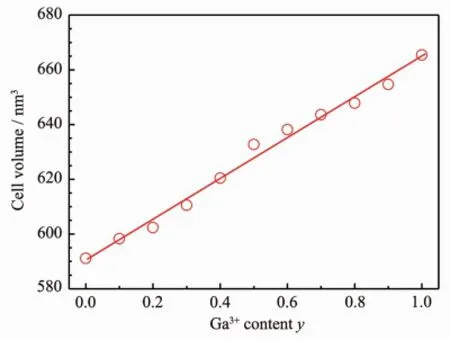
Fig.7 Dependence of the unit cell volume on the Ga3+content y in the SrAl11.91(1-y)Ga11.91yO19∶0.01Mn4+,0.08Mg2+system

Fig.8 Variation of the PL intensitywith the Ga3+content y in the SrAl11.91(1-y)Ga11.91yO19∶0.01Mn4+, 0.08Mg2+system
2.3PL propertiesof the Sr site replaced systems
As we know,CaAl12O19and BaAl12O19also have the iso-structure to SrAl12O19(JCPDS 38-470 for CaAl12O19and JCPDS 26-135 for BaAl12O19). Consequently,the PL properties of the Sr site replaced systems Sr1-zMzAl11.91O19∶0.01Mn4+,0.08Mg2+(M=Ca,Ba)were studied.Selected XRD patterns in the Ca2+and Ba2+replacing systems are represented in Fig.9and Fig.10,respectively.In the Ca2+replacing system,the patterns are in accordance with JCPDS 26-976 for SrAl12O19and JCPDS 38-0470 for CaAl12O19.The XRD peaks slightly shift to higher 2θ values.Based on these patterns,the unit cell volumes were carefully calculated,shown in Fig.11.The data indicate that in the Sr1-zCazAl11.91O19∶0.01Mn4+,0.08Mg2+system,only limited rather than the whole range solid solutions are formed:the solid solutions appear in the ranges 0.00<z<0.80 and 0.90<z<1.00,and the twophase range is narrow in the range 0.80<z<0.90.In the Ba2+replacing system,for the samples with the Ba2+content z near 0.00,the patterns agree with JCPDS 26-976 for SrAl12O19;while for the samples with the Ba2+content z near 1.00,the patterns agree with JCPDS 26-135 for BaAl12O19.For other samples,clearly the patterns are mixtures of those of SrAl12O19and BaAl12O19.The calculations for the unit cell volumes indicate that in the Sr1-zBazAl11.91O19∶0.01Mn4+, 0.08Mg2+system,the two-phase range become wider, 0.10<z<0.80 and the solid solutions appear only in the narrow ranges 0.00<z<0.10 and 0.80<z<1.00(Fig.11). In the solid solution ranges for the Ca2+replacing system,the cell volume decreases with the Ca2+content z,because Ca2+is smaller than Sr2+;while in the solid solution ranges for the Ba2+replacing system, with an increase of the Ba2+content z,the cell volume increases since Ba2+is larger than Sr2+.
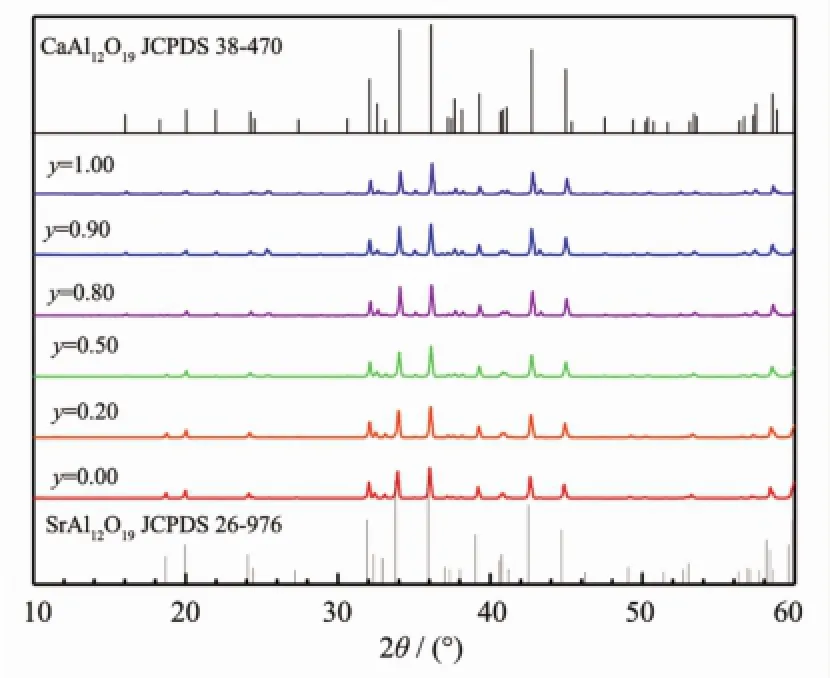
Fig.9 XRD patterns of selected samples in the Sr1-zCazAl11.91O19∶0.01Mn4+,0.08Mg2+system and related JCPDSdata
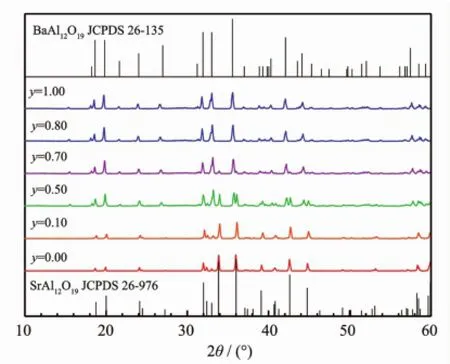
Fig.10 XRD patterns of selected samples in the Sr1-zBazA l11.91O19∶0.01Mn4+,0.08Mg2+system and related JCPDS data

Fig.11 Dependences of the unit cell volumes on the M content z in the Sr1-zMzAl11.91O19∶0.01Mn4+, 0.08Mg2+(M=Ca,Ba)systems

Fig.12 Variations of the PL intensitieswith the M content z in the Sr1-zMzAl11.91O19∶0.01Mn4+,0.08Mg2+(M=Ca,Ba)systems
The PL intensity is observed to increase constantly with increasing the Ca2+content z(Fig.12) and the peak wavelength has a slight blue shift:the main peak shifts from 659 nm for the Sr phase to 655 nm for the Ca phase(the inset in Fig.12).Since Mn4+doped BaAl12O19does not presentany luminescence,in the Ba2+replacing system Sr1-zBazAl11.91O19∶0.01Mn4+, 0.08Mg2+,the PL intensity falls steadily(Fig.12)and no peak wavelength shift is observed.
3 Conclusions
The luminescence properties were investigated for the doping systems on the Al site(SrAl11.99-xMxO19∶0.01Mn4+,M=Mg,Zn,Ge)and replacing systems on the Al site[SrAl11.91(1-y)M11.91yO19∶0.01Mn4+,0.08Mg2+,M= Ga)]and the Sr site(Sr1-zMzAl11.91O19∶0.01Mn4+,0.08Mg2+, M=Ca,Ba).When Mg2+or Zn2+was doped into the lattice,the PL intensity increased significantly(~60%),compared with the sample without the Mg2+or Zn2+doping,since this doping helped Mn4+enter the lattice:MO+MnO2⇌MAl′+MnAl·+3OO×(M=Mg,Zn), which was supported by EPR spectra.Ga3+could replace Al3+to form whole range solid solutions. Whereas doping small amount of Ga3+at the Al site could increase the PL intensity(~13%);however further increasing the Ga3+content,the PL intensity decreases dramatically.The solid solutions only appeared in limited ranges for the Ca2+and Ba2+replacing systems.For the Ca2+replacing system,the PL intensity increased with the Ca2+content,but for the Ba2+replacing system,the PL intensity decreased with Ba2+content.In general,the Eu2+activated(oxy) nitride phosphorsand Mn4+activated fluoride phosphors have better luminescence properties than the Mn4+doped aluminate phosphor studied in the work for the W-LED applications.However,the raw materials of this aluminate phosphors are cheap and the synthesis process for them is simple and convenient,thus the optimized SrAl12O19∶Mn4+would be used as a red phosphor for the low-priceW-LED products.
Acknow ledgments:We are thankful for financial supports from Osram(China)Ltd.,National Natural Science Foundations of China(Grant No.20861005 and 21371015)and Natural Science Foundations of Inner Mongolia Science Foundation (Grant No.2010 MS 0204).Thanks are also given to Ms.GUO Yu-Feng and Ms.WANG Ming-Xiao of Tsinghua University forEPRmeasurements.
References:
[1]Nakamura S,Mukai T,Senoh M.Appl.Phys.Lett.,2009,64: 1687-1689
[2]Jang H S,Won Y H,Jeon D Y.Appl.Phys.B,2009,95:715-720
[3]Nakamura S,Fasol G.The Blue Laser Diode:GaN Based Light Emitters and Lasers.Berlin:Springer,1997:216
[4]Liu J,Lian H,Shi C,et al.Chem.Lett.,2005,34:1340-1341
[5]Gao G J,Reibstein S,Wondraczek L,et al.J.Mater.Chem., 2011,21:3156-3161
[6]Guo H,Zhang H,Li F,et al.Opt.Express,2010,18:27257 -27262
[7]Schubert E F,Kim JK.Science,2005,308:1274-1278
[8]Smet P F,Parmentier A B,and Poelman D.J.Electrochem. Soc.,2011,158:R37-R54
[9]WangW N,Ogi T,Okuyama K,etal.J.Mater.Chem.,2011, 21:5183-5189
[10]Chiang C C,TsaiM S,Hon M H.J.Alloys Compd.,2007, 431:298-302
[11]Neeraj S,Kijima N,Cheetham A K.Chem.Phys.Lett.,2004, 387:2-6
[12]Wang JG,Jing X P,Lin JH,et al.J.Electrochem.Soc., 2005,152:G186-G188
[13]Pang M L,Lin J,Yu M.J.Solid State Chem.,2004,177: 2237-2241
[14]Macalik L,Maczka M,Majchrowski A,et al.J.Alloys Compd.,2004,380:248-254
[15]Lehmann W,Ryan F M.J.Electrochem.Soc.,1971,118: 477-482
[16]Soules T F,Beers W W,Duggal A R,et al.US Patent, 6252254 B1,2001-06-26
[17]Wang Z L,Liang H B,Su Q,et al.Electrochem.Solid-State Lett.,2005,8:H33-H35
[18]Wang JG,Jing X P,Lin JH,etal.J.Lumin.,2006,121:57-61
[19]Zhu H,Liu R S,Chen X Y,et al.Nature Comm.,2014,5: 4312(1-10)
[20]Xie R J,Hirosaki N,Mitomo M,et al.Chem.Mater.,2006, 18:5578-5583
[21]Han BY,Sohn K S.Electrochem.Solid-State Lett.,2010,13: J62-J64
[22]Wang X M,Wang C H,Jing X P,et al.Inorg.Chem.,2012, 51:3540-3547
[23]Kemeny G,Haake CH.J.Chem.Phys.,1960,33:783-788
[24]Klasens H A.Philips Res.Rep.,1954,9:377-390
[25]Pan Y X,Liu G K.J.Lumin.,2011,131:465-468
[26]ShuW,Jiang L L,Ding JW,etal.Mater.Sci.Eng.,B,2012, 177:274-277
[27]Wang L,Xu Y D,Wang Y H,et al.Phys.Status Solidi A, 2013,210:1433-1437
[28]Xu Y D,Wang D,Qi S,et al.J.Alloys Compd.,2013,550: 226-230
[29]Bergstein A,White W B.J.Electrochem.Soc.,1971,118: 1166-1171
[30]Steigerwald DA,Bhat JC,RudazSL,etal.IEEE J.Quantum. Electron.,2002,8:310-320
[31]Adivarahan V,Chitnis A,Shur M S,et al.App l.Phys.Lett., 2001,79:4240-4242
[32]Kimura K,OhgakiM,Marumo F,et al.J.Solid State Chem., 1990,87:186-194
[33]Shannon R D.Acta Crystallogr.A,1976,A32:751-767
[34]Tanabe Y,Sugano S.J.Phys.Soc.Jpn.,1956,11:864-877
[35]Tamatani M.Phosphor Handbook.Shionoya S,Yen W M Ed.,Boca Raton(USA):CRC Press,1998:153-176
[35]Brik M G,Pan Y X,Liu G K.J.Alloys Compd.,2011;509: 1452-1456
[37]Brik M G,Srivastava A M,Avram N M.Opt.Mater.,2011, 33:1671-1676
[38]Murata T,Tanoue T,Hase T,et al.J.Lumin.,2005,114: 207-212
Doping and Replacing Effects on the Lum inescent Properties of SrAl12O19∶M n4+Red Phosphor
XIN Xiao-Dong1WEIHeng-Wei2ZHAOWen-Hui3LIU Zhong-Shi4LIWen-Xian*,1JIAO Huan*,2JING Xi-Ping*,3
(1College of Chemistry and Chemical Engineering,Inner Mongolia University,Hohhot 010021,China)
(2Key Laboratory of Macromolecular Science of Shaanxi Province,College of Chemistry and Chem ical Engineering, Shaanxi Normal University,Xi′an 710062,China)
(3State Key Laboratory of Rare Earth Materials and Applications,College of Chemistry and Molecular Engineering, Peking University,Beijing 100871,China)
(4Osram(China)Ltd.,Shanghai200082,China)
SrAl12O19∶Mn4+is a potential candidate of red phosphor for high color rendering white LEDs.In this work,doping effects of Mg2+,Zn2+or Ge4+and replacing effects of Ga3+,Ca2+or Ba2+on the SrAl12O19∶Mn4+red phosphor were investigated.The sampleswere prepared by conventional solid state reactions at 1 250~1 500℃. The phase purities of the samples were investigated by X-ray diffraction and their luminescence properties were characterized by photoluminescence(PL)excitation and emission spectra.The results indicated that compared with the sample without the Mg2+or Zn2+doping,the Mg2+or Zn2+doping enhanced the PL intensity significantly (~60%),since this doping helped Mn4+enter the lattice:MO+MnO2⇌MAl′+MnAl·+3OO×(M=Mg,Zn),which wassupported by electron paramagnetic resonance spectra.The Ge4+doping lead to a decrease of PL intensity clearly. Ga3+could replace Al3+to form whole range solid solutions.When small amount of Ga3+was doped,the PL intensity increased(~13%),however when further increasing the Ga3+content,the PL intensity decreased dramatically.The solid solutions only appeared in limited ranges for the Sr2+replaced systems by Ca2+and Ba2+. For the Ca2+replacing system,the PL intensity increased with an increase of Ca2+content,but for the Ba2+replacing system,the PL intensity decreased with Ba2+content.
red phosphor;white-LEDs;SrAl12O19;Mn4+doping
TB34
A
1001-4861(2016)07-1199-08
10.11862/CJIC.2016.169
2015-09-30。收修改稿日期:2016-06-07。
国家自然科学基金(No.21371015)资助项目。
*通信联系人。E-mail:E-mail:nmglwx@163.com,jiaohuan@snnu.edu.cn,xpjing@pku.edu.cn;会员登记号:S06N3161M1407。
——高建新教授

