A novel technique using hydrophilic polymers to promote axonal fusion
Ravinder Bamba, D. Colton Riley, Nathaniel D. Kelm, Mark D. Does, Richard D. Dortch, Wesley P. Tayer
1 Department of Plastic Surgery, Vanderbilt University Medical Center, Nashville, TN, USA
2 Department of Surgery, Georgetown University, Washington, DC, USA
3 Georgetown University School of Medicine, Washington, DC, USA
4 Vanderbilt University Institute of Imaging Science, Nashville, TN, USA
5 Department of Radiology and Radiological Sciences, Vanderbilt University, Nashville, TN, USA
INVITED REVIEW
A novel technique using hydrophilic polymers to promote axonal fusion
Ravinder Bamba1,2,*, D. Colton Riley1,3, Nathaniel D. Kelm4, Mark D. Does4, Richard D. Dortch5, Wesley P. Tayer1
1 Department of Plastic Surgery, Vanderbilt University Medical Center, Nashville, TN, USA
2 Department of Surgery, Georgetown University, Washington, DC, USA
3 Georgetown University School of Medicine, Washington, DC, USA
4 Vanderbilt University Institute of Imaging Science, Nashville, TN, USA
5 Department of Radiology and Radiological Sciences, Vanderbilt University, Nashville, TN, USA
The management of traumatic peripheral nerve injury remains a considerable concern for clinicians. With minimal innovations in surgical technique and a limited number of specialists trained to treat peripheral nerve injury, outcomes of surgical intervention have been unpredictable. The inability to manipulate the pathophysiology of nerve injury (i.e., Wallerian degeneration) has left scientists and clinicians depending on the slow and lengthy process of axonal regeneration (~1 mm/day). When axons are severed, the endings undergo calcium-mediated plasmalemmal sealing, which limits the ability of the axon to be primarily repaired. Polythethylene glycol (PEG) in combination with a bioengineered process overcomes the inability to fuse axons. The mechanism for PEG axonal fusion is not clearly understood, but multiple studies have shown that a providing a calcium-free environment is essential to the process known as PEG fusion. The proposed mechanism is PEG-induced lipid bilayer fusion by removing the hydration barrier surrounding the axolemma and reducing the activation energy required for membrane fusion to occur. This review highlights PEG fusion, its past and current studies, and future directions in PEG fusion.
peripheral nerve injury; polyethylene glycol; axonal fusion; nerve transection; traumatic neuropathy
Introduction
Nerve injury is not fatal but can severely impact the quality of life. Peripheral nerve injuries are commonly caused by trauma to the upper limbs and are present in an estimated 2—3% of all patients admitted to a Level 1 trauma center (Noble et al., 1998). The economic impact of nerve injuries can be large with operative costs, hospital charges, rehabilitation visits, and lost time at work. Only subtle improvements to peripheral nerve repair have been made recently, and our current knowledge of nerve physiology and regeneration vastly exceeds our current repair capabilities.
Poor outcomes of peripheral nerve injury are largely due to the slow process of axonal outgrowth. After nerve injury, severed proximal axons with intact cell bodies can grow up to 1mm/day. Regenerating axons preferentially target appropriate end organ receptors but do not always take a direct route to their target (Fox et al., 2012). Thus, functional recovery is not always obtained due to slow growth and poor alignment of motor and sensory fibers. Muscle atrophy initiates immediately after muscle denervation, and if motor axons do not reach their target muscle within a critical time window, muscle tissue is less receptive to re-innervation.
The most common current strategies to augment recovery after nerve injury such as decellularized nerve allografts, tissue matrices and nerve growth guides involve techniques to enhance axonal regeneration and decrease surrounding inflammation. Despite these advancements, the combination of slow axonal outgrowth, Wallerian degeneration, and muscle atrophy are still barriers to significant progress.
Polyethylene Glycol (PEG) Fusion Mechanism
Invertebrate axonal fusion after nerve injury is a natural mechanism that promotes rapid and highly specific recovery of neurons. Given the mechanism of axonal fusion is possible, PEG has been investigated as an agent to promote axonal fusion in vertebrates. PEG has traditionally been used to make hybridomas via membrane fusion. Bittner et al. pioneered initial studies using PEG to fuse crayfish and giant earthworms axons with improvement in axonal fusion when axonal endings were exposed to a calcium free saline solution (Bittner et al., 1986; Krause et al., 1990). The addition of an anti-oxidant such as methylene blue (MB) was noted to have an additional positive impact in rat sciatic nerve repair (Spaeth et al., 2012).
The mechanism for PEG axonal fusion is not clearly understood. The proposed mechanism is PEG-induced lipid bilayer fusion by removing the hydration barrier surrounding the axolemma and reducing the activation energy requiredfor membrane fusion to occur. In an axonal injury without PEG, axonal endings seal after an influx of calcium, preventing axonal fusion (Yoo et al., 2003). Figure 1 demonstrates the bioengineered process of PEG fusion. When severed axonal endings are exposed to calcium-free hypotonic saline and an antioxidant (i.e., methylene blue or melatonin), vesicle-mediated sealing is decreased, keeping membrane leaflets open. PEG is then applied to artificially induce closely apposed membranes of severed axonal ends to flow into each other and fuse. This produces a partial repair of the plasmalemmal membranes that are then perfused with calcium containing saline, which causes vesicles to accumulate and seal remaining holes at the injury site. This process is known as PEG fusion.
The success of the PEG-fusion technique is based on multiple steps and factors. PEG, a hydrophilic compound, enhances axonal fusion in either severed or crushed settings and restores the ability to generate compound action potentials across the site of injury. PEG potentially facilitates lipid bilayer fusion by removing water molecules from the lipid bilayer at or near the damage site (Figure 1). Calcium plays a critical role in mediating PEG-fusion of axons. Sealing of axolemmal damage occurs through a calcium-dependent accumulation of membranous structures that interact with nearby undamaged membrane to form a plug. In severed nerves, this calcium-dependent system plugs the cut ends of axons preventing them from fusing with an adjacent axonal stump. Our studies have taken advantage of this calcium-dependent process by incubating in a calcium-free solution prior to nerve fusion, which prevents axolemmal sealing thereby enhancing PEG based fusion (Sexton et al., 2012, 2015; Riley et al., 2015). Oxidative damage has also been shown to play a role in ischemia reperfusion injury after peripheral nerve injury. Methylene blue has been previously shown to slow axolemmal sealing and thereby enhance behavioral recovery after nerve injury (Spaeth et al., 2012). In our own studies, methylene blue has shown a protective effect if applied in the correct sequence (i.e., after nerve severance and before PEG based fusion). Taken together, these studies have shown that PEG, with carefully controlled modification of the periaxonal environment can fuse simple nerve transections. The protocol has been designed to take advantage of three factors to optimize fusion. We have used the fusogenic properties of PEG, the advantages of a calcium-free irrigation solution, and the antioxidant properties of methylene blue to demonstrate a rapid and decisive recovery of completely severed sciatic nerves in a commonly accepted mammalian model of peripheral nerve injury.
In Vivo Studies
Most current research efforts have focused on investigating PEG fusion in a rat sciatic nerve model. The rat sciatic nerve is easily accessible through a 2—3 cm incision posterior to the femur. After a small cutdown to the nerve, the perineural tissue can be dissected easily to free the nerve for transection and repair. Our group has demonstrated the efficacy of PEG fusion after neurotmesis with direct repair, nerve autografts, and nerve allografts. This demonstrates that PEG fusion is a technique that is widely applicable. We have investigated the efficacy of PEG fusion using a variety of methods.
Compound action potentials (CAP) are measurements of nerve conductivity and can be readily measured across healthy nerves. After nerve transection, CAPs can no longer be obtained due to axonal discontinuity. CAPs are not restored after a direct suture repairdue to lack of axonal fusion secondary to axolemmal sealing after injury. However, after PEG axonal fusion, CAPs are restored. Bittner et al. published CAP data after direct suture repair with and without PEG (Bittner et al., 2012). Baseline CAPs were obtained in all rats prior to nerve injury (mean 4.1 ± 0.16 mV). Postoperatively, CAPs were not measurable immediately after repair in the control group (direct suture repair). However, CAPs were restored within minutes after repair (mean 3.3 ± 0.23 mV) in the PEG fusion group. In our experience with PEG fused autografts (Sexton et al., 2012), baseline CAPs were obtained in all rats prior to nerve injury (mean 4.131 ± 2.25 mV, n = 20). Postoperatively, CAPs were not measurable immediately after repair or after 72 hours in the control group (direct suture repair). However, CAPs were restored immediately after repair (mean 3.533 ± 1.61 mV, n = 10) and after 72 hours (mean 2.051 ± 0.857 mV, n = 10) in the PEG fusion group. Our experience with CAP restoration in the PEG fusion group was similar when using PEG fused allografts (Riley et al., 2015).
Our rat behavioral testing includes both the Sciatic Function Index (SFI) and Foot Fault Asymmetry Score (FF). Behavioral recovery was superior in the PEG fused group in direct repair at 12 weeks (Bittner et al., 2012) as well as the PEG fused group in nerve allografts at 6 weeks (Riley et al. 2015). Our nerve autograft study evaluated rats up to 3 days, and the PEG fused group already had superior behavioral outcomes (Sexton et al., 2012). Figure 2 demonstrates longterm behavioral outcomes after a standard microsurgical repair compared to a PEG fusion repair after nerve transection. When considering the electrophysiology and behavioral data together, it suggests at least a partial inhibition of Wallerian degeneration after PEG fusion.
After axonal injury, motor and sensory nerve counts are decreased distal to the injury site. Immunohistochemical staining using commercial antibodies against Carbonic Anhydrase II (CA2) and Choline Acetyletransferase is a method to stain for sensory and motor neurons respectively. Our research has shown increased sensory and motor axon counts in PEG-treated nerve repairs (Figure 3). Toluidine blue staining of PEG fused nerves shows higher proportions of healthy myelinated axons compared to negative control nerves. Additionally, our lab has demonstrated the expression of NPY1, a marker of Wallerian degeneration, in negative control nerves, whereas this is not expressed in PEG-fused nerves. These findings imply that we have delayed or even inhibited Wallerian degeneration by fusing axons and maintained both axonal viability and function.
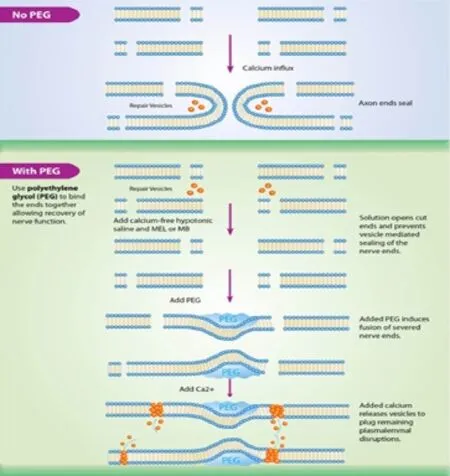
Figure 1 Mechanism of polyethylene glycol (PEG) axonal fusion.
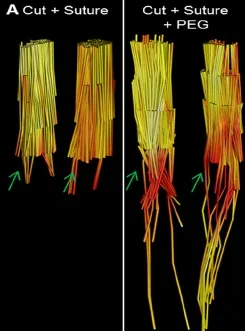
Figure 4 Diffusion tensor tractography of fixed rat sciatic nerves.

Figure 2 Behavioral assessments of sciatic nerve function according to the sciatic functional index (A) and foot-fault asymmetry test (B).
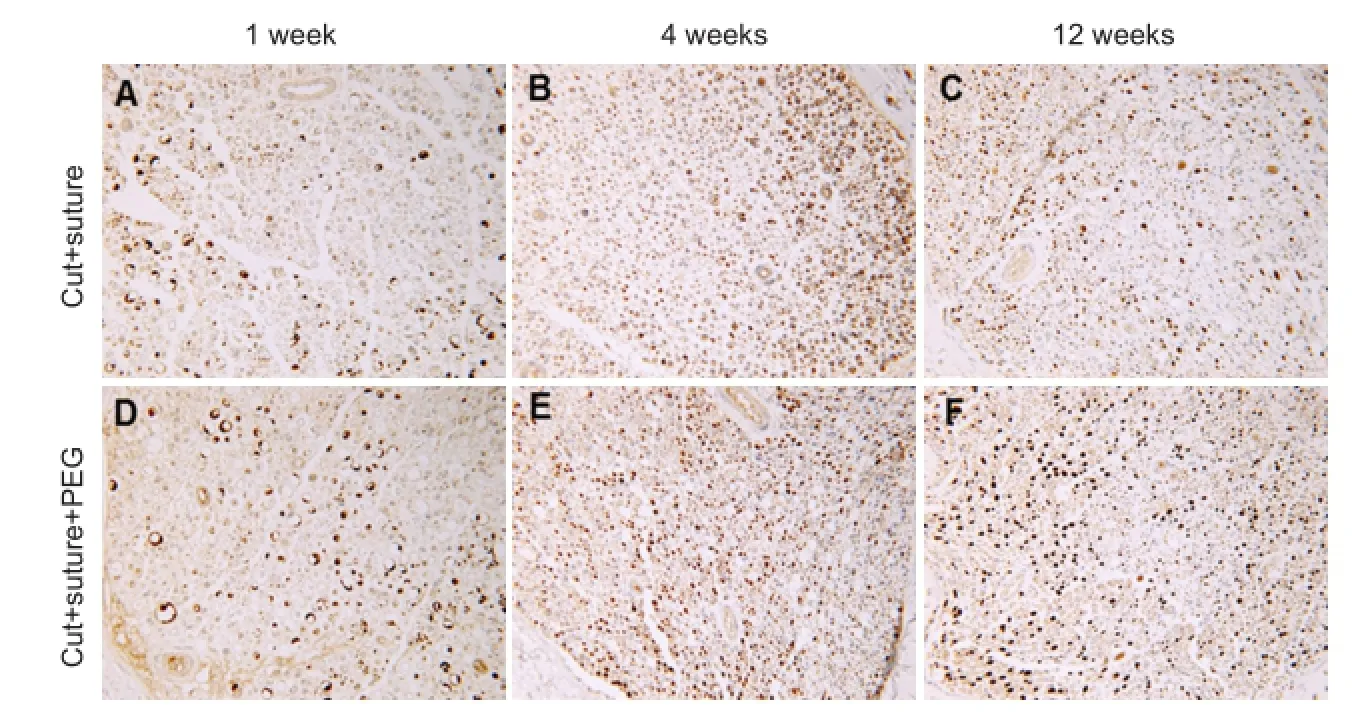
Figure 3 Representative photomicrographs of paraffin embedded cross section used for counts generated in Figure 4.
Diffusion tensor imaging (DTI) is a magnetic resonance imaging (MRI) technique, which is an emerging diagnostictool in nerve injury. The Institute of Imaging Science at our institution has developed a novel imaging protocol using DTI for peripheral nerves. Utilizing this protocol, we have been able to demonstrate the restoration of axonal continuity after PEG fusion repair (Figure 4). Representative tractography images show that axonal tracts do not extend more than a couple millimeters distal to the repair site in control nerves (cut + suture, n = 6). In PEG fused nerves (n = 6), the PEG group had a statistically significant increase in the number of tracts that traveled through the repair site (data not published, manuscript in progress). Our MRI data supports the interpretation that PEG-fused nerves establish axonal fusion which has been previously evaluated through behavioral testing and axon counts.
Future Directions
Despite the effectiveness we have observed with PEG fusion, there can be inconsistencies in morphological and functional recovery of PEG-fused animals. As demonstrated in Figure 4, PEG fusion has not fully restored DTI characteristics in these nerves, perhaps suggesting that not all axons were fused. We speculate that a variability in the number of fused axons may in turn be responsible for the variabilities in morphological and functional recovery. We also suspect factors such as the quality of repair, time of surgical intervention, and a surgeon’s level of experience with the technique play a critical role in achieving successful PEG fusion. Our studies have included applying the PEG solution manually through a syringe with the needle held at the surface of the neurorrhaphy. This method, potentially, could result in small movements of the needle resulting in sub-optimal delivery of the PEG solution or disturbance of the neurorrhaphy.
Our lab is currently investigating the development of a PEG delivery device. Our current efforts in developing a device are focused on achieving a consistent nerve repair with uniform PEG application to the neurorrhaphy site. We recently described our experience with PEG fusion using a nerve tube, which helps eliminate the need for sutures at the repair site (Sexton et al., 2015). Our ultimate goal is to develop a simple coaptation system that will allow acute repair of severed nerves, with or without segmental injury, with minimal microsurgical training.
Our lab has focused its efforts on small animal models, and the study of PEG fusion has occurred across multiple laboratories and animal models. We have demonstrated repeated success in working in a rat sciatic nerve model. Bittner et al. has demonstrated the efficacy of PEG fusion in invertebrate and small animal models in the aforementioned studies (Bittner et al., 1986; Krause et al., 1990; Spaeth et al., 2012). In addition, PEG fusion has been investigated in large animal sciatic nerve and ex-vivo central nerve models in other laboratories demonstrating reproducibility of the technique (Donaldson et al., 2002; Nehrt et al., 2010). Looking forward, we are currently conducting a Phase I clinical trial of PEG fusion in human nerve repair, and we were also hoping to move towards more large animal studies in the near future.
Conclusion
In summary, PEG fusion is an exciting new method in nerve repair. The ability to fuse severed nerves and regain rapid functional recovery could result in a paradigm shift in all aspects of patient care following peripheral nerve damage. Because this technique involves the use of current microsurgical techniques, with the simple addition of PEG, it could rapidly be incorporated into a neurorrhaphy and autografting regimen. Our current clinical trial will investigate efficacy and safety of PEG fusion in humans, and our development of a PEG delivery device will make PEG fusion reproducible with minimal training required to achieve superior results.
Bittner GD, Ballinger ML, Raymond MA (1986) Reconnection of severed nerve axons with polyethylene glycol. Brain Res 367:351-355.
Bittner GD, Keating CP, Kane JR, Britt JM, Spaeth CS, Fan JD, Zuzek A, Wilcott RW, Thayer WP, Winograd JM, Gonzalez-Lima F, Schallert T (2012) Rapid, effective, and long-lasting behavioral recovery produced by microsutures, methylene blue, and polyethylene glycol after completely cutting rat sciatic nerves. J Neurosci Res 90:967-980.
Donaldson J, Shi R, Borgens R (2002) Polyethylene glycol rapidly restores physiologic functions in damaged sciatic nerves of guinea pigs. Neurosurgery 50:147-156.
Fox IK, Brenner MJ, Johnson PJ, Hunter DA, Mackinnon SE (2012) Axonal regeneration and motor neuron survival after microsurgical nerve reconstruction. Microsurgery 32:552-562.
Krause TL, Bittner GD (1990) Rapid morphological fusion of severed myelinated axons by polyethylene glycol. Proc Natl Acad Sci U S A 87:1471-1475.
Nehrt A, Hamann K, Ouyang H, Shi R (2010) Polyethylene glycol enhances axolemmal resealing following transection in cultured cells and in ex vivo spinal cord. J Neurotrauma 27:151-161.
Noble J, Munro CA, Prasad VS, Midha R (1998) Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma 45:116-122.
Riley DC, Bittner GD, Mikesh M, Cardwell NL, Pollins AC, Ghergherehchi CL, BhupanapaduSunkesula SR, Ha TN, Hall BT, Poon AD, Pyarali M, Boyer RB, Mazal AT, Munoz N, Trevino RC, Schallert T, Thayer WP (2015) Polyethylene glycol-fused allografts produce rapid behavioral recovery after ablation of sciatic nerve segments. J Neurosci Res 93:572-583.
Rodriguez-Feo CL, Sexton KW, Boyer RB, Pollins AC, Cardwell NL, Nanney LB, Shack RB, Mikesh MA, McGill CH, Driscoll CW, Bittner GD, Thayer WP (2013) Blocking the P2X7 receptor improves outcomes after axonal fusion. J Surg Res 184:705-713.
Sexton KW, Pollins AC, Cardwell NL, Del Corral GA, Bittner GD, Shack RB, Nanney LB, Thayer WP (2012) Hydrophilic polymers enhance early functional outcomes after nerve autografting. J Surg Res 177:392-400.
Sexton KW, Rodriguez-Feo CL, Boyer RB, Del Corral GA, Riley DC, Pollins AC, Cardwell NL, Shack RB, Nanney LB, Thayer WP (2015) Axonal fusion via conduit-based delivery of hydrophilic polymers. Hand (N Y) 10:688-694.
Spaeth CS, Robison T, Fan JD, Bittner GD (2012) Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neurosci Res 90:955-966.
Yoo S, Nguyen MP, Fukuda M, Bittner GD, Fishman HM (2003) Plasmalemmal sealing of transected mammalian neurites is a gradual process mediated by Ca(2+)-regulated proteins. J Neurosci Res 74:541-551.
10.4103/1673-5374.180724 http://www.nrronline.org/
How to cite this article: Bamba R, Riley DC, Kelm ND, Does MD, Dortch RD, Thayer WP (2016) A novel technique using hydrophilic polymers to promote axonal fusion. Neural Regen Res 11(4):525-528.
Funding: This work was supported by the Department of Defense: Grant Number OR120216--Development of Class II Medical Device for Clini cal Translation of a Novel PEG Fusion Method for Immediate Physiological Recovery after Peripheral Nerve Injury.
*Correspondence to: Ravinder Bamba, M.D., Ravinder.bamba@vanderbilt.edu.
orcid: 0000-0002-5432-2764 (Ravinder Bamba)
Accepted: 2016-02-26
- 中国神经再生研究(英文版)的其它文章
- Counteraction of Nogo-A and axonal growth inhibitors by green tea polyphenols and other natural products
- Gait deterioration due to neural degeneration of the corticoreticular pathway: a case report
- Local translation of cell adhesion molecules in axons
- Examining the properties and therapeutic potential of glial restricted precursors in spinal cord injury
- The choroid plexus-cerebrospinal fluid interface in Alzheimer’s disease: more than just a barrier
- Physical interactions between activated microglia and injured axons: do all contacts lead to phagocytosis?

