糜子叶片防御酶系及抗氧化物质对黑穗病菌胁迫的响应
周 瑜,刘佳佳,张盼盼,屈 洋,张骥如飞,朱明旗,冯佰利
(1西北农林科技大学农学院/旱区作物逆境生物学国家重点实验室,陕西杨凌 712100;2黑龙江八一农垦大学/国家杂粮工程技术中心,黑龙江大庆 163319;3陕西省宝鸡市农业科学研究所,陕西岐山 722400)
糜子叶片防御酶系及抗氧化物质对黑穗病菌胁迫的响应
周 瑜1,刘佳佳1,张盼盼2,屈 洋3,张骥如飞1,朱明旗1,冯佰利1
(1西北农林科技大学农学院/旱区作物逆境生物学国家重点实验室,陕西杨凌 712100;2黑龙江八一农垦大学/国家杂粮工程技术中心,黑龙江大庆 163319;3陕西省宝鸡市农业科学研究所,陕西岐山 722400)
【目的】黑穗病是威胁糜子产量的重要病害,防治黑穗病最有效的方法是种植抗病品种。本研究测定黑穗病菌胁迫对糜子叶片防御酶系活性及抗氧化物质含量的影响,筛选鉴定糜子黑穗病抗性的生理生化指标,为选育抗黑穗病的糜子品种提供理论支撑。【方法】以不同糜子资源为材料,田间种植条件下采用种子饱和接种法接种黑穗病菌,2012—2013年进行糜子黑穗病抗性鉴定,筛选不同抗性的糜子品种。2014年研究不同抗性糜子苗期(SS)、拔节期(ES)、抽穗期(HS)、灌浆期(FS)叶片防御酶系及抗氧化物质对黑穗病菌胁迫的响应,防御酶系测定苯丙氨酸解氨酶(PAL)、抗坏血酸过氧化物酶(APX)、谷胱甘肽还原酶(GR)活性,抗氧化物质测定抗坏血酸(AsA)、还原型谷胱甘肽(GSH)含量。【结果】经连续两年糜子黑穗病抗性鉴定,黑虼蚤(R1)、驴驼川(R2)和小麦糜子(R3)平均发病率分别为0、0和0.73%,为抗病品种;黄硬黍(S1)、宁04-262(S2)和Ym0965(S3)平均发病率分别为19.71%、19.86%和32.28%,为感病品种。接种糜子黑穗病菌后,感病品种糜子叶片PAL活性变化幅度大于抗病品种,表现在拔节期PAL活性为3 610.8 U·g-1FW,显著高于抗病品种的2 520.7 U·g-1FW,而灌浆期为2 425.0 U·g-1FW,显著低于抗病品种的2946.0 U·g-1FW。抗、感品种糜子叶片APX活性均呈先降低后升高的变化趋势,拔节期显著最低;感病品种糜子叶片APX活性在抽穗期和灌浆期(分别为461.1 U·g-1FW和516.7 U·g-1FW)显著高于抗病品种(分别为361.5 U·g-1FW和428.2 U·g-1FW)。2类品种叶片GR活性变化呈先升高后降低趋势,抽穗期GR活性显著高于其他3个时期;且抽穗期感病品种叶片GR活性显著高于抗病品种,其中感病和抗病品种糜子叶片平均GR活性分别为271.9和167.4 U·g-1FW。糜子受黑穗病菌胁迫后,6个品种叶片AsA含量在147.7—344.8 μg·g-1FW范围内波动,无明显规律,且抗、感品种间无显著差异。抗病品种糜子叶片GSH含量从苗期到抽穗期显著降低后到灌浆期又显著升高,而感病品种糜子叶片GSH含量从苗期到抽穗期显著降低后到灌浆期并无显著变化,并且灌浆期抗病品种叶片GSH含量为984.7 μg·g-1FW,显著高于感病品种的676.0 μg·g-1FW。【结论】不同糜子品种对黑穗病的抗性不同,黑穗病菌胁迫可引起糜子叶片防御酶活性及抗氧化物质含量变化,拔节期和灌浆期PAL活性、抽穗期和灌浆期APX活性、抽穗期GR活性、灌浆期GSH含量在抗病品种和感病品种间存在显著差异,可作为鉴定糜子对黑穗病抗性的生理生化指标。
糜子;黑穗病;防御酶系;抗氧化物质
0 引言
【研究意义】糜子(Panicum miliaceum L.)生育期短、耐旱、耐瘠薄,生育时期与降雨季节相吻合,水分利用效率较高,在干旱半干旱地区粮食生产和种植业结构调整中具有重要意义[1-2]。糜子营养丰富、药食同源,在现代功能性食品开发中具有很大潜力[3-4],消费量不断增加。但是在中国糜子的主要生产地区,黑穗病是威胁糜子产量的重要因素,发病率一般在5%—10%,病情严重的地区发病率高达40%[5],严重影响糜子产量。目前,糜子主产地区多采用药剂拌种和轮作等农艺措施防治糜子黑穗病,但是选育抗病品种是最经济有效的防治途径[6]。糜子品种抗病性的选择主要是通过品种育成后进行抗病性鉴定,其在选育过程中没有进行相关的抗病性生理生化指标的鉴定,因此,糜子品种选育对主要病害的抗性没有针对性。确定抗病品种对糜子黑穗病侵染保护机制的生理生化指标,对糜子抗黑穗病品种选育具有重要意义。【前人研究进展】植物可通过自身物理或化学的屏障、防御基因的表达和相关酶的激活实现对病害的抗性[7-10]。研究表明,当植物受到病原菌侵染后,酚类氧化酶的活性会发生变化,与植物的抗病性密切相关[10-11]。苯丙氨酸解氨酶(phenylalanice ammonia lyase, PAL)是一种常见的酚类氧化酶,是苯丙烷代谢途径的关键酶和限速酶,催化L-苯丙氨酸产生肉桂酸,肉桂酸是重要的次级代谢产物,是酚类、植保素、木质素合成前体,因此,PAL能发挥重要的抗病作用[7,9,13-14]。另外,病原菌胁迫[15-16]如同非生物胁迫[17-20]都会导致植物体内活性氧的产生和消除平衡遭到破坏,对细胞造成伤害,为减轻活性氧的伤害,植物体内存在一系列复杂的防御体系,包括超氧化物歧化酶(superoxide dismutase,SOD)、过氧化物酶(peroxidase,POD)、抗坏血酸过氧化物酶(ascorbate peroxidase,APX)、谷胱甘肽还原酶(glutathione reductase,GR)等抗氧化保护酶系及抗坏血酸(ascorbate,AsA)、还原型谷胱甘肽(reduced glutathione,GSH)等抗氧化物质,抗氧化保护酶系和抗氧化物质协同作用,在直接和间接消除活性氧毒害中发挥重要作用。SOD是应对活性氧胁迫的第一道防线[21],它催化超氧阴离子形成过氧化氢和氧气,产生的过氧化氢进而由POD等酶清除[22]。张骥如飞等[23]研究发现,糜子接种黑穗病菌后苗期叶片SOD活性升高,POD活性变化规律不同,并且SOD活性和POD活性与黑穗病发病率呈负相关关系。APX、GR、AsA、GSH是抗坏血酸——谷胱甘肽循环中清除活性氧的重要物质,该循环中,APX是第一个关键酶,它利用AsA作为电子供体催化氧化还原反应来消除过氧化氢,AsA生成脱氢抗坏血酸(dehydroascorbate,DHA),GSH与DHA反应生成AsA和氧化型谷胱甘肽(oxidised glutathione,GSSG);GR是该循环中最后一个关键酶,它消耗NADPH催化GSSG再生成GSH[24-25]。此外,作为重要的抗氧化剂,AsA和GSH可以直接清除活性氧[26-27],还有研究表明AsA可参与信号转导诱导植物抗病性[28-29],GSH是维持细胞氧化还原平衡的重要成分,参与诱导防卫基因的表达[15]。【本研究切入点】目前,关于糜子黑穗病的研究停留在抗病资源的鉴定和防治方法方面,对糜子黑穗病抗性的生理生化机制和关键生理指标尚不明确。针对糜子接种黑穗病菌后叶片防御酶活性及抗氧化物质含量的研究报道较少,尤其是PAL、APX、GR活性的变化及AsA、GSH含量的变化尚未见报道。【拟解决的关键问题】本研究以不同糜子资源为材料,田间种植条件下采用种子饱和接种法接种黑穗病菌冬孢子,进行黑穗病抗性鉴定,筛选不同抗性糜子品种,进而研究其不同生育时期叶片防御酶PAL、APX、GR活性变化及抗氧化物质AsA、 GSH含量变化,探究糜子黑穗病的抗性机制,筛选鉴定糜子黑穗病抗性的生理生化指标,为糜子抗黑穗病品种选育提供依据。
1 材料与方法
1.1 材料
供试糜子材料为小杂粮课题组从国内外收集的430份糜子资源及区域试验品种。供试病原菌为糜子黑穗病菌冬孢子,采于山西大同田间发病植株,将冬孢子堆采下后阴干保存,拌种前过筛。
1.2 试验设计
试验于2012—2014年在西北农林科技大学农作一站进行,采用种子饱和接种法接种黑穗病菌冬孢子。2012—2013年对糜子资源进行田间黑穗病抗性鉴定,筛选出6个抗病差异显著的品种,于2014年进行生理指标测定,即在糜子生长的苗期、拔节期、抽穗期、灌浆期取样,每个重复随机选择3株糜子剪取整株叶片混合,样品置于冰盒带回实验室后保存于-40℃冰箱。试验均在大田条件下种植,3次重复。
1.3 酶液提取
酶液提取时随机选取已混合的糜子叶片,擦干表面水分,去除主脉,剪碎混匀,称取约0.5 g,在预冷的研钵中加液氮研磨,加入预冷缓冲液在冰浴中研磨成匀浆后定容(PAL的提取采用0.1 mol·L-1硼酸缓冲液,定容至10 mL;APX的提取采用50 mmol·L-1的PBS缓冲液,内含1 mmol·L-1的EDTA和1 mmol·L-1的AsA,定容至5 mL;GR的提取采用0.1 mol·L-1的Tricine-NaOH缓冲液,定容至5 mL),在4℃下10 000 r/min离心15 min,上清液即为待测酶液,于4℃保存备用。
1.4 PAL活性测定
参照高俊凤[30]的方法。反应体系为4 mL,包括0.02 mol·L-1L-苯丙氨酸1.0 mL、0.1 mol·L-1硼酸缓冲液(pH8.8)2.0 mL、稀释10倍的酶液1.0 mL,摇匀后置于30℃恒温水浴中保温反应60 min,之后加入6 mol·L-1HCl溶液0.2 mL终止反应,在290 nm波长处测定吸光度A290nm值。以反应液每小时A290nm增加0.01为一个酶活单位(U),结果表示为U·g-1FW。
1.5 APX活性测定
采用抗坏血酸氧化法[31]。取酶液0.1 mL,依次加入50 mmol·L-1的PBS缓冲液(pH7.0)1.8 mL、15mmol·L-1的AsA溶液0.1 mL、0.3 mmol·L-1的H2O21 mL。对照以0.1 mL的蒸馏水代替酶液。加入0.3 mmol·L-1的H2O2后立即用分光光度计测定10—60 s内A290nm值的变化。以1 min内A290nm值变化0.01定义为1个酶活单位(U),结果表示为U·g-1FW。
1.6 GR活性测定
采用辅酶Ⅱ法[31]稍作改变。反应体系为3 mL,依次加入1 mmol·L-1的NADPH溶液 0.3 mL、0.1mol·L-1的Tricine-NaOH缓冲液1.8 mL、稀释10倍的酶液0.6 mL、5 mmol·L-1的GSSG溶液0.3 mL,测定A340nm值的变化。以1 min内A340nm值变化0.01定义为1个酶活单位(U),结果表示为U·g-1FW。
1.7 AsA含量测定
采用二联吡啶法[31]并稍作改变。首先制作标准曲线,即配制不同浓度梯度的AsA标准液(0、0.1、0.2、0.3、0.4、0.5、0.6和0.7 mmol·L-1),分别取不同浓度的AsA标准液20 μL于不同的试管,之后分别加入150 mmol·L-1的NaH2PO4溶液200 μL、H2O 200 μL,混匀,30 s后再加入10%的TCA溶液400 μL、44% H3PO4溶液400 μL、4% 2,2-二联吡啶溶液400 μL、3% FeCl3溶液200 μL,混匀后在37℃水浴中保温反应60 min,测定A525nm值。结果以AsA浓度为横坐标,A525nm值为纵坐标绘制标准曲线。AsA提取时将糜子叶片去除叶脉后剪碎混匀,称取约0.5 g,在预冷的研钵中加液氮研磨,加入5%三氯乙酸5 mL,研磨成匀浆,之后15 000 r/min离心10 min,上清液定容至5 mL即为提取液。AsA含量测定时取提取液0.25 mL,测定方法同上,测定A525nm值根据标准曲线计算AsA含量,结果表示为μg·g-1FW。
1.8 GSH含量测定
采用DTNB法[31]稍作改变。首先配制不同浓度梯度的GSH标准液(0、0.02、0.04、0.06、0.08、0.10和0.12 mmol·L-1),取各标准液0.25 mL,分别加入150 mmol·L-1的NaH2PO4缓冲液2.6 mL,混匀后再分别加入DTNB试剂0.15 mL,摇匀后,30℃保温反应5 min,测定A412nm值,测定结果以GSH浓度为横坐标,A412nm值为纵坐标绘制标准曲线。GSH的提取方法同AsA的提取方法。GSH含量测定时取提取液0.25 mL,反应液加入方法同上,测定A412nm值,根据标准曲线计算GSH含量,结果表示为μg·g-1FW。
1.9 数据处理
试验数据采用Excel 2003软件进行统计,SPSS 22.0软件进行方差分析,SigmaPlot 12.5软件制图。
2 结果
2.1 糜子品种黑穗病抗性的鉴定
经过连续2年糜子黑穗病抗性鉴定,筛选出不同抗性的6个糜子品种,其中,黑虼蚤(R1)、驴驼川(R2)、小麦糜子(R3)平均发病率分别为0、0和0.73%,为抗病品种;黄硬黍(S1)、宁04-262(S2)、Ym0965(S3)平均发病率分别为19.71%、19.86%和32.28%,为感病品种(表1)。
2.2 黑穗病菌胁迫对糜子叶片防御酶活性的影响
糜子接种黑穗病菌后,抗、感品种叶片PAL活性呈现出明显不同的变化趋势,且同组品种同一时期的测定值相近(图1-A)。苗期各品种糜子叶片PAL活性在1 384.6—1 436.1 U·g-1FW范围内,处于较低水平,且抗、感品种间无显著差异,到拔节期2类品种PAL活性均显著升高,感病品种平均升高到3 610.8 U·g-1FW,显著高于抗病品种(平均为2 520.7 U·g-1FW),之后感病品种 PAL活性显著降低,而抗病品种仍为升高趋势,最终在灌浆期感病品种PAL活性平均降至2 425.0 U·g-1FW,显著低于抗病品种(平均为2 946.0 U·g-1FW)。
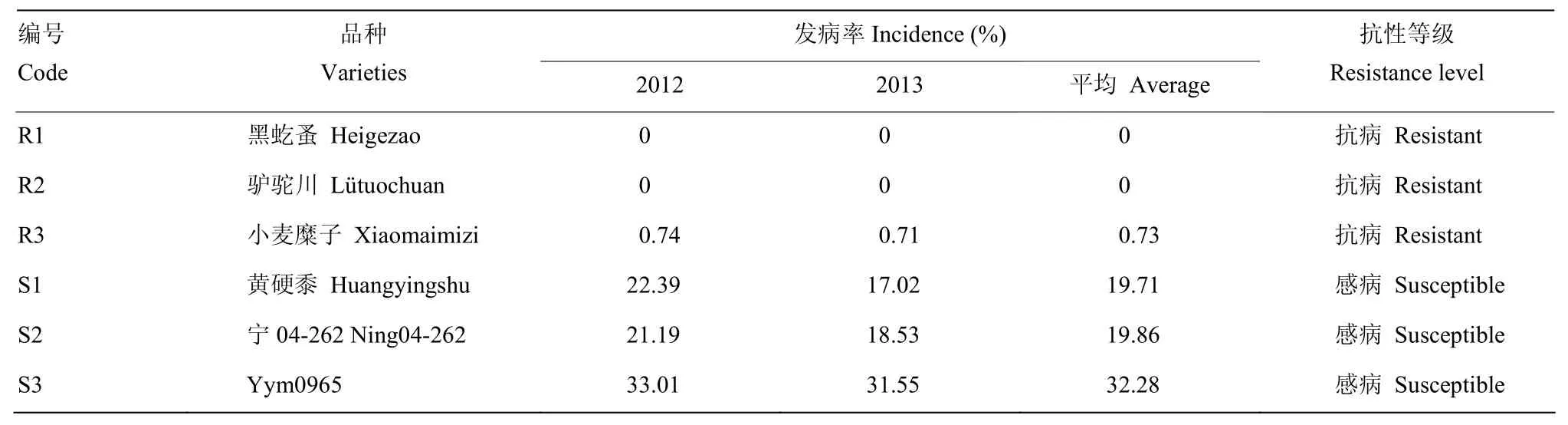
表1 糜子品种黑穗病发病率与抗性水平Table 1 The smut incidence and resistance level of broomcorn millet cultivars
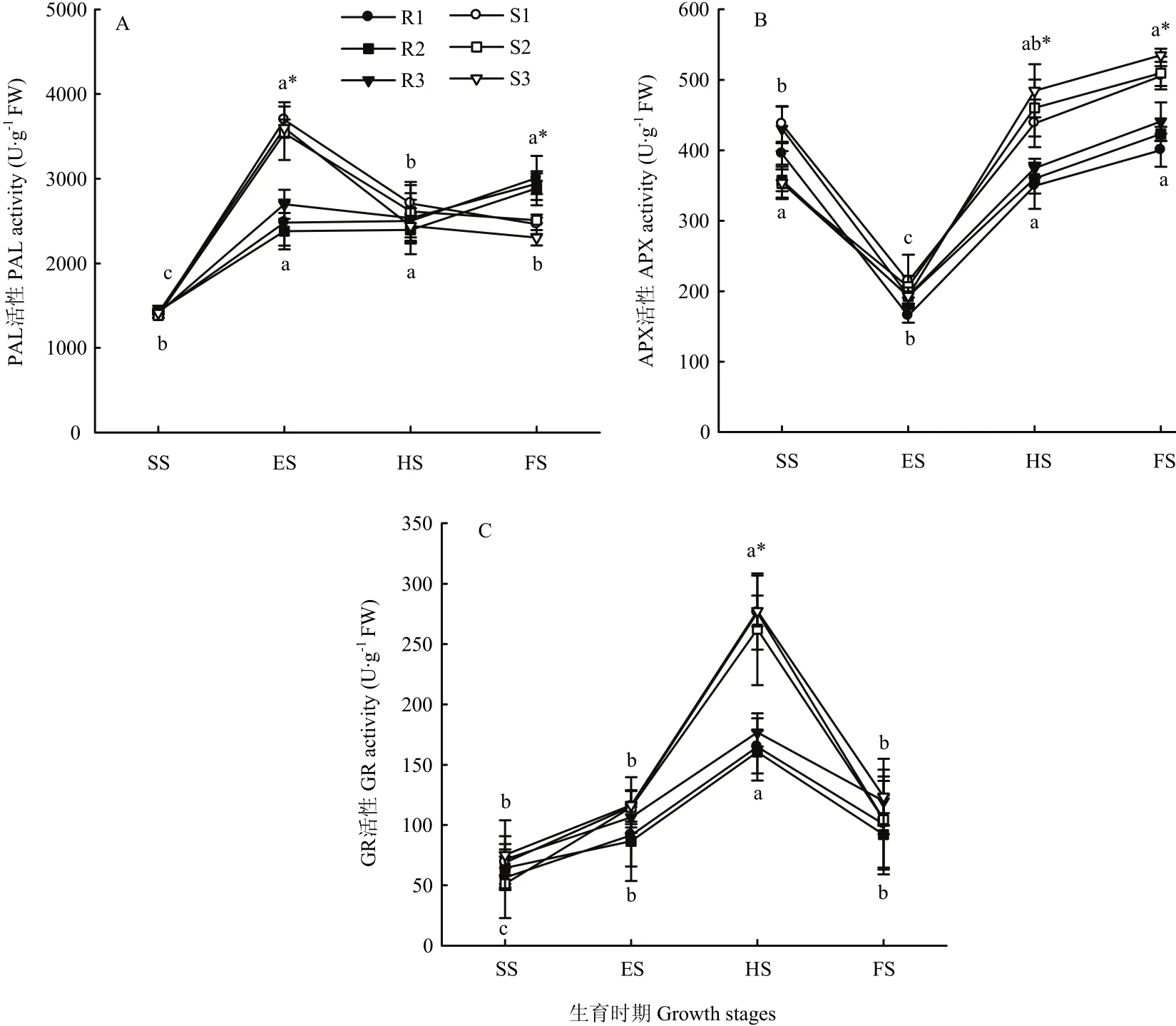
图1 黑穗病菌胁迫对糜子叶片防御酶活性的影响Fig. 1 Effects of smut fungus stress on defensive enzymes activity in broomcorn millet
由图1-B可知,黑穗病菌胁迫下抗、感品种糜子叶片APX活性均呈先降低后升高的变化趋势,拔节期APX活性显著最低。苗期和拔节期2类品种间APX活性无显著差异,到抽穗期和灌浆期感病品种叶片APX活性显著高于抗病品种,其中感病品种叶片平均APX活性分别为461.1和516.7 U·g-1FW,抗病品种为361.5和428.2 U·g-1FW。
接种黑穗病菌后2类品种糜子叶片GR活性均呈先升高后降低趋势,抽穗期GR活性显著高于其他3个时期,并且感病品种GR活性变化幅度大于抗病品种。抗、感品种糜子叶片GR活性显著差异出现在抽穗期,此时抗病品种平均GR活性为167.4 U·g-1FW,显著低于感病品种(271.9 U·g-1FW)。
2.3 黑穗病菌胁迫对糜子叶片抗氧化物质含量的影响
糜子接种黑穗病菌后,苗期到灌浆期各品种叶片AsA含量在147.7—344.8 μg·g-1FW范围内波动,无明显规律,并且抗、感品种间差异不显著(图2-A)。
黑穗病菌胁迫导致抗病品种糜子叶片GSH含量从苗期到抽穗期显著降低,抽穗期到灌浆期又显著升高,而感病品种叶片GSH含量从苗期到抽穗期显著降低后,抽穗期到灌浆期并无显著变化。灌浆期抗病品种叶片平均GSH含量为984.7 μg·g-1FW显著高于感病品种(平均为676.0 μg·g-1FW,图2-B)。
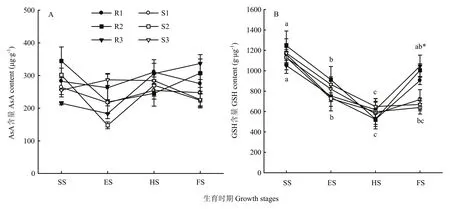
图2 黑穗病菌胁迫对糜子叶片抗氧化物质含量的影响Fig. 2 Effects of smut fungus stress on antioxidant content in broomcorn millet
3 讨论
关于PAL活性变化与植物抗病性的研究有很多报道。宋培玲等[32]研究发现油菜幼苗接种黑胫病菌后,PAL活性变化与油菜抗病性呈正相关;王萱[33]研究成株期辣椒与白粉病菌互作,认为辣椒白粉病抗性与PAL活性呈正相关关系;贺字典等[34]研究成株期玉米与黑穗病菌互作发现,抗病品种PAL活性升高幅度大于感病品种;刘丽等[35]研究发现拔节期玉米接种纹枯病菌后,高耐材料PAL活性表现为下降,高感材料PAL活性变化不明显;张淑珍等[36]研究大豆幼苗与疫霉根腐病菌互作发现抗、感品种PAL活性变化幅度均较小。因此,PAL活性与植物抗病性的关系可能会因不同寄主和病原菌组合而改变,也可能会因为取样时期的不同而存在差异。与前人只研究某一时期PAL活性与抗病性关系不同,本试验测定了糜子接种黑穗病菌后整个生育期内PAL活性的变化,探究PAL活性变化与抗病性关系。本试验中,抗病品种PAL活性虽然在拔节期显著低于感病品种,但最终在灌浆期显著高于感病品种,原因可能是感病品种在病原菌侵染后PAL活性迅速增加,然而随着生育进程的推进,感病品种由于自身缺乏抗性基因座引起PAL活性逐渐降低,PAL的保护作用明显减弱,最终在糜子黑穗病发病关键时期(灌浆期)丧失活力,导致黑穗病发生。反之,抗病品种存在抗性基因座,随着生育进程的推进,PAL的保护作用逐渐增强,最终在灌浆期达到峰值,有效地防止糜子黑穗病的发生。
关于APX活性变化与植物抗病性的关系存在不同的观点。刘丽等[35]研究拔节期玉米与纹枯病菌互作时发现APX活性与抗病性呈正相关;关西贞等[37]研究小麦近等基因系与白粉病菌互作后认为感病品系叶片APX活性在后期显著升高,高于抗病品系;刘会宁等[38]研究葡萄与黑痘病菌互作后认为APX活性与抗病性呈正相关还是负相关没有定论。本试验中,糜子接种黑穗病菌后抗、感品种在苗期和拔节期APX活性变化没有显著差异,但是到抽穗期和灌浆期感病品种叶片APX活性均显著高于抗病品种,与关西贞等[37]的观点相近。原因可能是糜子黑穗病发生后导致上部叶片丛生,不能正常抽穗,引起库源关系发生变化,前人研究认为库的缺失或库源比增大会减缓叶片的衰老[39-40],而叶片的衰老最终会导致APX活性降低[41],因此,导致抗病品种叶片APX活性低于感病品种。也可能是感病品种糜子发生黑穗病后穗部被充满病菌孢子的菌瘤所取代,导致糜子生理代谢失衡,活性氧积累加剧,引起应激反应,从而诱导APX活性大幅度提高。
GR是抗坏血酸——谷胱甘肽循环中最后一个关键酶,在应对氧化胁迫中具有重要作用。本试验接种黑穗病菌后,抗病品种和感病品种糜子叶片GR活性呈先升高后降低的趋势,与谢晓娜等[42]的研究结果一致。并且感病品种GR活性升高幅度大于抗病品种,特别是在抽穗期,感病品种叶片GR活性显著高于抗病品种,可能是因为感病品种对黑穗病菌胁迫更敏感,导致活性氧积累更多,通过诱导GR活性的提高来应对逆境胁迫,符合关西贞等[37]的观点。
陈利锋等[43]和马雪瑞等[44]认为对AsA与植物抗病性的关系存在3种不同的意见,一是AsA有利于抗病,抗病品种AsA积累;二是AsA可抑制抗病性的表达,感病品种积累较多的AsA;三是AsA与植物的抗病性无关。本试验中,糜子接种黑穗病菌后,抗、感病品种之间AsA含量变化无明显区别,由此推测,AsA可能与糜子对黑穗病的抗性无关,与WORKMAN等[45]在马铃薯黑斑病和软腐病的研究结果基本一致。
作为重要的抗氧化物质,GSH在应对活性氧胁迫中有重要作用。本试验中糜子受黑穗病菌胁迫后苗期到抽穗期GSH含量呈降低趋势,与植物受到其他胁迫后GSH含量变化趋势类似[15,46-47]。然而,抽穗期到灌浆期抗病品种叶片GSH含量显著升高而感病品种却没有显著变化,与余文英等[48]研究甘薯与疮痂病菌互作时GSH含量变化趋势一致,这说明抗病品种能够维持较高水平的GSH含量来有效抵御黑穗病菌胁迫。值得一提的是,在抗坏血酸——谷胱甘肽循环中,GR消耗NADPH将GSSG还原为GSH,以维持较高水平的GSH含量促进该循环高效运转,来应对活性氧胁迫[49]。但是本试验中GR活性的升高或降低并没有引起GSH含量相应地升高或降低。KUŹNIA等[15]研究番茄与霜霉病菌互作时,发现GR活性与GSH含量呈负相关关系,与本试验观点类似。另外,接种黑穗病菌后抗病品种灌浆期叶片GSH含量有显著升高,可能是因为GSH的生物合成得到提高[50],说明抗病品种能通过GSH生物合成来提高GSH含量,提高对逆境的抵御能力。
4 结论
不同品种糜子对黑穗病的抗性不同,黑穗病菌胁迫可引起糜子叶片防御酶活性及抗氧化物质含量变化,拔节期和灌浆期PAL活性、抽穗期和灌浆期APX活性、抽穗期GR活性、灌浆期GSH含量在抗病品种和感病品种间存在显著差异,可作为鉴定糜子对黑穗病抗性的生理生化指标。AsA含量变化能否作为抗性鉴定指标有待于进一步研究。
[1] 柴岩. 糜子. 北京: 中国农业出版社, 1999: 68-69. CHAI Y. Broomcorn Millet. Beijing: China Agriculture Press, 1999: 68-69. (in Chinese)
[2] 程炳文. 旱地先锋作物—糜子. 银川: 宁夏人民出版社, 2009: 10-11. CHENG B W. Pioneer Dry-land Crop—Broomcorn Millet. Yinchuan: Ningxia People's Press, 2009: 10-11. (in Chinese)
[3] 柴岩, 冯佰利, 王宏岩. 中国黄米食品. 杨凌: 西北农林科技大学出版社, 2012: 85-89. CHAI Y, FENG B L, WANG H Y. Chinese Millet Food. Yangling: Northwest Agriculture and Forestry University of Science and Technology Press, 2012: 85-89. (in Chinese)
[4] 林汝法, 柴岩. 中国小杂粮. 北京: 中国农业出版社, 2002: 85-90. Lin R F, Chai Y. Minor Grain Crops in China. Beijing: China Agriculture Press, 2002: 85-90. (in Chinese)
[5] 王星玉. 中国黍稷. 北京: 中国农业出版社, 1996: 59. WANG X Y. Chinese Broomcorn Millet. Beijing: China Agriculture Press, 1996: 59. (in Chinese)
[6] 王纶, 王星玉, 温琪汾, 赵卫红, 刘金玉. 中国黍稷种质资源抗黑穗病鉴定评价. 植物遗传资源学报, 2008, 9(4): 497-501. WANG L, WANG X Y, WEN Q F, ZHAO W H, LIU J Y. Identification and evaluation of resistance to dustbrand in Chinese prosomillet germplasm rescources. Journal of Plant Genetic Resources, 2008, 9(4): 497-501. (in Chinese)
[7] NGADZE E, ICISHAHAYO D, COUTINHO T A, WAALS J E. Role of polyphenol oxidaase, peroxidase, phenylalanine ammonia lyase, chlorogenic acid, and total soluble phenols in resistance of potatoes to soft rot. Plant Disease, 2012, 96(2): 168-192.
[8] VANITHA S C, NIRANJANA S R, UMESHA S. Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. Journal of Phytopathology, 2009, 157: 552-557.
[9] FORTUNATO A A, DEBONA D, BERNARDELI A M A, RODRIGUES F A. Defence-related enzymes in soybean resistance to target spot. Journal of Phytopathology, 2015, 163: 731-742.
[10] 李唯. 植物生理学. 北京: 高等教育出版社, 2012: 75-76, 401-404. LI W. Phytophysiology. Beijing: Higher Education Press, 2012: 75-76, 401-404. (in Chinese)
[11] ARMAS R, SANTIAGO R, LEGAZ M E, VICENTE C. Levels of phenolic compounds and enzyme activity can be used to screen for resistance of sugarcane to smut (Ustilago sciaminea). AustralasianPlant Pathology, 2007, 36: 32-38.
[12] BAI X H, TENG L H, LÜ D Q, QI H Y. Co-treatment of EFF and 1-MCP for enhancing the shelf-life and aroma volatile compounds of oriental sweet melons (Cucumis melo var. makuwa Makino). Journal of Integrative Agriculture, 2014, 13(1): 217-227.
[13] DUAN L, LIU H B, LI X H, XIAO J H, WANG S P. Multiple phytohormones and phytoalexins are involved in disease resistance to Magnaporthe oryzae invaded from roots in rice. Physiologia Plantarum, 2014, 152(3): 486-500.
[14] ZHANG X B, LIU C J. Multigaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Molecular Plant, 2015, 8: 17-27.
[15] KUŹNIA K E, SKŁODOWSKA M. Ascorbae, glutathione and related enzymes in chloroplasts of tomato leaves infected by Botrytis cinerea. Plant Science, 2001, 160: 723-731.
[16] 马莹莹, 贾娇, 苏前富, 孟玲敏, 高洁, 晋齐鸣. 玉米抵御玉蜀黍尾孢菌侵入的生理机制. 植物保护学报, 2015, 42(3): 340-346. MA Y Y, JIA J, SU Q F, MENG L M, GAO J, JIN Q M. The physiological mechanism of corn against Cercospora zeae-maydis infection. Journal of Plant Protection, 2015, 42(3): 340-346. (in Chinese)
[17] TANG B, XU S Z, ZOU X L, ZHENG Y L, QIU F Z. Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of waterlogging-tolerant and waterlogging-sensitive maize genotypes at seedling stage. Agriculture Science in China, 2010, 9(5): 651-661.
[18] 张盼盼, 冯佰利, 王鹏科, 高小丽, 拓菊梅, 柴岩, 宋慧. 干旱条件下糜子叶片衰老与保护酶活性变化. 干旱地区农业研究, 2010, 28(2): 99-103, 108. ZHANG P P, FENG B L, WANG P K, GAO X L, TA J M, CHAI Y, SONG H. Leaf senescence and protective enzyme system of broomcorn millet under drought condition. Agricultural Research in the Arid Areas, 2010, 28(2): 99-103, 108. (in Chinese)
[19] 王国骄, 王嘉宇, 马殿荣, 苗微, 赵明辉, 陈温福. 不同耐冷性杂草稻和栽培稻抗氧化系统对冷水胁迫的响应. 中国农业科学, 2015, 48(8): 1660-1668. WANG G J, WANG J Y, MA D R, MIAO W, ZHAO M H, CHEN W F. Response of antioxidant system to cold water stress in weedy and cultivated rice with different chilling sensitivity. Scientia Agricultura Sinica, 2015, 48(8): 1660-1668. (in Chinese)
[20] MA P, BAI T H, WANG X Q, MA F W. Effects of light intensity on photosynthesis and photoprotective mechanisms in apple under progressive drought. Journal of Integrative Agriculture, 2015, 14(9): 1755-1766.
[21] GAO J J, LI T, YU X C. Gene expression and activities of SOD in cucumber seedlings were related with concentrations of Mn2+, Cu2+, or Zn2+under low temperature stress. Agricultural Science in China, 2009, 8(6): 678-684.
[22] MELONI D A, OLIVA M A, MARTINEZ C A, CAMBRAIA J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environmental and Experimental Botany, 2003, 49(49): 69-76.
[23] 张骥如飞, 周瑜, 杨璞, 王鹏科, 高金锋, 高小丽, 冯佰利. 糜子感染黑穗病菌后的生理变化及与抗病性关系. 中国农业大学学报, 2015, 20(3): 108-113. ZHANG J R F, ZHOU Y, YANG P, WANG P K, GAO J F, GAO X L, FENG B L. Study on physiological changes and correlation with resistance level to the head smut of broomcorn millet after an infection with Sphacelotheca destruen. Journal of China Agricultural University, 2015, 20(3): 108-113. (in Chinese)
[24] XU R, YAMADA M, FUJIYAMA H. Lipid peroxidation and antioxidative enzymes of two turfgrass species under salinity stress. Soil Science Society of China, 2013, 23(2): 213-222.
[25] HUANG G Y, WANG Y S, SUN C C, DONG J D, SUN Z X. The effect of multiple heavy metals on ascorbate, glutathione and related enzymes in two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). International Journal of Oceanography and Hydrobiology, 2010, 39(1): 11-25.
[26] MA Y H, MA F W, WANG Y H, ZHANG J K. The responses of the enzymes related with ascorbate-glutathione cycle during drought stress in apple leaves. Acta Physiology Plant, 2011, 33: 173-180.
[27] BLOKHINA O, VIROLAINEN E, FAGERSTEDT K V. Antioxidants,oxidative damage and oxygen deprivation stress: A review. Annals of Botany, 2003(91): 179-194.
[28] KAWANO T, MUTO S. Mechanism of peroxidase actions for salicylic acid-induced generation of active oxygen species and an increase in cytosolic calcium in tobacco cell suspension culture. Journal of Experimental Botany, 2000, 345: 685-693.
[29] 马春花, 李明军, 李翠英, 邵建辉, 马锋旺. 不同抗性苹果砧木叶片抗坏血酸代谢对干旱胁迫的响应. 西北植物学报, 2011, 3(8): 1596-1602. MA C H, LI M J, LI C Y, SHAO J H, MA F W. Response of ascorbic acid metabolism in apple rootstocks leaves under drought stress. Acta Botanica Boreali-Occidentalia Sinica, 2011, 3(8): 1956-1602. (in Chinese)
[30] 高俊凤. 植物生理学实验指导. 北京: 高等教育出版社, 2006: 219-220.GAO J F. Plant physiology Experiment Instruction. Beijing: Higher Education Press, 2006: 219-220. (in Chinese)
[31] 陈建勋. 植物生理学实验指导. 广州: 华南理工大学出版社, 2006: 70-71, 75-77. CHEN J X. Plant Physiology Experiment Instruction. Guangzhou: South China University of Technology Press, 2006: 70-71, 75-77. (in Chinese)
[32] 宋培玲, 张键, 郝丽芬, 皇甫海燕, 袁喜丽, 包玉英, 李子钦. 不同抗性油菜品种接种黑胫病菌防御酶活性变化研究. 华北农学报, 2015, 30(2): 110-115. SONG P L, ZHANG J, HAO L F, HUANGFU H Y, YUAN X L, BAO Y Y, LI Z Q. Changes in activities of defense enzymes in different rapeseed cultivars infected by Leptosphaeria biglobosa. Acta Agriculturae Boreali-Sinica, 2015, 30(2): 110-115. (in Chinese)
[33] 王萱. 辣椒白粉病抗性与苯丙氨酸解氨酶活性的关系. 中国农学通报, 2009, 25(3): 193-196. WANG X. Relationship between resistance to leveillula taurica and PAL activity in pepper. Chinese Agricultural Science Bulletin, 2009, 25(3): 193-196. (in Chinese)
[34] 贺字典, 高增贵, 庄敬华, 陈捷, 郑俊强, 唐树戈. 玉米丝黑穗病菌对寄主防御相关酶活性的影响. 玉米科学, 2006, 14(2): 150-151, 155. HE Z D, GAO Z G, ZHUANG J H, CHEN J, ZHENG J Q, TANG S Y. Effect of maize head smut pathology (Sphacelotheca reiliana) on the major defensive enzymes of host. Journal of Maize Science, 2006, 14(2): 150-151, 155. (in Chinese)
[35] 刘丽, 马永毅, 张志明, 冷鹏飞, 潘光堂, 赵茂俊. 玉米不同防卫酶系对纹枯病作用的研究. 玉米科学, 2009, 17(3): 99-102, 106. LIU L, MA Y Y, ZHANG Z M, LENG P F, PAN G T, ZHAO M J. Analysis function of different defense enzymes on maize. Journal of Maize Science, 2009, 17(3): 99-102, 106. (in Chinese)
[36] 张淑珍, 靳立梅, 徐鹏飞, 陈维元, 吴俊江, 李文滨, 邱丽娟, 常汝镇. 野生大豆接种大豆疫霉根腐病后苯丙氨酸解氨酶(PAL)活性的变化. 大豆科学, 2009, 28(6): 1044-1048. ZHANG S J, JIN L M, XU P F, CHEN W Y, WU J J, LI W B, QIU L J, CHANG R Z. Response of activity to phytophthora sojae inoculation in glycine soja. Soybean Science, 2009, 28(6): 1044-1048. (in Chinese)
[37] 关西贞, 张卫东, 田纪春. 小麦近等基因系与白粉病菌互作的生理指标研究. 华北农学报, 2010, 25(1): 217-221. GUAN X Z, ZHANG W D, TIAN J C. Physiological indicators of near-isogenic wheat lines in interaction with powdery mildew. Acta Agriculturae Boreali-Sinica, 2010, 25(1): 217-221. (in Chinese)
[38] 刘会宁, 唐华程. 葡萄抗黑痘病与3种酶活性的关系. 长江大学学报(自然科学版), 2013, 10(35): 8-12. LIU H N, TANG H C. Relationship between resistance of grape to Sphaceloma ampelinum and 3 enzymes activity. Journal of Yangtze University (Natural Science Edition), 2013, 10(35): 8-12. (in Chinese)
[39] 段留生, 韩碧文, 何钟佩. 器官间关系对叶片衰老的影响. 植物学通报, 1998, 15(1): 43-49. DUAN L S, HAN B W, HE Z P. The effects of corelation between leaf and other organs on leaf senescence. Chinese Bulletin of Botany, 1998, 15(1): 43-49. (in Chinese)
[40] 黄升谋. 水稻源库关系与叶片衰老的研究. 江西农业大学学报, 2001, 23(2): 171-173. HUANG S M. A study on the relationship between the leaf senescence and source sink ratio in hybrid rice. Acta Agriculturae Universitatis Jiangxiensis, 2001, 23(2): 171-173. (in Chinese)
[41] ŠPUNDOVÁ M, SLOUKOVÁ K, HUNKOVÁ M, NAUŠ J. Plant shading increases lipid peroxidation and intensifies senescenceinduced changes in photosynthesis and activities of ascorbate peroxidase and glutathione reductase in wheat. Photosynthetica, 2005, 43(3): 403-409.
[42] 谢晓娜, 张小秋, 梁永检, 杨丽涛, 李杨瑞. 宿根矮化病菌侵染后甘蔗防御酶活性变化. 南方农业学报, 2014, 45(9): 1551-1557. XIE X N, ZHANG X Q, LIANG Y J, YANG L T, LI Y R. Changes of defense-related enzyme activities in sugarcane under ratoon stuntiong disease stress. Journal of Southern Agriculture, 2014, 45(9): 1551-1557. (in Chinese)
[43] 陈利锋, 叶茂炳, 陈永幸, 徐朗莱, 徐雍皋. 抗坏血酸与小麦抗赤霉病性的关系. 植物病理学报, 1997, 27(2): 113-118. CHEN L F, YE M B, CHEN Y X, XU L L, XU Y G. The relationship between ascorbic acid and resistance of wheat to scab. Acta Phytopathologica Sinica, 1997, 27(2): 113-118. (in Chinese)
[44] 马雪瑞, 段玉玺, 陈立杰, 刘大伟, 王媛媛, 朱晓峰. 2011.利用抗坏血酸揭示小粒黑豆对胞囊线虫抗性的研究. 大豆科学, 2011, 30(1): 123-126. MA X R, DUAN Y X, CHEN L J, LIU D W, WANG Y Y, ZHU X F. Revealing resistance of Xiaoliheidou to soybean cyst nematode by ascorbic acid. Soybean Science, 2011, 30(1): 123-126. (in Chinese)
[45] WORKMAN M, HOLM D G. Potato clone variation in blackspot and soft rot susceptibility, redox potential, ascorbic acid, dry matter and potassium. American Potato Journal, 1984, 61(12): 726-733.
[46] 丁玲, 吴雪, 杜长霞, 徐艳丽, 樊怀福. 短期干旱胁迫对黄瓜幼苗叶片抗氧化系统的影响. 浙江农林大学学报, 2015, 32(2): 285-290. DING L, WU X, DU C X, XU Y L, FAN H F. An antioxidantsystem in cucumber seedling leaves with short term drought stress. Journal of Zhejiang Agriculture and Forestry, 2015, 32(2): 285-290. (in Chinese)
[47] 王俊力, 王岩, 赵天宏, 曹莹, 刘玉莲, 段萌. 臭氧胁迫对大豆叶片抗坏血酸-谷胱甘肽循环的影响. 生态学报, 2011, 31(8): 2068-2075. WANG J L, WANG Y, ZHAO T H, CAO Y, LIU Y L, DUAN M. Effects of ozone on AsA-GSH cycle in soybean leaves. Acta Ecologica Sinica, 2011, 31(8): 2068-2075. (in Chinese)
[48] 余文英, 潘延国, 柯玉琴, 艾育芳, 阮妙鸿. 甘薯抗疮痂病的活性氧代谢研究. 河南科技大学学报(农学版), 2003, 23(3): 1-6. YU W Y, PAN Y G, KE Y Q, AI Y F, RUAN M H. Active oxygen metabolism of sweet potato under the stress of sweet potato scab. Journal of Henan University of Science and Technology (Agricultural Scicence), 2003, 23(3):1-6. (in Chinese)
[49] GILL S S, ANJUM N A, HASANUZZAMAN M, GILL R, TRIVEDI D K, AHMAD I, PEREIRA E, TUTEJA N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiology and Biochemistry, 2013, 70(1): 204-212.
[50] MOTOS J R A, VIVANCOS P D, ALVAREZ S, GARCIA M F, BLANCO M J S, HERNANDEZ J A. Physiological and biochemical mechanisms of the ornamental Eugenia myrtifolia L. plants for coping with NaCl stress and recovery. Planta, 2015, 242: 829-864.
(责任编辑 李莉)
Response of Leaf Defensive Enzymes and Antioxidant to Smut Fungus Stress in Broomcorn Millet
ZHOU Yu1, LIU Jia-jia1, ZHANG Pan-pan2, QU Yang3, ZHANG Ji-ru-fei1, ZHU Ming-qi1, FENG Bai-li1
(1College of Agronomy, Northwest A&F University/State Key Laboratory of Crop Stress Biology for Arid Areas, Yangling 712100, Shaanxi;2Heilongjiang August First Agricultural University/National Coarse Cereals Engineering Research Center, Daqing 163319, Heilongjiang;3Baoji Academy of Agricultural Sciences, Qishan 722400, Shaanxi)
【Objective】 Head smut is an important disease that threats the yield of broomcorn millet seriously and the best way to control the disease is to plant resistant varieties. In order to screen the physiological and biochemical indexes of broomcorn millet for resistance to head smut, the activity of defensive enzymes and content of antioxidant were measured under the smut fungus stress. Furthermore, this will also provide theoretical supports for the breeding of broomcorn millet smut resistant varieties. 【Method】Artificial inoculation of seed saturated inoculation was adopted to infect broomcorn millet and inoculated broomcorn millet plants were planted in field. A field experiment of resistance levels identification to screen the varieties with different resistance was conducted in 2012-2013. In 2014, the broomcorn millet varieties with different resistance levels were used to measure the activity of defensive enzymes and content of antioxidant at seedling stage (SS), elongation stage (ES), heading stage (HS) and filling stage (FS) under the smut fungus stress. The defensive enzymes include the phenylalanine ammonia lyase (PAL), ascorbate peroxide (APX), glutathione reductase (GR) and the antioxidant contained the ascorbate (AsA) and glutathione (GSH). 【Result】 After 2 consecutive years of resistance identification, the average incidence rate of Heigezao (R1), Lvtuochuan (R2) and Xiaomaimizi (R3) were 0, 0 and 0.73%, respectively, indicating that they were disease-resistant varieties; the average incidence rate of Huangyingshu (S1), Ning04-262 (S2) and Yym0965 (S3) was 19.71%, 19.86% and 32.28%, respectively, therefore, they belong to disease-susceptible varieties. With the stress of smut fungus, the PAL activity of susceptible varieties changed greater than that in resistant varieties, since the PAL activity of susceptible varieties was significantly higher at elongation stage (3 610.8 U·g-1FW), but significantly lower at filling stage (2 425.0 U·g-1FW) compared to that (2 520.7 and 2 946.0 U·g-1FW, respectively) of resistant varieties. The APX activity showed the same trend in both kinds of varieties, which was decreased first and then increased; the minimum value appeared at the elongation stage. At heading stage and filling stage, the APX activity of susceptible varieties was 461.1 U·g-1FW and 516.7 U·g-1FW, which was significantly higher than that (361.5 U·g-1FW and 428.2 U·g-1FW) in resistant varieties. The GR activity was increased first and then decreased in all the varieties. At heading stage, the value of GR activity was significantly higher than that of other 3 stages. The difference between the 2 kinds of varieties was that the GR activity of susceptible varieties was obviously higher than that of resistant varieties at heading stage, the value of which was 271.9 U·g-1FW and 167.4 U·g-1FW, respectively. After inoculation with smut fungus, the content of AsA ranged from 147.7 μg·g-1FW to 344.8 μg·g-1FW without obvious regularity in all the varieties. Also there was no significant difference between resistant and susceptible varieties. The GSH content of resistant varieties was decreased significantly from seedling stage to heading stage, and then increased significantly at filling stage. The GSH content of susceptible varieties was also decreased significantly from seedling stage to heading stage, but there was no significant difference compared to filling stage. Therefore, the GSH content was obviously higher in resistant varieties (984.7 μg·g-1FW) than that in susceptible varieties (676.0 μg·g-1FW) at filling stage. 【Conclusion】 Different broomcorn millet varieties have different resistance levels to head smut disease. Smut fungus stress can cause the changes of defensive enzymes activity and antioxidant content in leaves of broomcorn millet. The PAL activity of broomcorn millet leaves at elongation stage and filling stage, APX activity at heading stage and filling stage, GR activity at heading stage, GSH content at filling stage are obviously different between resistant and susceptible varieties, which can be used as the physiological and biochemical indexes to identify head smut resistant germplasms in broomcorn millet.
broomcorn millet; head smut; defense enzymes; antioxidant
2016-03-30;接受日期:2016-06-01
国家“十二五”科技支撑计划(2014BAD07B03)、国家自然科学基金(31371529)、国家谷子糜子产业技术体系(CARS-07-A9)、陕西省科技统筹创新工程计划(2014KTZB02-03)
联系方式:周瑜,E-mail:yuzhou@nwsuaf.edu.cn。刘佳佳,E-mail:ljjzl2014@163.com。周瑜和刘佳佳为同等贡献作者。通信作者冯佰利,E-mail:7012766@163.com

