3,5-双(4-羧基-苯氧基)苯甲酸与1,3-双(咪唑基)丙烷构筑的两个Cd(Ⅱ)金属有机骨架材料的合成、结构及荧光性质
赵 仑 陈瑞战 王子忱
(1吉林大学化学学院,长春130012) (2长春师范大学化学学院,长春130032)
3,5-双(4-羧基-苯氧基)苯甲酸与1,3-双(咪唑基)丙烷构筑的两个Cd(Ⅱ)金属有机骨架材料的合成、结构及荧光性质
赵仑1,2陈瑞战2王子忱*,1
(1吉林大学化学学院,长春130012) (2长春师范大学化学学院,长春130032)
以3,5-双(4-羧基-苯氧基)苯甲酸(H3BCPBA)、1,3-双(咪唑基)丙烷(bip)与硝酸镉为反应物,在水热、溶剂热条件下以不同的反应温度和不同的降温速率,分别合成出了2种金属-有机骨架(MOF)化合物:{[Cd1.5(BCPBA)(bip)]·H2O·DMF}n(1)和[Cd1.5(BCPBA) (bip)0.5(H2O)]n(2)。通过X射线单晶衍射(XRD)确定其结构,并用红外光谱(IR)、元素分析、热重(TG)和粉末X射线衍射仪(PXRD)等对其进行表征,并测试了2种化合物的荧光属性。结构表明化合物1中Cd1原子先与3个BCPBA3-配体配位,形成一个一维链结构,2个相邻的一维链通过Cd2原子由羧基氧桥接在一起。然后由含氮配体将一维的双链连结成一个二维的层结构。化合物2是一个由新颖的篮子型六连结的三核Cd(Ⅱ)二级结构单元(SBUs)构筑的二维层结构。
3,5-双(4-羧基-苯氧基)苯甲酸;1,3-双(咪唑基)丙烷;金属有机骨架材料;荧光
0 Introduction
In recent years,multifunctional metal-organic frameworks(MOFs)have become an attractive prospect[1].The major task for synthesizing such functional materials is to choose appropriate ligands (because of their inherent advantages of organic linkers)and inorganic metal cations.Multicarboxylate ligands are often selected as multifunctional organic linkers due to their abundant coordination modes to metal ions,allowing for various structural topologies[2]. Flexibly N-bridging ligands as auxiliary ligands can freely rotate and adopt a variety of conformations according to the restrictions imposed by the coordination geometry of themetal ions[3].
The length of a flexible ligand are always inconsistent in different MOFs.As a result,a large number of interesting frameworks incorporating multicarboxylate ligands and flexible ligands have been reported recently[4].For example,in accord with the subjects,3,5-bis(4-carboxy-phenoxy)benzoic acid (H3BCPBA)[2b,5]and 1,3-bis(imidazolyl)propane(bip)[6]were investigated for the construction ofmetal-organic coordination polymers.
In this article,with the aim of understanding the coordination chemistry of this versatile ligand and preparing new materials with interesting structural topologies and physical properties,we only focused our attention on the reactions with different solvents and investigated the influence of reaction temperature and decreased temperature rates on the structure of the resultant complexes.In this paper we describe the syntheses,crystal structures and properties of the MOFs,namely,{[Cd1.5(BCPBA)(bip)]·H2O·DMF}n(1), [Cd1.5(BCPBA)(bip)0.5(H2O)]n(2).Two compounds have been characterized by X-ray crystallography,IR spectra,TG and elemental analysis in detail.

Scheme 1 Molecular structures of ligands
1 Experimental
1.1M aterials and m ethods
All chemicals were of reagent grade quality obtained from commercial sources and used without further purification.TGA was performed on a Perkin-Elmer TG-7 analyzer heated from 30 to 650℃under nitrogen.The luminescent properties of these compounds were measured on a HITACHI F-7000 spectrometer.Elemental analyses(C,H,N)were performed with a Perkin-Elmer 240c elemental analyzer.Powder X-ray diffraction(PXRD)patterns were collected on a Bruker D2 PHASER X-ray diffractometer using Cu Kα(λ=0.154 18 nm)at a scan speed of 0.1°·s-1in the range of 5°~50°.IR spectra were obtained from KBr pellets on a Perkin-Elmer 580B IR spectrometer in the 400~4 000 cm-1region.
1.2Synthesis of{[Cd1.5(BCPBA)(bip)]·H2O· DM F}n(1)
A mixture of Cd(NO3)2·4H2O(0.030 8 g,0.1 mmol),H3BCPBA(0.039 4 g,0.1 mmol)and bip (0.017 6 g,0.1 mmol)were added to DMF(8 mL) and H2O(2mL),which was placed in a Teflon reactor (20 mL)and heated at 80℃for 3 days.After gradually cooling to room temperature at a rate of 10℃·h-1,large quantities of colorless block crystals were obtained and the crystals were filtered off, washed with anhydrous ethyl,and dried under ambient conditions.Yield:52%based on bip.Anal. Calcd.for C65H61Cd3N9O19(%):C,48.51;H,3.82;N, 7.83.Found(%):C,48.43;H,3.91;N,7.76 FTIR (KBr,cm-1):3 133(m),1 665(s),1 593(s),1 532(s),1 489(m),1 362(s),1 313(s),1 279(s),1 070(s),960 (m),784(s),656(m).
1.3Synthesis of[Cd1.5(BCPBA)(bip)0.5(H2O)]n(2)
A mixture of Cd(NO3)2·4H2O(0.030 8 g,0.1 mmol),H3BCPBA(0.039 4 g,0.1 mmol),bip(0.017 6 g,0.1 mmol)and H2O(10 mL)was placed in a Teflon reactor(20mL)and heated at 160℃for 3 days.After gradually cooling to room temperature at a rate of 20℃·h-1,large quantities of colorless block crystals were obtained and the crystals were filtered off,washed with ethanol,and dried under ambient conditions. Yield:61%based on bip.Anal.Calcd.for C51H38Cd3N4O18(%):C,45.98;H,2.88;N,4.21.Found (%):C,46.78;H,2.79;N,4.15.FTIR(KBr,cm-1): 3 133(m),1 596(s),1 665(s),1 592(s),1 365(s), 1 070(m),944(m),837(s),783(m),657(m),531(m).

Scheme 2 Crystallographically established coordinationmodes of the carboxylic groups in compounds 1 and 2
1.4X-ray crystallography
Single-crystal XRD data for compounds 1 and 2 were recorded on a Bruker Apex CCD diffractometer with graphite-monochromatized Mo Kαradiation(λ= 0.071 073 nm)at 293(2)K.Absorption corrections were applied using the multiscan technique.All the structures were solved by directmethod of SHELXS-97 and refined by the full-matrix least-squares techniques by using the SHELXL-97[7]program within WINGX.No-hydrogen atoms were refined with anisotropic temperature parameters.The hydrogen atoms of the organic ligands were refined as rigid groups.The detailed crystallographic data and structure refinement parameters for 1 and 2 are summarized in Table1.
CCDC:1052091,1;1052166,2.

Table1 Crystal data and structure refinement for 1 and 2

Table2 Selected bond lengths(nm)and angles(°)for com pounds 1 and 2
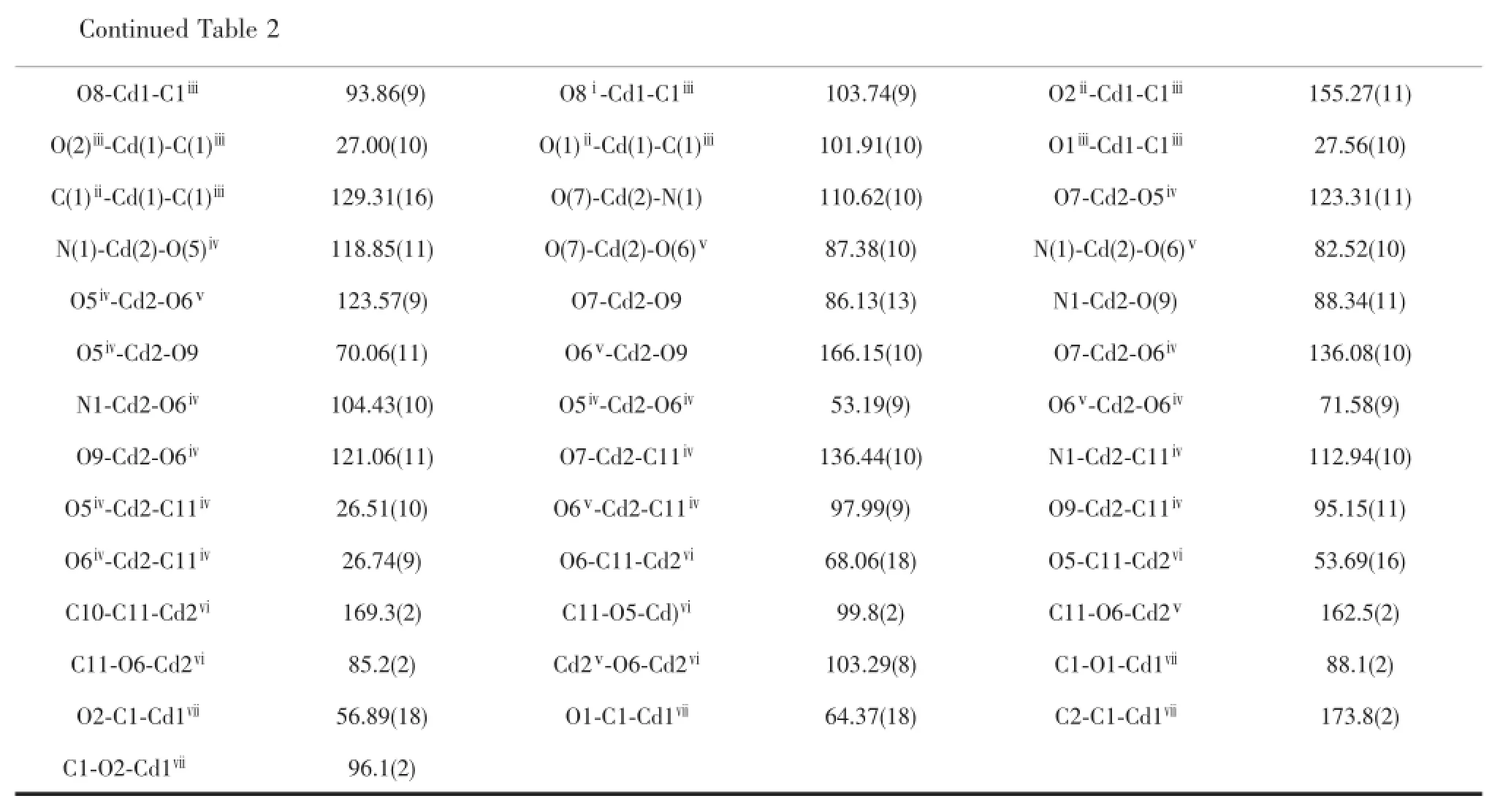
Symmetry codes:ⅰ-x+1,-y,-z+3;ⅱx+1/2,-y,z+1/2;ⅲx+1,y,z+1;ⅳx-1,y,z-1;ⅴx-1/2,-y,z-1/2;ⅵ-x+5/2,y,-z+7/2 for1;ⅰ-x-1,y,-z+1/2;ⅱ-x,y,-z+1/2;ⅲx-1,y,z;ⅳx-1/2,-y+1/2,z-1/2;ⅴ-x-1/2,-y+1/2,-z+1;ⅵx+1/2,-y+1/2,z+1/2;ⅶx+1,y,z for2
2 Results and discussion
2.1Structure description of 1
The crystal structure determination reveals that compound 1 crystallizes in a monoclinic space group P2/n.The asymmetric unit contains one and half Cd(Ⅱ)cation,one bip ligand,one deprotonated BCPBA3-ligand,one uncoordinated DMF and one H2O molecule,which are omitted for clarity(Fig.1a).The hydrogen atoms of OW1 were not located.In the asymmetric unit,the Cd1 cation is seven-coordinated by six oxygen atoms belonging to three separated BCPBA3-ligands as well as one nitrogen atom from bip ligands.While the other Cd2 cation is bismonodentate coordinated by two carboxylic O atoms from two BCPBA3-ligands and two nitrogen atoms from two bip ligands to form a distorted tetrahedron geometry(Fig.1a).In an asymmetric unit,three carboxylate groups of the BCPBA3-ligands take the different coordination modes,other two carboxylate groups take the same coordination modes.They all take a chelating in bidentate mode to bridge Cd center while the other carboxylate group adopts aμ3-chelating-bidentate tridentatemode(Scheme 2a).The bond lengthsofCd-O bond distance vary in the range of 0.228 4(4)~0.246 7(5)nm and the Cd-N bond distance is0.216 6(5)~0.226 1(5)nm.All the distancesand bond anglesarewithin thenormal range(Table2).
The Cd1 cation is coordinated by three carboxylic O atoms from three BCPBA3-ligands to form a 1D chain(Fig.1b).Then two adjacent 1D chain are bridged together by carboxylic oxygen and Cd2 atom(Fig.1c,d).Then the N ligands join all infinite 1D doublechains into a 2D sheets(Fig.1e).The sheets are stacked fashion and form a 3D framework.
2.2Structure description of 2
The crystal structure determination reveals that compound 2 crystallizes in a monoclinic space group C2/c.The asymmetric unit contains one and half Cd(Ⅱ)cation,a half of bip ligand and one deprotonated BCPBA3-ligand and one coordinated H2O molecule. In the asymmetric unit,the Cd1 cation is coordinated by six carboxylic O atoms from four BCPBA3-ligands forming a distorted octahedron environment.While the Cd2 cation exists in a distorted octahedron geometry, being ligated by five oxygen atoms from four BCPBA3-ligands and one H2Omolecule and one nitrogen atom from one bip ligand(Fig.2a).

Fig.1(a)Coordination environmentof the Cd(Ⅱ)ions in compound 1;(b)View of 1D chain;(c,d)View of double chain;(e)A schematic view of the 2D sheet

Fig.2(a)Coordination environment of the Cd(Ⅱ)ions in compound 2;(b)Trinuclear Cd(Ⅱ)SBUs;(d)Simp lified topological consideration of the 2D sheet;(c,e,f)A schematic view of the 2D sheet
In an asymmetric unit,the wholly deprotonated BCPBA3-anion adopts different coordination modes tobridge Cd centers(Scheme 2b).The bond lengths of Cd-O bond distance vary in the range of 0.220 3(2)~0.256 4(3)nm and the Cd-N bond distance is 0.222 9(3)nm.All the distances and bond angles are within the normal range(Table2).In this compound, the BCPBA3-ligands bridge three Cd(one Cd1 and two Cd2)centers to form[Cd3(CO2)6]clusters(Cd1-Cd2 0.401 3 nm;Cd2-Cd2 0.393 4 nm).Then,the two Cd2 atom are connected by the bip ligand to form a baskets clusters which can be compared with the structures reported previously(Fig.2b)[8].
The trinuclear Cd(Ⅱ)SBUs can be regarded as 6-connected nodes with the BCPBA3-ligands acting as linkers to form a 2D layered network(Fig.2c,d).The sheets are stacked fashion and form a 3D framework.
2.3Therm al analysis
The thermal stabilities of compounds 1 and 2 were investigated by thermogravimetric analyses(TGA) (Fig.3).The experiments were performed on samples consisting of numerous single crystals of 1 or 2 under nitrogen atmosphere with a heating rate of 10℃· min-1.The compound 1 showed a slow weight loss form 30 to 255℃,corresponding to the exclusion of uncoordinated DMF and H2O molecules.The destruction of the framework occured at ca.300℃. The remaining weight might correspond to the formation of CdO(Obsd.24.91%,Calcd.23.94%). The Compound 2 showed a slow weight loss form 30 to 300℃,corresponding to the loss of coordinated H2O molecules.Then its framework began to destruct at ca.400℃,and finally the stoichiometric amount of CdO formed as residue(Obsd.29.00%,Calcd. 28.92%).

Fig.3 TGA curves of compounds 1 and 2
2.4Photolum inescence properties
The luminescent properties of the compounds 1, 2,H3BCPBA and bip were investigated separately in the solid state at room temperature,as shown in Fig.4. The excitation maxima for H3BCPBA and bip are at 370 and 345 nm,and themaxima emission of spectra are at 424 and 440 nm(shoulder peak).The emission peaks were at 424 nm for compound 1(λex=360 nm), while compound 2 shows a red-shift in emission at λem=438 nm whenλex=343 nm,whichmay be assigned to ligand tometal charge transfer(LMCT)[9].
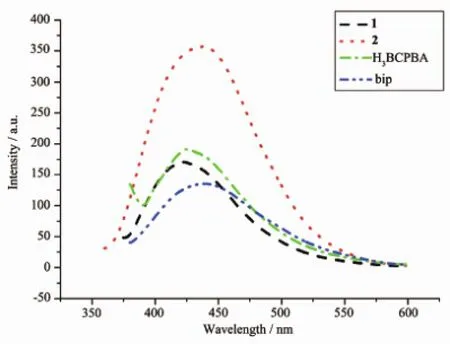
Fig.4 Solid-state emission spectra of compounds 1,2, H3BCPBA and bip at room temperature
2.5XRD analysis
To confirm the phase purity and to examine the crystallinity of bulk samples,the X-ray powder diffraction patterns of the compounds were recorded. As shown in Fig.5,the peak positions of simulated and experimental patterns are in good agreement with each other,demonstrating the phase purity of the product.The dissimilarities in intensitymay be due to the preferred orientation of the crystalline powder sam ples.
3 Conclusions
In this study,twometal-organic frameworks have been synthesized successfully under hydrothermal conditions with different solvents,temperatures and decreasing temperature rates by using the flexible carboxylic acid and the N-donor ligands as auxiliary ligands.Their structural differences indicates that the solvothermal conditions have remarkable influence onthe resultant topologies.The results also show that the flexible carboxylic acid are good candidates for the construction of high dimensional MOFs.

Fig.5 Experimental(a)and simulated(b)XRD patterns of compound 1 and 2
Acknow ledgements:The authors gratefully acknowledge the financial support for thiswork from the Program of the 12th Five-Year Plan for Science and Technology Research of Jilin Province Department of Education(Grant No.2013248).
References:
[1](a)He Y B,Li B,OKeeffe M,etal.Chem.Soc.Rev.,2014, 43:5618-5656
(b)Zhao M,Ou S,Wu C D.Acc.Chem.Res.,2014,47:1199 -1207
(c)Yang W T,Guo M,Yi F Y,et al.Cryst.Growth Des., 2012,12:5529-5534
(d)Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012, 112:1126-1162
(e)Zhao L,Guo H D,Tang D,et al.CrystEngComm,2015, 17:5451-5467
(f)Garibay S J,Wang ZQ,Tanabe K K,et al.Inorg.Chem., 2009,48:7341-7349
(g)Maspoch D,RuizM D,Veciana J.Chem.Soc.Rev.,2007, 36:770-818
[2](a)An D L,Chen Y Q,Tian Y.Z.Anorg.Allg.Chem.,2014, 640;1776-1781
(b)Chen M,Wang ZW,Zhao H,etal.Inorg.Chem.Commun., 2014,45:84-88
(c)Chen M,Wang ZW,Sanudo E C,et al.Inorg.Chem. Commun.,2014,43:121-125
(d)Wang H,Safarifard V,Wang SY,etal.RSCAdv.,2014, 4:1423-11429
(e)WANG Peng-Fei(汪鹏飞),FANG Qin(方勤),WU Guo-Zhi(吴国志),et al.Chinese J.Inorg.Chem.(无机化学学报),2013,29:2521-2527
(f)Xu X R,Pan X B,Tang SF,et al.Z.Anorg.A llg.Chem., 2014,639:967-973
(g)Hu D H,Sun C Y,Liu FH,etal.CrystEngComm,2013, 15:6769-6775
[3](a)Liu L L,Yu C X,Zhou Y,et al.Inorg.Chem.Commun., 2014,40:194-199
(b)Chai Y,Yan Z,Sun W,et al.Inorg.Chim.Acta,2014, 410:76-81
(c)Yang Y,Yang J,Du P,et al.CrystEngComm,2014,16: 6380-6390
(d)Wang W,Yang J,Kan W Q,et al.CrystEngComm, 2013,15:5844-5852
(e)Wang JG,ChaiN,Wang SC,etal.Inorg.Chem.Commun., 2013,30:143-146
(f)Qin JH,Ma L F,Hu Y,et al.CrystEngComm,2012,14: 2891-2898
(g)LIUHong-Wen(刘宏文),LUWen-Guan(卢文冠).Chinese J.Inorg.Chem.(无机化学学报),2011,27(1):152-156
(h)ZHAO Yue(赵越),ZHAILing-Ling(翟玲玲),SUNWei-Yin(孙为银).Chinese J.Inorg.Chem.(无机化学学报), 2014,30(1):99-105
[4](a)Hu JY,Yao H C,Bai Y,etal.Polyhedron,2014,78:1-9
(b)Beheshti A,Lalegani A,Bruno G,et al.J.Mol.Struct., 2014,1071:18-22
(c)Wang X L,LiN,Tian A X,et al.Inorg.Chem.,2014,53: 7118-7129
(d)Lin Z J,Lu J,Hong M C,et al.Chem.Soc.Rev.,2014, 43:5867-5895
(e)Schneemann A,Bon V,Schwedler I,et al.Chem.Soc. Rev.,2014,43:6062-6096
[5](a)Wei X H,Yang L Y,Liao SY,et al.Inorg.Chim.Acta, 2014,416:207-214
(b)Wei X H,Yang L Y,Liao S Y,et al.Dalton Trans., 2014,43:5793-5800
(c)Cui JH,Yang Q X,Li Y Z,et al.Cryst.Growth Des.,2013,13:1694-1702
(d)Cui JH,Li Y Z,Guo Z J,et al.Chem.Commun.,2013, 49:555-557
[6](a)Chang X H,Zhao Y,Han M L,et al.CrystEngComm, 2014,16:6417-6426
(b)Ma LF,Han M L,Qin JH,etal.Inorg.Chem.,2012,51: 9431-9442
(c)Kan W Q,Ma J F,Liu B,et al.CrystEngComm,2012, 14:286-299
(d)Ma L F,Li X Q,Wang L Y,et al.CrystEngComm, 2011,13:4625-4634
[7]Sheldrick G M.Acta Crystallogr.,Sect.A,2008,64:112-122
[8](a)Ma Y S,Cai W S,Chen B,et al.CrystEngComm, 2013,15:7615-7619
(b)Cui J.Inorg.Chem.Commun.,2014,50:54-57
[9](a)Ma L F,Meng Q L,LiC etal.Cryst.Growth Des.,2010, 10:3036-3043
(b)Guo H D,Fu Z A,Guo X M,et al.J.Mol.Struct., 2013,1038:8-11
(c)Zhou J L,Wang Y Y,Qin L,et al.CrystEngComm, 2013,15:616-627
(d)Cui JH,Yanh Q X,Li Y H,et al.Cryst.Growth Des. 2013,13:694-1702
Syntheses,Characterizations and Luminescence Properties of Two Cd(Ⅱ)Metal-O rganic Frameworks Based on 3,5-Bis(4-carboxy-phenoxy)benzoic Acid w ith N-donor Ligand 1,3-Bis(im idazolyl)propane
ZHAO Lun1,2CHEN Rui-Zhan2WANG Zi-Chen*,1
(1Colledge of Chemistry,Jilin University,Changchun 130012,China) (2Colledge of Chemistry,Changchun Normal University,Changchun,130032,China)
Twometal-organic frameworks(MOFs),namely,{[Cd1.5(BCPBA)(bip)]·H2O·DMF}n(1)and[Cd1.5(BCPBA) (bip)0.5(H2O)]n(2)(H3BCPBA=3,5-bis(4-carboxy-phenoxy)benzoic acid,bip=1,3-bis(imidazolyl)propane),have been synthesized under hydrothermal/solvothermal conditions with different reaction temperatures and decreasing temperature rates.Their structures have been determined by single-crystal X-ray diffraction analyses and further characterized by infrared spectra(IR),elemental analyses,and thermogravimetric analyses(TGA).In compound 1, the Cd1 cation is coordinated by three BCPBA3-ligands to form a 1D chain,then two adjacent 1D chain are bridged together by carboxylic oxygen and Cd2 atom.Then the bip ligands join all infinite 1D doublechains into a 2D sheets.In compound 2,trinuclear Cd(Ⅱ)secondardedy building units(SBUs)can be regarded as 6-connected nodeswith the BCPBA3-ligands acting as linkers to form a 2D layered network.Photoluminescence properties of 1 and 2 in solid state at room temperaturewere studied.CCDC:1052091,1;1052166,2.
3,5-bis(4-carboxy-phenoxy)benzoic acid;1,3-bis(imidazolyl)propane;metal-organic frameworks;luminescence
O614.24+2文献标示码:A
1001-4861(2016)07-1190-09
10.11862/CJIC.2016.144
2015-11-26。收修改稿日期:2016-04-24。
吉林省教育厅十二五规划项目(吉教科合字[2013]第248号)资助。
*通信联系人。E-mail:wangzc@jlu.edu.cn

