Immunomodulatory approaches to CNS injury: extracellular matrix and exosomes from extracellular matrix conditioned macrophages
PERSPECTIVE
Immunomodulatory approaches to CNS injury: extracellular matrix and exosomes from extracellular matrix conditioned macrophages
After central nervous system (CNS) injury, a pro-inflammatory, innate immune response contributes to permanently lost neuronal function by promoting changes in the micro-environment and extracellular matrix (ECM) that lead to CNS neuronal degeneration and death and permanent scarring. This pro-inflammatory immune response is largely due to activated resident microglia and infiltrating macrophages adopting a predominantly pro-inflammatory, M1-like, phenotype. Activated, M1-like, microglia and macrophages release additional pro-inflammatory factors, including cytokines and microvesicles like exosomes, that recruit additional cell types, including activated microglia and astrocytes and naïve blood macrophages, M0, that enter the injured tissue and polarize toward M1-like phenotypes that can lead to secondary tissue damage and increased scarring (Figure 1A). After the initial inflammatory response resolves, M1-like macrophages and microglia persist due to pro-inflammatory cytokine signaling cascades that act by both autocrine and paracrine signaling mechanisms to maintain pro-inflammatory signaling, ultimately leading to permanent scarring. Scar tissue also appears to promote persistent inflammatory signaling. Thus, a logical approach to treating CNS injuries is to develop immunomodulatory strategies that interrupt cyclical pro-inflammatory signaling cascades while promoting anti-inflammatory, M2-like, signaling, ultimately altering the default healing response in the CNS from tissue destruction and scarring toward positive tissue and ECM remodeling.
Immunomodulation is increasingly recognized as a promising approach in mitigating CNS trauma and disease. However, current clinically approved immunomodulatory approaches rely on broad based steroids that suppress both the pro- and anti-inflammatory activities of the innate immune response. Generalized immunosuppression may limit secondary tissue damage due to decreased inflammation. However, general immunosuppression may also suppress beneficial anti-inflammatory activities of the innate immune response necessary to promote positive tissue remodeling in the CNS. Therefore, a more targeted immunomodulatory approach that promotes anti-inflammatory, pro-repair microglial and macrophage signaling may lead to positive tissue remodeling and better functional outcomes (Figure 1B). To modulate the innate immune system toward an anti-inflammatory phenotype in adult mammals, a number of new approaches are showing promise in suppressing pro-inflammatory signaling. Here we discuss immunomodulation using ECM technology and the potential for using ECM technology directly and indirectly to advance macrophage pre-polarization therapies and exosome based approaches (Figure 2).
Extracellular matrix technology: ECM is the unique, tissue-specific protein and glycosaminoglycan matrix located around cells in all tissues and organs. Regenerative medicine strategies using ECM technology have been widely successful, both pre-clinically and clinically, in promoting positive tissue repair in various organs and tissues throughout the body, including muscle, connective, epithelial, and peripheral nervous system tissues. Initial studies using ECM technology in nervous system tissues are also now showing encouraging results (Massensini et al., 2015). ECM technology uses xenogeneic ECM bioscaffolds, derived by decellularizing healthy tissues or organs from primarily porcine or equine sources, to decrease scarring and to promote site-appropriate tissue repair in tissues the body cannot repair by default. ECM provides mechanical and biochemical support, facilitates intercellular communication, and modulates cellular and metabolic functions in a tissue-appropriate manner. When prepared properly, ECM is a flexible biocompatible platform that can be derived from numerous tissue sources and used in various forms, including powders, sheets, and injectable hydrogels, which can all be further tuned mechanically and biochemically based on the nature and severity of the injury (Keane and Badylak, 2015).
Though the mechanisms are not completely understood, ECM appears to fundamentally alter the default healing response, in part, by releasing factors that modulate the innate immune response toward an anti-inflammatory phenotype. Specifically, ECM is hypothesized to increase anti-inflammatory signaling at the injury site by increasing the M2/M1 macrophage ratio at the injury site; a low ratio M2/M1 is thought to promote inflammation and scarring, whereas a high M2/M1 ratio appears to promote positive tissue remodeling. ECM appears to increase the M2/M1 macrophage ratio, in part, by releasing protein degradation products, termed matricryptic peptides (Agrawal et al., 2011), as infiltrating immune system cells degrade ECM bioscaffolds. In addition to modulating the innate immune response, matricryptic peptides are hypothesized to regulate several components of the healing response, either directly or indirectly, including angiogenesis, stem cell recruitment and differentiation, and neurogenesis, with ECM from younger homologous tissue sources often increasing positive tissue remodeling over older, non-homologous tissue sources. Macrophages polarized toward an alternatively activated, M2-like, phenotype secrete cytokines and neurotrophic factors, and express cell-surface markers consistent with antigen-presenting activity, indicating ECM bioscaffolds have the potential to influence nerve, immune, and glial cell activities by modulating macrophage phenotypes. In agreement with this idea, pre-clinical investigation by us and others suggest ECM hydrogel bioscaffolds can promote positive tissue remodeling in rodent CNS injury models.
A long-standing question in the use of ECM bioscaffolds in tissue remodeling, including the CNS, is whether homologous tissue sources are more effective at promoting site appropriate cellular migration, differentiation, and tissue remodeling. We have successfully derived ECM from fetal porcine CNS tissues, including brain, spinal cord, retina, and optic nerve (Ren et al., 2015) and are currently investigating whether fetal tissue derived ECMs increase primary CNS neuron survival and axon regeneration, as well as site-appropriate tissue remodeling in the CNS over the default healing response scarring. Indeed, in preliminary studies, fetal homologous tissues appear to contain higher hyaluronic acid and sulfated glycosaminoglycan levels compared to adult versions, typical of pro-developmental tissues. Moreover, fetal derived CNS and non-CNS ECMs appear to significantly reduce glial fibrillary acidic protein (GFAP), an activated astrocyte marker, expression following optic nerve crush, suggesting fetal ECMs are successful in mediating the immune response after injury, though further studies are required to determine whether ECMs can also increase primary CNS neuron survival and promote axon regrowth in vivo. Nevertheless, these initial observations are consistent with previous studies comparing young versus older tissue derived ECM bioscaffolds (Sicari et al., 2012) and suggest fetal ECMs, derived from developing tissues, have the potential to advance positive tissue remodeling in the CNS.
ECM and autologous monocyte/macrophage polarization technology: ECM’s ability to positively modulate macrophage polarization toward an M2-like phenotype supports the idea that using autologous macrophages polarized ex vivo by ECM and then reintroduced to the donor subject may be a viable immunomodulatory approach where ECM bioscaffolds alone are not optimal, such as in treating diseases or injuries via the circulatory, ventricular, or lymphatic systems. In previous autologous monocyte/macrophage conditioning studies, monocytes purified from blood, have been conditioned ex vivo with regenerative tissues, like skin or anti-inflammatory cytokines, toward an anti-inflammatory macrophage phenotype. These conditioned or pre-polarized macrophages haveshown some pre-clinical and clinical success when re-injected directly into the spinal cord (Schwartz and Yoles, 2006) or indirectly intrathecal into the ventricular system of the CNS to treat difficult to access regions of the CNS, as in stroke (Chernykh et al., 2015). Once injected, pre-polarized macrophages are hypothesized to target the injury site and release factors that promote an anti-inflammatory, pro-repair response in the microenvironment, like anti-inflammatory cytokines and growth factors. However, injecting cells in general are fraught with caveats including cellular dedifferentiation, non-targeted localization, improper timing, and the inability of pre-polarized macrophages to properly respond to molecular cues released in the microenvironment from injured tissues, which may help explain recent inconsistencies in clinical trials (Lammertse et al., 2012). Pro-inflammatory cytokines secreted at the injury site may simply repolarize macrophages toward an M1-like phenotype. Since ECM can polarize macrophages toward an M2 phenotype, injecting pre-polarized macrophages within an ECM matrix may be a solution for at least direct tissue injection applications.
ECM and exosome therapy: Another, perhaps, more attractive option is to use the anti-inflammatory secreted products from ECM conditioned macrophages, like microvesicles or exosomes. An emerging concept in neuro-protection, -inflammation, and -regeneration is that microvesicle crosstalk within and between cellular populations mediates tissue level remodeling. Thus, an alternative approach to injecting cells directly into CNS tissue is injecting the positive immunomodulatory factors released from ECM polarized macrophages, like microvesicles, which are increasingly recognized as regulators of neuroinflammation and the default healing response in the CNS. Thus, instead of using ECM with or without live cells, microvesicles like exosomes may enable the beneficial secreted products released from anti-inflammatory, pro-repair microglia or macrophages to be delivered without the negative side-effects often associated with using whole cells or difficulties of injecting ECM directly into circulatory fluids.
Exosomes are nanometer sized (10—100 nm) microvesicles originally described as secreted products from lymphocytes (Raposo et al., 1996) but are now known to be secreted from virtually all cells, including CNS neurons and glia, under both normal and pathological conditions. Exosomes play critical roles in intercellular communication by transferring nucleic acids, lipids, and cytosolic proteins, not only locally, but also through fluids like the lymphatic, ventricular, and circulatory systems. Once internalized, exosomes can release their unique cargoes, delivering bioactive signaling molecules that can significantly alter the cellular activities and phenotypes of recipient cells. Macrophage and microglial secreted exosomes change in composition under inflammatory conditions and recent studies suggest microglial and macrophage derived exosomes directly regulate astrocyte activation and the secretion of scar tissues components into the ECM, thus regulating wound healing (Gupta and Pulliam, 2014).
How do ECM bioscaffolds known to regulate microglia and macrophage phenotypes also regulate exosome signaling within immune system cells and glial populations in the CNS to regulate healing, and can distinct exosome populations be used to regulate inflammation and healing in the CNS? One of the consistent issues with persistent inflammation in the CNS is the disruption of the cyclical pro-inflammatory signaling cascades, which makes the minimally invasive “anti-inflammatory” exosomes or microvesicle-based therapies attractive approaches for modulating CNS inflammation and healing. In theory, by adding anti-inflammatory exosomes to injured CNS tissues, we may be able to downregulate the typical M1-like, pro-inflammatory response, preventing ECM degradation and scarring, while also promoting functional tissue reconstruction by promoting the M2-like pro-repair response in CNS tissues. Though a description of specific studies is beyond this perspective, an increasing number of studies have shown that exosomes can deliver cargoes to recipient cells that can regulate neuronal growth and function. In agreement, our preliminary data suggest exosomes derived from specific embryonic tissues can significantly increase axon regeneration at least in vitro. Exosomes are internalized by CNS neuron growth cones, transported into growing axons, and exosomes derived from different cellular sources differentially modulate axon growth rates. Exosomes are easily transfected and thus can be engineered to deliver specific nucleic acid and signaling molecule cargoes that can potentially modulate inflammation and/ or axon growth in a minimally invasive platform via regulation of the expression of cell surface receptors and intracellular signaling (Lee et al., 2014). Thus, combinatorial therapies employing engineered and/or ECM induced microvesicles or exosomes secretions may provide a minimally invasive, more comprehensive tissue level approach to treating CNS injury.
Conclusion: The innate immune response plays a large role in the inability of the CNS to regenerate after injury in higher mammals. New immunomodulatory technologies that take advantage of naturally derived biologic factors are emerging as potential, rapidly translatable tools for preventing or even restoring lost neurologic function in the CNS. By ameliorating the pro-inflammatory response to injury in the CNS without suppressing the anti-inflammatory, positive tissue remodeling activities of the immune system, functional recovery in the CNS is increasingly becoming an attainable goal. Though combinatorial therapies focused on targeting multiple intracellular signaling pathways within specific cellular populations have shown that CNS neurons can regenerate long distances to re-innervate targets in the brain, these approaches fail to address changes within the ECM microenvironment that prohibit axon growth and lead to scarring. Thus, combinatorial therapies acting on multiple cell types to alter the default healing response may lead to a more robust and comprehensive level of tissue remodeling. Additionally, just as ECM can be loaded with macrophages or other cell types, ECM can also potentially be loaded with exosomes engineered to target different cellular populations. One of the major hurdles in treating neuro-inflammatory disorders in the CNS has been the lack of an optimized delivery strategy that allows the drugs to cross the blood brain barrier or penetrate deeper regions of the CNS. ECM stimulated exosomes have the potential to serve as an attractive drug delivery vehicle owing to their desirable properties such as low immunogenicity, ability to effectively transport a range of biomolecules, interaction with a host of target cells, and importantly, delivery of their cargoes by systemic or even intrathecal injection into the CNS.
We gratefully acknowledge funding from the Department of Defense office of the Congressionally Directed Medical Research Programs and the Clinical and Rehabilitative Medicine Research Program (MBS, MR130444), Competitive Medical Research Fund, University of Pittsburgh (MBS), Pennsylvania Lions Sight Conservation & Eye Research Foundation (MBS), Sterling Lions Club of Pennsylvania and Dance for Sight (MBS), Start-up funds, Department of Ophthalmology, University of Pittsburgh (MBS), National Institutes of Health CORE Grant P30 EY008098, Eye and Ear Foundation of Pittsburgh, PA, Unrestricted Grant from Research to Prevent Blindness, New York, NY.
Yolandi van der Merwe, Michael B. Steketee*
Department of Bioengineering, Swanson School of Engineering, University of Pittsburgh, Pittsburgh, PA, USA (van der Merwe Y) Department of Ophthalmology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA; McGowan Institute for Regenerative Medicine, University of Pittsburgh, Pittsburgh, PA, USA (van der Merwe Y, Steketee MB) Center for Neuroscience University of Pittsburgh, University of Pittsburgh, Pittsburgh, PA, USA (Steketee MB)
*Correspondence to: Michael B. Steketee, Ph.D., SteketeeM@UPMC.edu.
Accepted: 2016-03-22
orcid: 0000-0002-0269-8671 (Yolandi van der Merwe) 0000-0002-0227-9178 (Michael B. Steketee)
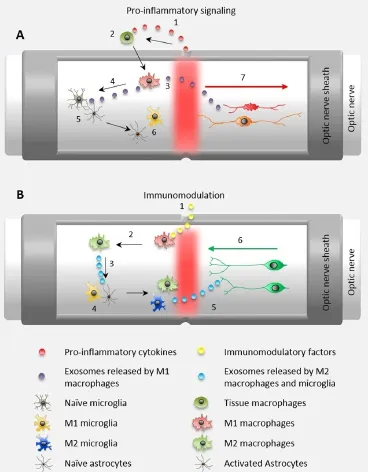
Figure 1 Optic nerve model of acute CNS injury.
Agrawal V, Tottey S, Johnson SA, Freund JM, Siu BF, Badylak SF (2011) Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A 17:2435-2443.
Chernykh ER, Shevela EY, Starostina NM, Morozov SA, Davydova MN, Menyaeva EV, Ostanin AA (2015) Safety and therapeutic potential of M2-macrophages in stroke treatment. Cell Transplant doi: http://dx.doi. org/10.3727/096368915X690279.
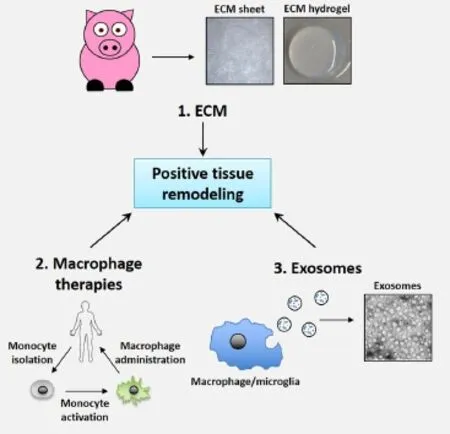
Figure 2 Immunomodulatory approaches to treating CNS trauma.
Gupta A, Pulliam L (2014) Exosomes as mediators of neuroinflammation. J Neuroinflammation 11:68.
Keane TJ, Badylak SF (2015) The host response to allogeneic and xenogeneic biological scaffold materials. J Tissue Eng Regen Med 9:504-511.
Lammertse DP, Jones LA, Charlifue SB, Kirshblum SC, Apple DF, Ragnarsson KT, Falci SP, Heary RF, Choudhri TF, Jenkins AL, Betz RR, Poonian D, Cuthbert JP, Jha A, Snyder DA, Knoller N (2012) Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord 50:661-671.
Lee HD, Kim YH, Kim DS (2014) Exosomes derived from human macrophages suppress endothelial cell migration by controlling integrin trafficking. Eur J Immunol 44:1156-1169.
Massensini AR, Ghuman H, Saldin LT, Medberry CJ, Keane TJ, Nicholls FJ, Velankar SS, Badylak SF, Modo M (2015) Concentration-dependent rheological properties of ECM hydrogel for intracerebral delivery to a stroke cavity. Acta Biomater 27:116-130.
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161-1172.
Ren T, van der Merwe Y, Steketee MB (2015) Developing Extracellular Matrix Technology to Treat Retinal or Optic Nerve Injury(1,2,3). eNeuro 2.
Schwartz M, Yoles E (2006) Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J Neurotrauma 23:360-370.
Sicari BM, Johnson SA, Siu BF, Crapo PM, Daly KA, Jiang H, Medberry CJ, Tottey S, Turner NJ, Badylak SF (2012) The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33:5524-5533.
10.4103/1673-5374.180733 http://www.nrronline.org/
How to cite this article: van der Merwe Y, Steketee MB (2016) Immunomodulatory approaches to CNS injury: extracellular matrix and exosomes from extracellular matrix conditioned macrophages. Neural Regen Res 11(4):554-556.
- 中国神经再生研究(英文版)的其它文章
- Local translation of cell adhesion molecules in axons
- Gait deterioration due to neural degeneration of the corticoreticular pathway: a case report
- Repositioning again of zonisamide for nerve regeneration
- A novel technique using hydrophilic polymers to promote axonal fusion
- Examining the properties and therapeutic potential of glial restricted precursors in spinal cord injury
- The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier

